Abstract
Fosfomycin (C3H7O4P) is a phosphonic acid derivative representing an epoxide class of antibiotics. The drug is a re-emerging bactericidal antibiotic with a wide range of actions against several Gram-positive and Gram-negative bacteria. Among the existing antibacterial agents, fosfomycin has the lowest molecular weight (138 Da), which is not structurally associated with other classes of antibiotics. In intensive care unit (ICU) patients, severe soft tissue infections (STIs) may lead to serious life-threatening problems, and therefore, appropriate antibiotic therapy and often intensive care management (ICM) coupled with surgical intervention are necessary. Fosfomycin is an antibiotic primarily utilized for the treatment of STIs in ICUs. Recently, fosfomycin has attracted renewed interest for the treatment of serious systemic infections caused by multidrug-resistant Enterobacteriaceae. In some countries, intravenous fosfomycin has been prescribed for various serious systemic infections, such as acute osteomyelitis, nosocomial lower respiratory tract infections, complicated urinary tract infections, bacterial meningitis, and bacteremia. Administration of intravenous fosfomycin can result in a sufficient concentration of the drug at different body regions. Dose modification is not required in hepatic deficiency because fosfomycin is not subjected to enterohepatic circulation.
Introduction
Fosfomycin (C3H7O4P) is a phosphonic acid derivative representing an epoxide class of antibiotics. Fosfomycin is a re-emerging bactericidal antibiotic with a wide range of actions against several Gram-positive and Gram-negative bacteria, including vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus, and carbapenem-resistant Enterobacteriaceae. Some non-fermenters such as Acinetobacter are inherently resistant to fosfomycin, whereas Pseudomonas is susceptible to the antibiotics in vitro.Citation1–Citation3 In the past decade, fosfomycin was isolated from some strains of Streptomyces, but nowadays it is produced synthetically. Among the existing antibacterial agents, fosfomycin has the lowest molecular weight (138 Da), which is not structurally associated with other classes of antibiotics.Citation4,Citation5
In intensive care unit (ICU) patients, severe soft tissue infections (STIs) may lead to serious life-threatening problems, and therefore, appropriate antibiotic therapyCitation6 and often intensive care management (ICM) coupled with surgical intervention are necessary. Fosfomycin is considered an antibiotic primarily appropriate for the treatment of STIs in ICUs.Citation7
Some appropriate properties of fosfomycin in healthy volunteers (eg, identical concentrations of the drug in the soft tissues and plasma) have made the drug a frequently administered antibiotic in the treatment of patients with sepsis and/or STI especially in Europe.Citation8 Nevertheless, in USA, the Food and Drug Administration (FDA) has approved fosfomycin only for the management of uncomplicated cystitis.
Recently, the use of fosfomycin has attracted renewed interest for the treatment of serious systemic infections caused by multidrug-resistant Enterobacteriaceae.Citation3 In some countries, intravenous fosfomycin has been prescribed for various serious systemic infections, such as acute osteomyelitis, nosocomial lower respiratory tract infections, complicated urinary tract infections, bacterial meningitis, and bacteremia.Citation3,Citation9 Fosfomycin trometamol, an oral formulation of fosfomycin, is available in USA and in Europe (in additional to the parenteral disodium compound) as used for therapy of cystitis in one 3 g dose or multiple oral doses of 3 g administered every other day.
Mechanism of action
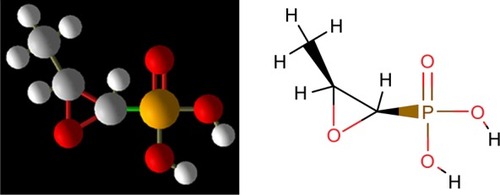
Generally, antibiotics have the bactericidal and/or bacteriostatic activities and affect the vital functions essential for bacteria, including cell wall synthesis, protein translation, DNA duplication, RNA transcription, and/or cell membrane organization. Fosfomycin mechanism of action is unique; the drug irreversibly inhibits the initial phase of microbial cell wall synthesis.Citation10 Chemical structure of fosfomycin is shown in .
Figure 1 Chemical structure of fosfomycin.
