Abstract
Aim: Malnutrition is one of the most common complications in patients with liver cirrhosis. Abnormal energy substrate metabolism may contribute to aggravation of malnutrition. Late evening snack (LESs) supplementation has been recommended as an intervention to reduce starvation time and improve nutritional status. Published studies have analyzed the effect of LESs on the branched-chain amino acid (BCAA)/tyrosine ratio (BTR) and oxidation rate of fat and carbohydrate in patients with liver cirrhosis.
Methods: We searched PubMed, Cochrane Library, Web of Science and Embase for relevant research from January 2000 to October 2018. The primary outcome for this analysis was changes in BTR and fat and carbohydrate oxidation in patients with liver cirrhosis.
Results: A total of 9 articles, containing 211 patients, were included in this analysis. The results supported that supplementation with BCAA-enriched LESs improved BTR, and long-term supplementation with BCAAs (>1 month) may be more beneficial than short-term supplementation (<1 month) in patients with liver cirrhosis. In addition, supplementation with BCAAs may increase the oxidation rate of carbohydrates and decrease the oxidation rate of fat. Furthermore, compared with liquid-enriched LESs, BCAA was a better choice for increasing the oxidation of carbohydrates and decreasing the rate of fat oxidation.
Conclusion: BCAA-enriched LES supplementation is an appropriate nutritional intervention to improve abnormal energy substrate metabolism, which may improve malnutrition in patients with liver cirrhosis. Further research is needed on the long-term benefit and improved survival in patients with liver cirrhosis.
Introduction
Malnutrition is one of the most common complications in patients with liver cirrhosis and is associated with an increased risk of morbidity and mortality.Citation1 As an important part of the comprehensive treatment of liver cirrhosis, nutritional intervention can help to improve the nutritional status and quality of life of patients with liver cirrhosis.Citation2 The liver is an important organ for maintaining normal energy and nutrient metabolism. Therefore, it is important to explore appropriate nutritional interventions for patients with liver cirrhosis.
Abnormal energy substrate metabolism is characteristic of patients with liver cirrhosis, which may aggravate malnutrition.Citation3 In particular, after overnight fasting, patients with liver cirrhosis show increased rates of fat oxidation and decreased rates of carbohydrate oxidation compared to normal controls. In addition, previous studies on the correlation between lifestyle and liver disease have shown that lower daily frequency of meals is associated with nonalcoholic fatty liver disease, which is a risk factor for the development of liver cirrhosis, and a high daily eating frequency is associated with healthy lifestyle.Citation4,Citation5 Therefore, increasing the frequency of eating is used as intervention. Late evening snacks (LESs), which add an extra meal before sleep, has been recommended as an intervention to reduce the starvation time and improve nutritional status.Citation6,Citation7
LESs are currently an effective method to improve the metabolic status of patients with end-stage liver disease.Citation6 However, published studies have had small sample sizes. It is also unclear whether long-term intervention with LESs and whether branched-chain amino acids (BCAAs) are more suitable for patients with liver cirrhosis. In patients with liver cirrhosis, due to the decline in glycogen reserves and activated glutamine synthesis in muscle, the consumption of BCAAs increases, which can lead to an imbalanced ratio of BCAA to aromatic amino acids (AAAs).Citation8 Clinically, the BCAA/tyrosine ratio (BTR) reflects the ratio of BCAAs to AAAs, and changes are closely related to liver dysfunction.Citation9 Previous studies have shown that BTR is a prognostic factor for hepatocellular carcinoma.Citation10 In addition, the rate of fat and carbohydrate oxidation is closely related to prognosis of cirrhosis.Citation11 Therefore, this study summarizes the currently published literature that has analyzed the effects of LESs on BTR and the oxidation rate of fat and carbohydrate in patients with liver cirrhosis. It may provide evidence for the clinical application of LESs in patients with liver cirrhosis.
