Abstract
Non-vitamin K oral anticoagulants (NOACs) are increasingly used as alternatives to conventional therapies and have considerable accumulated real-world clinical data in patients with non-valvular atrial fibrillation (NVAF) or venous thromboembolism (VTE). However, it is not easy to make a complete changeover to NOACs in real-world clinical practice because NOACs still have challenges in specific patient populations (eg, Asian patients, NVAF patients presenting with acute coronary syndrome [ACS], dialysis patients with NVAF, patients with cancer-associated VTE, etc.). Clinical data on the optimal dose of NOACs in Asian patients with NVAF are not sufficient. The intensity of NOAC and antiplatelet treatment and the duration of antiplatelet treatment should be adjusted according to the bleeding and thrombotic risk profiles of the individual NVAF patient presenting with ACS. Increased bleeding risk and unclear efficacy of NOACs in dialysis patients with NVAF should be considered when making decisions on whether to give NOACs for these patients. If dialysis patients with NVAF require anticoagulant for stroke prevention, then apixaban could be considered while awaiting more clinical efficacy and safety data. Additional studies are needed to determine the utility of continuing treatment with reduced-dose NOACs for long-term therapy after VTE. We have enough experiences in using NOACs in cancer patients showing the benefit of antithrombotic treatment counterbalanced the bleeding risk; however, some challenges of cancer-associated VTE management exist due to differences in cancer types or chemotherapy regimens and comorbidities. Different dosing regimens among NOACs may impact on medication adherence; thus, individual patient preference should be considered in choosing a particular NOAC. A significant proportion of patients remain on warfarin because of the high price of NOACs and variability in reimbursement coverage. To compensate clinical-evidence and achieve optimal use of NOACs, we should pay attention to the outcomes of ongoing studies and evaluate more real-world data.
Introduction
Atrial fibrillation (AF) affects 1–2% of the general population and is the most common type of arrhythmia, which is a problem with heart rhythm.Citation1,Citation2 Patients with AF fail to pump all of the blood in the atria into the ventricle, causing some blood to contribute to thrombus formation. If the thrombus detaches and migrates to an artery in the brain, it can block the blood stream and cause a stroke.Citation3 The Framingham study reported that the incidence of stroke is more than 5-fold higher in the presence of AF, a greater increase than that observed in subjects with coronary heart disease (2-fold), hypertension (3-fold), or cardiac failure (4-fold).Citation4 Currently, the prevalence of AF is lower in Asians compared to Caucasians.Citation5 However, Asia has a larger elderly population than countries with predominantly Caucasian populations; thus, the incidence of chronic disease in Asia is predicted to rise, and the prevalence of AF is thus expected to increase further.Citation5 The predicted number of Asian AF patients in 2050 is 72 million, more than twice as many as in the United States (US) and Europe, and almost 2.9 million Asian patients will experience an AF-associated stroke if they do not take oral anticoagulants (OACs).Citation6
Venous thromboembolism (VTE), requiring antithrombotic therapy as AF, comprises both deep vein thrombosis (DVT) and pulmonary embolism (PE).Citation7 In DVT, blood clots form in the deep veins of the body, especially the lower leg or thigh.Citation8 These clots can break off, travel through the bloodstream to the lungs, and suddenly block a pulmonary artery.Citation9 This condition is called PE. The overall annual incidence rates of VTE range between 1.07 and 1.83 per 1000 individuals and the incidence rate of VTE in Asians was about 10–20% of that reported in the Caucasian population.Citation10,Citation11 Although the incidence in the Asian population is lower than that in the Western population, the burden of VTE in the Asian population is expected to increase with the rapid aging and increased life expectancy of this population. In Korea, the incidence of VTE is gradually increasing yearly (eg, 0.21 and 0.29 per 1000 individuals in 2009 and 2013, respectively) and it is significantly higher in the older population than in the younger population.Citation12
Considering the mechanisms of stroke in AF and the goal of VTE treatment (eg, prevent thrombus extension and new thrombus formation), antithrombotic therapy is required in patients with these diseases. OACs such as vitamin K antagonists (VKAs) are necessary to prevent thromboemboli in AF patients.Citation13,Citation14 A parenteral anticoagulant overlapping with a VKA is a conventional antithrombotic therapy in patients with VTE.Citation15,Citation16 However, these standard therapies have numerous disadvantages. Warfarin requires frequent international normalized ratio (INR) monitoring and dose adjustments, has multiple interactions with diet and drugs; multiple genetic polymorphisms are associated with warfarin metabolism, and parenteral anticoagulants should be administered by injection.Citation17–Citation22
These weaknesses of conventional therapies drove the development of non-vitamin K oral anticoagulants (NOACs) with new mechanisms of action, eg, direct thrombin inhibitor, dabigatran, and factor Xa (FXa) inhibitors, rivaroxaban, apixaban, and edoxaban. NOACs demonstrated efficacy and safety in comparison with warfarin in patients with non-valvular atrial fibrillation (NVAF), as well as parenteral therapy + VKA or placebo in patients with VTE.Citation23–Citation36 Based on the clinical study data, NOACs are currently approved in various countries, eg, the US, Europe, Korea, and Japan, for the following indications: (1) reduction of the risk of stroke and systemic embolic events (SEE) in NVAF; (2) treatment of DVT and PE and reduced risk of recurrence of DVT and PE.Citation37 Approval status and dosing regimens in the label information of NOACs among the four regions are shown in .Citation37 The international clinical guidelines for NVAF or VTE management were updated to recommend NOACs as the preferred strategy for antithrombotic therapy.Citation14,Citation15,Citation38
Table 1 Approval status and dosing regimens in the label information of NOACs in the US, Europe, Korea, and JapanCitation37
Currently, NOACs are increasingly used as alternatives to conventional therapies and have considerable accumulated real-world clinical data in patients with NVAF or VTE for at least 3–10 years after they have been released to the market. However, it is not easy to make a complete changeover to NOACs in real-world clinical practice because NOACs still have challenges in specific patient populations (eg, Asian patients, NVAF patients presenting with ACS, dialysis patients with NVAF, patients with cancer-associated VTE, etc.). Therefore, this narrative review aims to review recent data on the use of NOACs and discuss challenges when using NOACs in specific patient populations with NVAF or VTE.