Fosfomycin must enter into the bacterial cytoplasm for the bactericidal activity. To reach the target cell, fosfomycin uses the bacterial hexose monophosphate transport system (stimulated by glucose-6-phosphate [G6P]) and the bacterial l-a-glycerophosphate transport system (activated by glycerol-3-phosphate [G3P]). The chemical structure of fosfomycin mimics both G6P and G3P.Citation11,Citation12
In the bacterial cytoplasm, fosfomycin binds UDP-GlcNAc enolpyruvyl transferase (MurA) and inactivates a vital enzyme such as enolpyruvyl transferase (MurA) involved in the peptidoglycan biosynthesis.Citation13 MurA catalyzes the first phase of the peptidoglycan biosynthesis.Citation14 Fosfomycin inhibits the peptidoglycan biosynthesis via preventing the formation of UDP-GlcNac-3-O-enolpyruvate from UDP-GlcNAc and phosphoenolpyruvate (PEP), resulting in bacterial cell destruction.Citation12 Fosfomycin acts as a PEP analog and competes with that.Citation15 Furthermore, fosfomycin reduces penicillin-binding proteins (PBPs).Citation10
Pharmacodynamic (PD) characteristics of fosfomycin
It is not clear whether fosfomycin shows a concentration-dependent or a time-dependent bactericidal function.Citation16 In this regard, some studies have demonstrated that fosfomycin exhibits a concentration-dependent activity to destruct strains of Escherichia coli and Proteus mirabilis in vitro as well as strains of Streptococcus pneumoniae in vivo.Citation17,Citation18 However, other studies have indicated a time-dependent bactericidal action of fosfomycin to destroy strains of S. aureus in vitro.Citation4,Citation19
Depending on the applied concentration of fosfomycin, the drug may exhibit an extended post-antibiotic effect (PAE) (between 3.4 and 4.7 h) against strains of E. coli and P. mirabilis in vitro.Citation17 However, a comparatively smaller PAE has been seen against S. aureus strains (0.5–1.4 h).Citation20
Pharmacokinetic (PK) characteristics of fosfomycin
Fosfomycin disodium is a very hydrophilic agent. Approximately 3% of the drug is bound to serum proteins and permits favorable tissue availability. The low molecular weight warrants high diffusibility of the drug.Citation3
After intravenous administration, blood content of fosfomycina shows a rapid disposition phase followed by a slow distribution phase.Citation21 Following the administration of multiple doses, a cumulative effect is seen. Elimination half-life of fosfomycin disodium is 1.5–2 h.Citation22–Citation24 The Cmax calculated with the standard intravenous formulation of the drug ranges from 200 to 644 mg/L, which is 10–20 times greater than the oral dose.Citation25,Citation26 The volume of the drug distribution is 18–27 L at a steady state.Citation11
Administration of intravenous fosfomycin can result in a sufficient concentration of the drug at different body regions, such as bone, muscle, lung, appendix, cerebrospinal fluid, gallbladder, common bile duct, and heart valves.Citation11,Citation25 Dose modification is not required in the hepatic deficiency because fosfomycin is not subjected to enterohepatic circulation.Citation11 Approximately 93% of an administered dose undergoes the glomerular filtration in the kidney and is excreted unaltered in the urine.Citation11 For serious systemic infections, fosfomycin disodium is utilized between 12 and 24 g as 2–4 divided doses. Reduction of daily dose of the drug is necessary for creatinine clearance of <40 mL/min. Addition of a dose of 2 g after each session has been suggested for patients subjected to intermittent hemodialysis. No dose adjustment is needed in continuous renal replacement therapy (CRRT).Citation27
Adverse effects
A number of adverse effects, including mild and self-limited gastrointestinal disorders (eg, nausea, abdominal pain, diarrhea, and dyspepsia), have also been reported following the oral administration of fosfomycin.Citation27 Other side effects, including dizziness, headaches, vaginitis, respiratory infections, and microbial superinfections, may also occur. Laboratory changes involve alterations in the number of blood cells (eosinophils, neutrophils, red blood cells, and platelets) and increase in the liver enzymes and bilirubin but no change in the renal function.Citation28