Material and methods
Study selection
This meta-analysis was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).Citation12 We selected articles from January 2000 to October 2018 using the databases of PubMed, Cochrane Library, Web of Science and Embase. All of the articles were about LESs in patients with liver cirrhosis. The following search terms were used: (“late evening snack” [Title/Abstract] or “nocturnal nutritional supplementation” [Title/Abstract] or “nocturnal snack” [Title/Abstract] or “evening snack” [Title/Abstract] or “nocturnal meal” [Title/Abstract]) or (“bedtime snack” [Title/Abstract]) and (“Liver Cirrhosis” [Title/Abstract] or “Cirrhosis,Liver” [Title/Abstract]) or “Cirrhoses, Liver”[Title/Abstract] or “Liver Cirrhoses” [Title/Abstract] or “Hepatic Cirrhosis” [Title/Abstract] or “Cirrhoses, Hepatic” [Title/Abstract] or “Cirrhosis, Hepatic” [Title/Abstract] or “Hepatic Cirrhoses”[Title/Abstract]) or “Cirrhosis, Hepatic” [Title/Abstract] or “Hepatic Cirrhoses” [Title/Abstract] or “Fibrosis, Liver” [Title/Abstract] or “Fibroses, Liver” [Title/Abstract] or “Liver Fibroses” [Title/Abstract] or “Liver Fibrosis” [Title/Abstract]).
Two investigators (J.Y. and W.H.) conducted a preliminary search separately, deleted duplicate records, sifted through relevant headings and abstracts, and identified relevant terms for further evaluation. References to retrieved articles were also reviewed to identify other eligible studies.
The study protocol was approved by the Ethics Committee of Beijing YouAn Hospital, Beijing, China.
Definition and study endpoints
Liver cirrhosis was diagnosed by clinical and laboratory profiles and by histological examination of liver biopsy specimens.Citation13 The primary endpoint of this study was whether BTR and oxidation rate of carbohydrate and fat were affected by LESs.
Data extraction and quality assessment
Two investigators (J.Y. and W.H.) extracted the following information from the selected researches independently: first author, year of publication, intervention of experimental group, total numbers of patients enrolled, time of intervention for each event, levels of BTR, and oxidation rate of carbohydrate and fat before and after intervention. When research on the same patients appeared in multiple articles, to avoid duplication of information, we selected the study with the largest sample.
The US Agency for Healthcare Research and Quality (AHRQ) was used to evaluate bias risks in each study.
Study eligibility
Inclusion criteria: liver cirrhosis was diagnosed on the basis of pathological examination findings and Child–Pugh classification. Exclusion criteria: patients had a history of other organ diseases, such as chronic heart failure or chronic respiratory, pancreatic or renal diseases.
Statistical analysis
We used Review Manager 5.2 and Stata 12.0 software for statistical analysis. Differences were expressed as mean ± standard deviation with 95% CI. Heterogeneity was tested using the I2 statistic. Heterogeneity was considered to be low in studies with I2 25–50%, moderate in studies with I2 50–75%, and high in studies with I2>75%. I2>50% represented significant heterogeneity. A fixed-effects model was used when study heterogeneity was not significant and a random-effects model when heterogeneity was significant. Begg’s test was used to estimate publication bias and sensitivity analysis was used to test stability.
Results
Study selection and characteristics
The selection process is illustrated in . A total of 9 articles met the inclusion criteria.Citation14–Citation22 The main characteristics of the included studies are described in . The meta-analysis included 221 patients from Japan, aged 42–85 years. One study was a randomized controlled trial (RCT)Citation18 and the others were single-arm studies. Three of the 9 studies supplied a pack of LESs (210 kcal),Citation14,Citation17,Citation22 others gave a pack of LESs and 1 or more supplements during the daytime. Three of the 9 studies used long-term LESs (>1 month)Citation14,Citation15,Citation17 and the others used short-term LESs. Six of the 9 studies reported changes in BTRCitation14–Citation17,Citation19,Citation21 and 5 reported changes in fat and carbohydrate oxidation.Citation18–Citation22
Table 1 Characteristics of included researches
Quality assessment
Although one study was an RCT, the baseline characteristics of each group were not comparable. We, therefore, compared the changes before and after LES treatment in each group, and we divided this RCT into 3 groups of one-arm study. Assessment of the single-arm studies by the AHRQ methodology checklist is shown in . All included single-arm studies described the source of data and patient inclusion and exclusion criteria clearly, provided detailed explanation for excluded data, and presented measurements for the primary study endpoints.
Table 2 The US Agency for Healthcare Research and Quality checklist for quality assessment of one-arm research
Effects of less on BTR
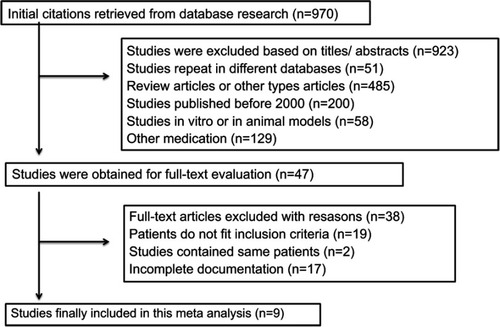
We selected 6 studies that measured the changes in BTR before and after LESs.Citation14–Citation17,Citation19,Citation21 BTR was increased after LESs (MD=0.79, 95% CI [0.15, 1.43]). The heterogeneity was significant (I2=90%) with publication bias (P<0.00001) (). In order to find the reason, subgroup analysis was performed by treatment period. Three of the studies had short-term LESs (<1 month) compared with >1 month in the others. BTR in the long-term LES group was increased with no heterogeneity (I2=0%), but BTR in the short-term LES group was increased with high heterogeneity (I2=96%) (). The differences between the groups were significant (P=0.02). The changes in BTR after long-term LESs were superior to that after short-term LESs.
Figure 2 Meta-analysis of the changes in BTR. (A) Comparisons of BTR before and after LESs. (B) Subgroup analysis of the influence of different intervention periods on BTR. (C) Subgroup analysis of the effects of different daily intervention times on BTR.Abbreviations: BTR, branched-chain amino acid/tyrosine ratio; LESs, late evening snacks.
In the long-term treatment subgroup, BACCs were given besides LESs in one study,Citation15 and BACCs were given once a day as LESs in 2 studies.Citation14,Citation17 Subgroup analysis showed that BTR was increased in the two groups, but there was no difference between these 2 treatments (P=0.48) ().
Analysis of sensitivity was conducted to evaluate the robustness of the effect. The results showed that sensitivity was low (MD=0.58, 95% CI [0.35, 0.81], the range for all articles was 95% CI [0.27, 0.93]). Begg’s test showed publication bias in the 6 studies, although it was not significant (Pr > |z| =0.296, continuity corrected).
Effects of less on oxidation rate of fat
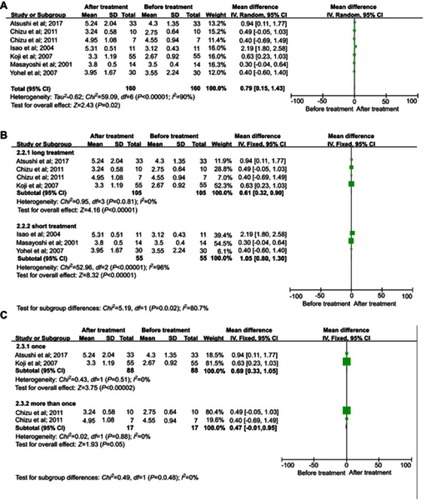
We selected 5 studies that measured changes in fat oxidation before and after LESs.Citation18–Citation22 Fat oxidation was decreased after LESs (MD= −13.21, 95% CI [−15.34, −11.07]) with low heterogeneity (I2=23%) ().
Figure 3 Meta-analysis of changes in the oxidation rate of fat. (A) Comparisons of the oxidation rate of fat before and after LESs. (B) Subgroup analysis of the effects of different interventions on fat oxidation.Abbreviation: LESs, late evening snacks.
Patients in two studiesCitation17,Citation22 were treated with a pack of liquid nutrients as LESs, and the others received a pack of BCAAs. Subgroup analysis showed that BCAAs and liquid nutrients decreased fat oxidation, although there was no significant difference between the 2 groups (P=0.15). There was no heterogeneity in the BCAA group, but the heterogeneity in the liquid nutrient group was significant (I2=73%) (), so this was an important source of heterogeneity. The results indicated BCAA was a better choice for reducing the oxidation rate of fat.
Analysis of sensitivity was conducted to evaluate the robustness of the effect. The results showed that sensitivity was low (mean estimate= −1.11, 95% CI [−1.42, −0.80]), and the range for all articles was 95% CI [−1.78, −0.65]). Begg’s test showed publication bias in the 5 studies, although it was not significant (Pr > |z| =0.086, continuity corrected).