Following intravenous administration of fosfomycin, the potential adverse effects such as hypokalemia and sodium overload may occur. Each gram of intravenous fosfomycin consists of 0.32 g of sodium.Citation29 Furthermore, fosfomycin may increase potassium renal excretion, resulting in hypokalemia. Other adverse effects, including infusion site reactions, heart failure, and hypertension (because of sodium overload), and increased alanine aminotransferase (ALT) may be developed by an intravenously administered fosfomycin dose.Citation30 Therefore, administration of potassium supplements is deemed to be necessary in patients receiving fosfomycin, and their levels should be monitored regularly. In patients with heart failure, caution is also essential.Citation27
Drug interaction
ICU patients are at a high risk for developing the resistant bacterial infections, and therefore, a combined antibacterial treatment is suggested for them.Citation31,Citation32 Fosfomycin has been reported to show a 100% synergistic effect after combining with other antibacterial drugs.Citation33
Preventing the different stages of cell wall synthesis may lead to the synergistic effect of fosfomycin and β-lactam antibiotics; fosfomycin prevents the first stage of the cell wall synthesis procedure, whereas β-lactam antibiotics inhibit the final phase.Citation34 The potency of fosfomycin to alter the function of PBPs may also induce the synergistic effect between fosfomycin and β-lactam antibiotics.Citation35,Citation36 The ciprofloxacin-mediated destruction of the bacterial outer membrane can enhance the penetration and action of fosfomycin and promote the synergistic effect between fosfomycin and ciprofloxacin.Citation37 For treating Pseudomonas aeruginosa infections, synergy between fosfomycin and a wide range of other antibiotics, including cefepime, amikacin, aztreonam, meropenem, imipenem, ceftazidime, gentamicin, and ciprofloxacin, has been reported.Citation38,Citation39 Fosfomycin combined with amikacin or sulbactam has a synergistic effect to fight against Acinetobacter baumannii strains and may consider an efficient combination therapy for the treatment of A. baumannii infections.Citation40,Citation41 With respect to methicillin-resistant S. aureus, Enterococcus, Streptococcus, and Enterobacteriaceae species, fosfomycin also has synergistic effects when combined with other antibacterial agents.Citation33,Citation34 In addition to high antibacterial effectiveness, fosfomycin can decrease toxicity related to other drugs (eg, glycopeptides, aminoglycosides, and polymyxin B) as lower doses of these antibiotics can be administered.Citation42–Citation44
Fosfomycin resistance
Some mechanisms of fosfomycin resistance have been described.Citation45 The chromosomal resistance is caused by mutations in the genes encoding the G6P transporter or the G3P transporter resulting in the reduced uptake of the drug by the pathogen.Citation46,Citation47 Another resistance mechanism is based on modifications in the targeted enzyme (Mur A) (point mutations at the binding site of the murA gene),Citation48 which decreases the affinity of fosfomycin. Increased expression of the murA gene also results in clinical resistance to fosfomycin.Citation49 The third mechanism of resistance is based on the inactivation of fosfomycin either by enzymatic cleavage of the epoxide ring or by phosphorylation of the phosphonate group. In the presence of the plasmid-mediated fosfomycin-modifying metalloenzymes (FosA, FosB, and FosX), the epoxide structure is cleaved.Citation50 Some kinases including FomA and FomB cause phosphorylation of fosfomycin to the diphosphate and triphosphate states leading to fosfomycin degradation.Citation51,Citation52
Fosfomycin in critically ill patients