Effects of less on oxidation rate of carbohydrate
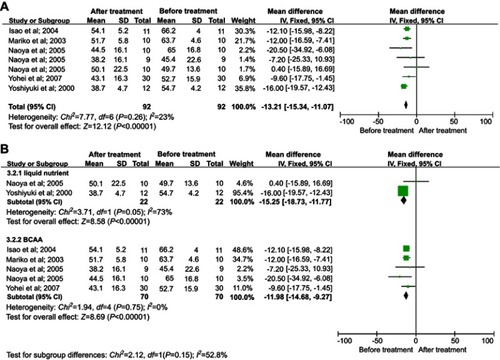
We selected 5 studies that measured the oxidation rate of carbohydrate before and after LESs.Citation18–Citation22 The level of carbohydrate oxidation was increased after LESs (MD=11.92, 95% CI [9.88, 13.96]) with no heterogeneity (I2=0%) ().
Figure 4 Meta-analysis of changes in the oxidation rate of carbohydrate. (A) Comparisons of oxidation rate of carbohydrate before and after LESs. (B) Subgroup analysis of the effect of different interventions on the oxidation rate of carbohydrate.Abbreviation: LESs, late evening snacks.
Patients from two studiesCitation17,Citation22 received LESs as liquid nutrients and the others received a pack of BCAAs. Subgroup analysis showed that BCAAs and liquid nutrients both increased the oxidation rate of carbohydrate, although there was no significant difference between the two treatment groups (P=0.18). There was no heterogeneity in the BCAA group, but the heterogeneity in the liquid nutrient group was significant (I2=59%) (), so this was an important source of heterogeneity. The result indicated that BCAA was a better choice for increasing the oxidation rate of carbohydrate.
Analysis of sensitivity was conducted to evaluate the robustness of the effect. The results showed that sensitivity was low (MD=1.09, 95% CI [0.78, 1.4]), the range for all articles was 95% CI [0.63, 1.67]). Begg’s test showed publication bias in the 5 studies, although it was not significant (Pr > |z| =0.086, continuity corrected).
Adverse effects of less
There were no adverse events related to LESs in any of the 9 articles.
Discussion
This study evaluated the published literature on the effects of LESs, especially BCAA-based LESs. The results of the analysis supported that supplementation with BCAAs can improve BTR, and long-term supplementation with BCAAs (>1 month) may be more beneficial than short-term supplementation (<1 month) in patients with liver cirrhosis. In addition, supplementation with BCAAs may increase the oxidation rate of carbohydrates and decrease the oxidation rate of fat, thereby significantly improving abnormal energy substrate metabolism in patients with liver cirrhosis. LESs are one of the commonly used nutritional interventions, but there is a scarcity of high-quality research among published studies. The results of the present study may provide evidence for BCAAs as a major component of LESs to improve substrate metabolism in patients with liver cirrhosis.
Since the glycogen reserve of liver cells in patients with cirrhosis is less than that of normal people, short-term hunger can induce gluconeogenesis.Citation3 Therefore, after a natural sleep cycle, fat and protein are the main energy-supplying substances, which is inclined to cause malnutrition in patients with liver cirrhosis.Citation23,Citation24 Therefore, to improve this condition, previous studies have proposed LESs as an intervention method, that is, giving a certain amount of calories at nighttime can reduce the time of hunger, thereby improving the abnormal substrate energy supply.Citation25 Similar to previous studies, the present study supports the idea that LESs may improve the oxidation rate of carbohydrates and reduce the oxidation rate of fat in patients with liver cirrhosis. Furthermore, the BCAA-based LESs are better than fat-based LESs for increasing carbohydrate oxidation rate and reducing fat oxidation rate. Therefore, BCAA is considered to be a good choice for LES intervention.
Patients with liver cirrhosis often have amino acid metabolism disorder, which is often associated with a variety of complications such as hepatic encephalopathy and sarcopenia.Citation14 Most studies have shown that BCAA supplementation improves amino acid profile, albumin level, and hepatic encephalopathy,Citation26 and may also promote regeneration of liver cells.Citation27 There is also research indicating that BCAAs can reduce the risk of liver cancer recurrence.Citation28 However, to date, there has been no large study on BCAA supplementation in patients with liver cirrhosis. A total of 9 studies were pooled in the present study. The results showed that in patients with liver cirrhosis, BTR was significantly higher after than before oral BCAA supplementation. More importantly, there has been no study on the duration of BCAA supplementation. We showed that long-term BCAA supplementation (>1 month) may be more beneficial than short-term supplementation (<1 month) in patients with liver cirrhosis.
There were some limitations to this study, most of which were related to the quality of the research papers included. First, only 1 of the 9 studies was an RCT and the others were single-arm studies. Second, most of the studies had small samples. Third, most of the patients included in this study were Asians. Therefore, a large, multicenter RCT is needed to further analyze the effects of LESs, especially BCAA-based LESs, on patients with liver cirrhosis.
Conclusion
LESs based on BCAAs can improve BTR, increase the oxidation rate of carbohydrates, and decrease the oxidation rate of fat in patients with liver cirrhosis, which may improve the malnutrition in these patients. Long-term supplementation with BCAAs is more efficient and better than fat-based LESs. Our results may provide evidence for the clinical application of LESs in patients with liver cirrhosis. When patients are supplemented with BCAAs, one should also consider the individual choice of the patient and the detailed status of their condition. Therefore, further research requires a larger sample of more individualized and standardized treatments.
Acknowledgments
This study was funded by the Shanxi Province 136 revitalization medical project (General Surgery Department); Science Foundation of China, Grant/Award Number: 81600561; National Key R&D Program of China (No. 2017YFA0103000); Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (No. ZYLX201806); “Beijing Municipal Administration of Hospitals” Ascent Plan (No. DFL20151601); National Science and Technology Key Project on “Major Infectious Diseases such as HIV/AIDS, Viral Hepatitis Prevention and Treatment” (Nos. 2012ZX10002004-006, 2017ZX10203201-005, 2017ZX10201201-001-001, 2017ZX10201201-002-002, 2017ZX10202203-006-001, 2017ZX10302201-004-002).
Disclosure
The authors report no conflicts of interest in this work.
References
- Tandon P, Raman M, Mourtzakis M, Merli M. A practical approach to nutritional screening and assessment in cirrhosis. Hepatology. 2017;65(3):1044–1057. doi:10.1002/hep.2900328027577
- Mandato C, Di Nuzzi A, Vajro P. Nutrition and Liver Disease. Nutrients. 2017;10:1. doi:10.3390/nu10010009
- Cheung K, Lee SS, Raman M. Prevalence and mechanisms of malnutrition in patients with advanced liver disease, and nutrition management strategies. Clinical Gastroenterol Hepatol. 2012;10(2):117–125. doi:10.1016/j.cgh.2011.08.016
- Trovato FM, Catalano D, Musumeci G, et al. 4Ps medicine of the fatty liver: the research model of predictive, preventive, personalized and participatory medicine-recommendations for facing obesity, fatty liver and fibrosis epidemics. Epma J. 2014;5(1):21. doi:10.1186/1878-5085-5-2025937854
- Trovato FM, Martines GF, Brischetto D, et al Fatty liver disease and lifestyle in youngsters: diet, food intake frequency, exercise, sleep shortage and fashion. Liver Int. 2016;36(3):427–433. doi:10.1111/liv.1295726346413
- Tsien CD, McCullough AJ, Dasarathy S. Late evening snack: exploiting a period of anabolic opportunity in cirrhosis. J Gastroenterol Hepatol. 2012;27(3):430–441. doi:10.1111/j.1440-1746.2011.06951.x22004479
- Plank LD, Gane EJ, Peng S, et al. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: a randomized 12-month trial. Hepatology. 2008;48(2):557–566. doi:10.1002/hep.2236718627001
- Holecek M. Ammonia and amino acid profiles in liver cirrhosis: effects of variables leading to hepatic encephalopathy. Nutrition. 2015;31(1):14–20. doi:10.1016/j.nut.2014.03.01625220875
- Furuichi Y, Imai Y, Miyata Y, et al Branched-chain amino acid-enriched nutrient increases blood platelet count in patients after endoscopic injection sclerotherapy. Hepatol Res. 2016;46(11):1129–1136. doi:10.1111/hepr.1266826857535
- Ishikawa T. Branched-chain amino acids to tyrosine ratio value as a potential prognostic factor for hepatocellular carcinoma. World J Gastroenterol. 2012;18(17):2005–2008. doi:10.3748/wjg.v18.i17.200522563186