In recent years, the multidrug resistance to the used antibiotics has increased. Therefore, there is a crucial need for the development of new antimicrobial candidates.Citation53–Citation61 Obtaining the appropriate concentrations of antibiotics at target sites is crucial to eliminate the relevant pathogens and clinical outcomes.Citation62–Citation64 Recent studies in patients with sepsis have shown that despite sufficient concentrations of antibiotics in plasma, their concentrations in the interstitial fluid of soft tissues may be inadequate. This may be due to deficiency in transcapillary transfer of antibiotics to target sites.Citation65–Citation69 Thus, most available antibiotics have a reduced tissue penetration in the septic patients. In this regard, the target site penetration of fosfomycin, an antibiotic mainly appropriate for the treatment of STIs in ICU patients, has been investigated. In nine patients with sepsis, the microbiologically active levels of fosfomycin were evaluated in the interstitial space fluid of skeletal muscle and correlated with the corresponding plasma concentrations.Citation7 The results demonstrated that fosfomycin concentrations in plasma and muscle interstitium exceeded the minimum inhibitory concentrations (MICs) for various clinically important pathogens (Streptococcus pyogenes, S. aureus, and P. aeruginosa). Thus, fosfomycin shows a tissue PK profile, which suggests a substitute for other broad-spectrum antibiotics in critically ill patients undergoing STI.Citation7
In a study on critically ill patients, the optimal dosage regimen of intravenous fosfomycin in combination with carbapenem and based on PK/PD targets was evaluated for the treatment of P. aeruginosa.Citation70 The P. aeruginosa isolates were recovered from various clinical specimens. MICs of all the isolates were determined, and PK parameters were obtained. Monte Carlo simulation was performed to determine the percentage of target attainment (PTA) and cumulative fraction of response (CFR). The results indicated that the extended infusion of fosfomycin 16–24 g combined with prolonged carbapenem infusion could be utilized in non-MDR P. aeruginosa treatment.Citation70
Gram-negative resistance is a crucial global crisis that has been illustrated by the rapid growth of carbapenem-resistant Enterobacteriaceae (CRE). For serious systemic infections induced by multidrug-resistant Enterobacteriaceae, the use of fosfomycin as an essential and beneficial option has been recently renewed. The new evidence on the hidden capacity of intravenous fosfomycin to destroy Gram-negative pathogens has been demonstrated elsewhere.Citation3 Although several hopeful evidence are available for fosfomycin as the last antibacterial option to treat severe Gram-negative infections, more investigations are still necessary before using the intravenous fosfomycin.Citation3
Along with harmonization of current breakpoints and according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) and Clinical and Laboratory Standards Institute (CLSI), fosfomycin has a high potency to treat serious systemic infections. However, breakpoints for Pseudomonas sp. need to be defined urgently. Dose of fosfomycin requires to be defined for serious infections where probably higher daily dosages (24 g/day) may be required to prevent the heteroresistant mutant selection. Well controlled and randomized studies comparing fosfomycin versus colistin and investigating mono and combination therapy are essential to identify optimal regimens of fosfomycin in critically ill populations with resistant Gram-negative infections. Until the abovementioned need gaps are clear, fosfomycin should not be used as a monotherapy option to treat severe systemic infections.Citation3
Disclosure
The authors report no conflicts of interest in this work.
References
- RazRFosfomycin: an old – new antibioticClin Microbiol Infect2012184710.1111/j.1469-0691.2011.03636.x21914036
- ThadenJTPogueJMKayeKSRole of newer and re-emerging older agents in the treatment of infections caused by carbapenem-resistant EnterobacteriaceaeVirulence20166114
- SaiprasadPVKrishnaprasadKExploring the hidden potential of fosfomycin for the fight against severe gram-negative infectionsIndian J Med Microbiol201634441610.4103/0255-0857.19537927934817
- GrifKDierichMPPfallerKMiglioliPAAllerbergerFIn vitro activity of fosfomycin in combination with various antistaphylococcal substancesJ Antimicrob Chemother20014820921711481290
- TessierFQuentinCIn vitro activity of fosfomycin combined with ceftazidime, imipenem, amikacin, and ciprofloxacin against Pseudomonas aeruginosaClin Microbiol Infec199716159162