- Meng QH, Hou W, Yu HW, et al Resting energy expenditure and substrate metabolism in patients with acute-on-chronic hepatitis B liver failure. J Clin Gastroenterol. 2011;45(5):456–461. doi:10.1097/MCG.0b013e31820f7f0221422948
- Moher D, Shamseer L, Clarke M, et al Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi:10.1186/2046-4053-4-125554246
- Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383(9930):1749–1761. doi:10.1016/S0140-6736(14)60121-524480518
- Hiraoka A, Michitaka K, Kiguchi D, et al Efficacy of branched-chain amino acid supplementation and walking exercise for preventing sarcopenia in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2017;29(12):1416–1423. doi:10.1097/MEG.000000000000098629016470
- Koreeda C, Seki T, Okazaki K, et al Effects of late evening snack including branched-chain amino acid on the function of hepatic parenchymal cells in patients with liver cirrhosis. Hepatol Res. 2011;41(5):417–422. doi:10.1111/j.1872-034X.2011.00795.x21518402
- Sakaida I, Tsuchiya M, Okamoto M, Okita K. Late evening snack and the change of blood glucose level in patients with liver cirrhosis. Hepatol Res. 2004;30S:67–72. doi:10.1016/j.hepres.2004.10.01015607142
- Aoyama K, Tsuchiya M, Mori K, et al Effect of a late evening snack on outpatients with liver cirrhosis. Hepatol Res. 2007;37(8):608–614. doi:10.1111/j.1872-034X.2007.00036.x17517075
- Okamoto M, Sakaida I, Tsuchiya M, et al Effect of a late evening snack on the blood glucose level and energy metabolism in patients with liver cirrhosis. Hepatol Res. 2003;27(1):45–50.12957206
- Yamauchi M, Takeda K, Sakamoto K, Ohata M, Toda. Effect of oral branched chain amino acid supplementation in the late evening on the nutritional state of patients with liver cirrhosis. Hepatol Res. 2001;21(3):199–204.11673104
- Katsumi N, Kawamura N, Yamaguchi Y, et al Effect of oral branched chain amino acid-rich nutrient administered during endoscopic injection sclerotherapy of cirrhotic patients. Hepatol Res. 2005;32(3):158–162. doi:10.1016/j.hepres.2005.03.01315970464
- Urata Y, Okita K, Korenaga K, et al The Geffect of supplementation with branched-chain amino acids in patients with liver cirrhosis. Hepatol Res. 2007;37(7):510–516. doi:10.1111/j.1872-034X.2007.00081.x17539993
- Miwa Y, Shiraki M, Kato M, et al Improvement of fuel metabolism by nocturnal energy supplementation in patients with liver cirrhosis. Hepatol Res. 2000;18(3):184–189.11058823
- Changani KK, Jalan R, Cox IJ, et al Evidence for altered hepatic gluconeogenesis in patients with cirrhosis using in vivo 31-phosphorus magnetic resonance spectroscopy. Gut. 2001;49(4):557–564.11559655
- Glass C, Hipskind P, Tsien C, et al Sarcopenia and a physiologically low respiratory quotient in patients with cirrhosis: a prospective controlled study. J Appl Physiol (1985). 2013;114(5):559–565. doi:10.1152/japplphysiol.01042.201223288550
- Suzuki K, Endo R, Kohgo Y, et al Guidelines on nutritional management in Japanese patients with liver cirrhosis from the perspective of preventing hepatocellular carcinoma. Hepatol Res. 2012;42(7):621–626. doi:10.1111/j.1872-034X.2012.00990.x22686857
- Kawaguchi T, Taniguchi E, Sata M. Effects of oral branched-chain amino acids on hepatic encephalopathy and outcome in patients with liver cirrhosis. Nutr Clin Pract. 2013;28(5):580–588. doi:10.1177/088453361349643223945292
- Beppu T, Nitta H, Hayashi H, et al Effect of branched-chain amino acid supplementation on functional liver regeneration in patients undergoing portal vein embolization and sequential hepatectomy: a randomized controlled trial. J Gastroenterol. 2015;50(12):1197–1205. doi:10.1007/s00535-015-1067-y25847401
- Nojiri S, Fujiwara K, Shinkai N, Iio E, Joh T. Effects of branched-chain amino acid supplementation after radiofrequency ablation for hepatocellular carcinoma: A randomized trial. Nutrition. 2017;33:20–27. doi:10.1016/j.nut.2016.07.01327908546