- DupontHMentecHSolletJPBleichnerGImpact of appropriateness of initial antibiotic therapy on the outcome of ventilator-associated pneumoniaIntensive Care Med20012735536210.1007/s00134000064011396279
- JoukhadarCKleinNDittrichPTarget site penetration of fosfomycin in critically ill patientsJ Antimicrob Chemother20035151247125210.1093/jac/dkg18712668580
- FrossardMJoukhadarCBurgmannHDistribution and antimicrobial activity of fosfomycin in the interstitial fluid of human soft tissuesAntimicrob Agents Chemother2000442728273210991852
- Docobo-PérezFDrusanoGLJohnsonAPharmacodynamics of fosfomycin: insights into clinical use for antimicrobial resistanceAntimicrob Agents Chemother2015595602561010.1128/AAC.00752-1526124169
- DijkmansACZacaríasNVBurggraafJFosfomycin: pharmacological, clinical and future perspectivesAntibiotics2017642410.3390/antibiotics6040024
- PopovicMSteinortDPillaiSJoukhadarCFosfomycin: an old, new friend?Eur J Clin Microbiol Infect Dis20102912714210.1007/s10096-009-0833-219915879
- KahanFMKahanJSCassidyPJKroppHThe mechanism of action of fosfomycin (phosphonomycin)Ann N Y Acad Sci197423536438610.1111/nyas.1974.235.issue-14605290
- BrownEDVivasEIWalshCTKolterRMurA (MurZ), the enzyme that catalyzes the first committed step in peptidoglycan biosynthesis, is essential in Escherichia coliJ Bacteriol19951774194419710.1128/jb.177.14.4194-4197.19957608103
- SilverLLFosfomycin: mechanism and resistanceCold Spring Harb Perspect Med20177a02526210.1101/cshperspect.a02526228062557
- KahanFMKahanJSCassidyPJKroppHThe mechanism of action of fosfomycin (phosphonomycin)Ann NY Acad Sci197423536438610.1111/nyas.1974.235.issue-14605290
- RoussosNKarageorgopoulosDESamonisGFalagasMEClinical significance of the pharmacokinetic and pharmacodynamic characteristics of fosfomycin for the treatment of patients with systemic infectionsInt J Antimicrob Agents200934650651510.1016/j.ijantimicag.2009.08.01319828298
- MazzeiTCassettaMIFallaniSArrigucciSNovelliAPharmacokinetic and pharmacodynamic aspects of antimicrobial agents for the treatment of uncomplicated urinary tract infectionsInt J Antimicrob Agents200628S35S4110.1016/j.ijantimicag.2006.05.01916829051
- RibesSTabernerFDomenechAEvaluation of fosfomycin alone and in combination with ceftriaxone or vancomycin in an experimental model of meningitis caused by two strains of cephalosporin-resistant Streptococcus pneumoniaeJ Antimicrob Chemother20065793193610.1093/jac/dkl04716507562
- PfauslerBSpissHDittrichPZeitlingerMSchmutzhardEJoukhadarCConcentrations of fosfomycin in the cerebrospinal fluid of neurointensive care patients with ventriculostomyassociated ventriculitisJ Antimicrob Chemother20045384885210.1093/jac/dkh15815056646
- Hamilton-MillerJMIn vitro activity of fosfomycin against ‘problem’ gram-positive cocciMicrobios199271951031453987
- DuezJMMoussonCSiéborEFosfomycin and its application in the treatment of multidrug-resistant Enterobacteriaceae infectionsClin Med Rev Ther20113123142
- FrossardMJoukhadarCErovicBMDistribution and antimicrobial activity of fosfomycin in the interstitial fluid of human soft tissuesAntimicrob Agents Chemother2000442728273210991852
- JoukhadarCKleinNDittrichPTarget site penetration of fosfomycin in critically ill patientsJ Antimicrob Chemother2003511247125210.1093/jac/dkg18712668580
- KwanKCWadkeDAFoltzELPharmacokinetics of phosphonomycin in Man. I. Intravenous administrationJ Pharm Sci1971606786855125763
- RoussosNKarageorgopoulosDESamonisGFalagasMEClinical significance of the pharmacokinetic and pharmacodynamic characteristics of fosfomycin for the treatment of patients with systemic infectionsInt J Antimicrob Agents20093450651510.1016/j.ijantimicag.2009.08.01319828298
- ReffertJLSmithWJFosfomycin for the treatment of resistant gram-negative bacterial infections. Insights from the society of infectious diseases pharmacistsPharmacotherapy20143484585710.1002/phar.143424782335
- FalagasMEVouloumanouEKSamonisGVardakasKZFosfomycinClin Microbiol Rev20162932134710.1128/CMR.00068-1526960938
- MichalopoulosASLivaditisIGGougoutasVThe revival of fosfomycinInt J Infect Dis201115e732e73910.1016/j.ijid.2011.07.00721945848
- Infectopharm Arzneimittel und Consilium GmbHFomicyt Package InsertHeppenheim, GermanyInfectopharm2015
- FlorentAChichmanianRMCuaEPulciniCAdverse events associated with intravenous fosfomycinInt J Antimicrob Agents201137828310.1016/j.ijantimicag.2010.09.00221074377
- DellitTHOwensRCMcGowanJEInfectious diseases society of America and the society for healthcare epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardshipClin Infect Dis20074415917710.1086/51039317173212
- SafdarNHandelsmanJMakiDGDoes combination antimicrobial therapy reduce mortality in gram-negative bacteraemia? A meta-analysisLancet Infect Dis2004451952710.1016/S1473-3099(04)01108-915288826
- KastorisACRafailidisPIVouloumanouEKGkegkesMatthewIDFalagasESynergy of fosfomycin with other antibiotics for gram-positive and gram-negative bacteriaEur J Clin Pharmacol20106635936810.1007/s00228-010-0794-520186407
- SamonisGMarakiSKarageorgopoulosDEVouloumanouEKFalagasMESynergy of fosfomycin with carbapenems, colistin, netilmicin, and tigecycline against multidrug-resistant Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa clinical isolatesEur J Clin Microbiol Infect Dis20123169570110.1007/s10096-011-1360-521805292
- GrossatoASartoriRFontanaREffect of non-b-lactam antibiotics on penicillin-binding protein synthesis of enterococcus hirae ATCC 9790J Antimicrob Chemother1991272632712037534
- TotsukaKUchiyamaTShimizuKKannoYTakataTYoshidaTIn vitro combined effects of fosfomycin and b-lactam antibiotics against penicillin-resistant Streptococcus pneumoniaeJ Infect Chemother19973495410.1007/BF02489184
- YamadaSHyoYOhmoriSOhuchiMRole of ciprofloxacin in its synergistic effect with fosfomycin on drug-resistant strains of Pseudomonas aeruginosaChemotherapy20075320220910.1159/00009841917356268
- OkazakiMSuzukiKAsanoNEffectiveness of fosfomycin combined with other antimicrobial agents against multidrug-resistant Pseudomonas aeruginosa isolates using the efficacy time index assayJ Infect Chemother20028374210.1007/s10156020000411957118
- TessierFQuentinCIn vitro activity of fosfomycin combined with ceftazidime, imipenem, amikacin, and ciprofloxacin against Pseudomonas aeruginosaEur J Clin Microbiol Infect Dis1997161591629105845
- Martinez-MartinezLRodriguezGPascualASuárezAIPereaEJIn vitro activity of antimicrobial agent combinations against multiresistant Acinetobacter baumanniiJ Antimicrob Chemother1996381107110810.1093/jac/38.6.11079023661
- SantimaleeworagunWWongpoowarakPChayakulPPattharachayakulSTansakulPGareyKWIn vitro activity of colistin or sulbactam in combination with fosfomycin or imipenem against clinical isolates of carbapenem-resistant Acinetobacter baumannii producing OXA-23 carbapenemasesSoutheast Asian J Trop Med Public Health20114289090022299471
- InouyeSWatanabeTTsuruokaTKitasatoIAn increase in the antimicrobial activity in vitro of fosfomycin under anaerobic conditionsJ Antimicrob Chemother19892465766610.1093/jac/24.5.6572599990
- YanagidaCItoKKomiyaIHorieTProtective effect of fosfomycin on gentamicin-induced lipid peroxidation of rat renal tissueChem Biol Interact200414813914710.1016/j.cbi.2004.05.00515276870
- NakamuraTKokuryoTHashimotoYInuiKIEffects of fosfomycin and imipenem-cilastatin on the nephrotoxicity of vancomycin and cisplatin in ratsJ Pharm Pharmacol19995122723210217324
- Castaneda-GarciaABlazquezJRodriguez-RojasAMolecular mechanisms and clinical impact of acquired and intrinsic fosfomycin resistanceAntibiotics2013221723610.3390/antibiotics202021727029300
- TsuruokaTYamadaYCharactertization of spontaneous fosfomycin (phosphonomycin)-resistant cells of Escherichia coli B in vitroJ Antibiot19752890691110.7164/antibiotics.28.9061104551
- KadnerRJWinklerHHIsolation and characterization of mutations affecting the transport of hexose phosphates in Escherichia coliJ Bacteriol19731138959004347928
- KimDHLeesWJKempsellKELaneWSDuncanKWalshCTCharacterization of a Cys115 to Asp substitution in the Escherichia coli cell wall biosynthetic enzyme UDP-GlcNAc enolpyruvyl transferase (MurA) that confers resistance to inactivation by the antibiotic fosfomycinBiochemistry1996354923492810.1021/bi952937w8664284
- HoriiTKimuraTSatoKShibayamaKOhtaMEmergence of fosfomycin-resistant isolates of shiga-like toxin-producing Escherichia coli O26Antimicrob Agents Chemother19994378979310.1128/AAC.43.4.78910103182
- RigsbyREFillgroveKLBeihofferLAArmstrongRNFosfomycin resistance proteins: A nexus of glutathione transferases and epoxide hydrolases in a metalloenzyme superfamilyMethods Enzymol200540136737916399398
- KobayashiSKuzuyamaTSetoHCharacterization of the fomA and fomB gene products from Streptomyces wedmorensis, which confer fosfomycin resistance on Escherichia coliAntimicrob Agents Chemother20004464765010681332
- KuzuyamaTKobayashiSO’HaraKHidakaTSetoHFosfomycin monophosphate and fosfomycin diphosphate, two inactivated fosfomycin derivatives formed by gene products of fomA and fomB from a fosfomycin producing organism Streptomyces wedmorensisJ Antibiot19964950250410.7164/antibiotics.49.5028682732
- FarhadiTHashemianSMRComputer-aided design of amino acid-based therapeutics: A reviewDrug Des Devel Ther2018121239125410.2147/DDDT.S159767
- FarhadiTFakharianAHashemianSMAffinity improvement of a humanized antiviral antibody by structure-based computational designInt J Pept Res Ther2017251181186
- FarhadiTRanjbarMMDesigning and modeling of complex DNA vaccine based on MOMP of Chlamydia trachomatis: an in silico approachNetw Model Anal Health Inform Bioinforma20176110.1007/s13721-016-0142-5
- FarhadiTOvchinnikovRSRanjbarMMIn silico designing of some agonists of toll-like receptor 5 as a novel vaccine adjuvant candidatesNetw Model Anal Health Inform Bioinforma2016531s1310.1007/721-016-0138-1
- FarhadiTNezafatNGhasemiYIn silico phylogenetic analysis of Vibrio cholera isolates based on three housekeeping genesInt J Comput Biol and Drug Des201581627410.1504/IJCBDD.2015.06878925869320
- HashemianSMRFarhadiTGanjparvarMLinezolid: a review of its properties, function, and use in critical careDrug Des Devel Ther20182018121759176710.2147/DDDT.S164515
- FarhadiTHashemianSMRConstructing novel chimeric DNA vaccine against Salmonella enterica based on SopB and GroEL proteins: an in silico approachJ Pharm Invest2018486639655
- FarhadiTFakharianAOvchinnikovRSVirtual screening for potential inhibitors of CTX-M-15 protein of Klebsiella pneumoniaeInterdiscip Sci: Comput Life Sci2018104694703
- FarhadiTIn silico designing of peptide inhibitors against pregnane X receptor: the novel candidates to control drug metabolismInt J Pept Res Ther2018243409420
- HyattJMMcKinnonPSZimmerGSSchentagJJThe importance of pharmacokinetic/pharmacodynamics surrogate markers to outcome. Focus on antibacterial agentsClin Pharmacokinet19952814316010.2165/00003088-199528020-000057736689
- SchentagJJNixDEAdelmanMHMathematical examination of dual individualization principles (I): relationships between AUC above MIC and area under the inhibitory curve for cefmenoxime, ciprofloxacin, and tobramycinDICP: Ann Pharmacother1991251050105710.1177/106002809102501003
- CrokaertFPharmacodynamics, a tool for a better use of antibiotics?Intensive Care Med20012734034310.1007/s00134010086511396276
- JoukhadarCFrossardMMayerBXImpaired target site penetration of beta-lactams may account for therapeutic failure in patients with septic shockCrit Care Med20012938539110.1097/00003246-200102000-0003011246321
- BrunnerMPernerstorferTMayerBXEichlerHGMüllerMSurgery and intensive care procedures affect the target site distribution of piperacillinCrit Care Med2000281754175910.1097/00003246-200006000-0000910890614
- JoukhadarCKleinNMayerBXPlasma and tissue pharmacokinetics of cefpirome in patients with sepsisCrit Care Med2002301478148210.1097/00003246-200207000-0001312130965
- De La PenaADalla CostaTTaltonJDPenetration of cefaclor into the interstitial space fluid of skeletal muscle and lung tissue in ratsPharm Res2001181310131411683245
- TegederISchmidtkoABräutigamLKirschbaumAGeisslingerGLötschJTissue distribution of imipenem in critically ill patientsClin Pharmacol Ther200271532533310.1067/mcp.2002.12252612011818
- AsuphonOMontakantikulPHoungsaitongJKiratisinPSonthisombatPOptimizing intravenous fosfomycin dosing in combination with carbapenems for treatment of Pseudomonas aeruginosa infections in critically ill patients based on pharmacokinetic/pharmacodynamic (PK/PD) simulationInt J Infect Dis201650232910.1016/j.ijid.2016.06.01727418581