Summary of pharmacological properties of NOACs compared with conventional treatments
Although warfarin has been approved for more than 60 years and is widely used in clinical practice, it has some characteristics hindering its compliance. The dose of warfarin is influenced by clinical factors, eg, age, race, concomitant medications, food, and genetic factors such as CYP2C9 and VKORC1 genotypes.Citation17 Warfarin inhibits multiple clotting factors in the coagulation cascade and has a slow onset of action, a narrow therapeutic range, and exhibits interactions with various drugs related to CYP450, dietary vitamin K, and botanical (herbal) products.Citation17 These features necessitate continuous INR monitoring.Citation17 Geriatric patients aged over 60 years exhibit increased anticoagulant effects, and they should have a lower dose of warfarin to have a beneficial level of anticoagulation.Citation17 Since Asian patients are more sensitive to warfarin, they require lower doses of warfarin and to have a lower INR range goal.Citation17,Citation39
Parenteral anticoagulants such as molecular-weight heparin (LMWH), unfractionated heparin (UFH), or fondaparinux are administered by subcutaneous or intravenous injection.Citation18–Citation22 LMWHs such as enoxaparin, tinzaparin, dalteparin, and UFH are co-inhibitors of FXa and IIa (thrombin) in the blood coagulation cascade and fondaparinux is a selective inhibitor of FXa.Citation18–Citation22 LMWH and fondaparinux need no routine monitoring of coagulation parameters.Citation18–Citation20,Citation22 On the other hand, UFH requires frequent monitoring of coagulation system status routinely done with the activated partial thromboplastin time (APTT) test.Citation21
To overcome weaknesses of conventional treatments, target-specific NOACs inhibit a specific single step (eg, rivaroxaban, apixaban, and edoxaban inhibit FXa, and dabigatran inhibits thrombin directly) and do not require routine monitoring of coagulation parameters or INR.Citation40–Citation47 NOACs are expected to replace parenteral anticoagulants because of their rapid onset/offset of action and possible switching of all-oral fixed dose regimens.Citation48 However, there will be clinical situations when a laboratory assessment of the anticoagulant effect of NOACs is required, although NOACs do not require routine monitoring (eg, a necessary reversal of anticoagulation or identification of subtherapeutic or supratherapeutic levels in special patients is needed, etc.).Citation49 The intensity of anticoagulation caused by dabigatran can be measured by the APTT and a dilute thrombin time can be used to measure dabigatran levels.Citation49 For the FXa inhibitor, a prothrombin time assay can measure the intensity of anticoagulation and anti-FXa assays can measure the FXa inhibitor level.Citation49
Furthermore, specific reversal agents for NOACs exist. Idarucizumab binds all dabigatran with high affinity and neutralizes dabigatran activity as a monoclonal antibody fragment.Citation50 Today, idarucizumab is approved as a medicine to neutralize the effects of dabigatran in the US, Europe, Korea, and Japan. Andexanet alfa, which binds to the direct FXa inhibitors, is approved under Accelerated Approval in the US for patients treated with rivaroxaban and apixaban when reversal of anticoagulation is needed.Citation51 Those specific reversal agents are expected to reverse the anticoagulation effect of NOACs rapidly when life-threatening/uncontrolled bleeding occurs or an emergent surgery/urgent procedure is needed.
The inhibition sites of conventional treatments and NOACs in the blood coagulation cascade are shown in and their pharmacological properties are outlined in .
Figure 1 Inhibition sites of conventional treatments and NOACs in the blood coagulation cascade
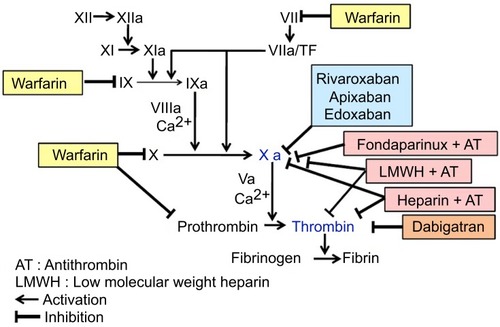
Table 2 Outline of the pharmacological properties of conventional treatments and NOACs
Summary of clinical efficacy and safety of NOACs
The main efficacy and safety data of NOACs in premarketing development programs require review when used in specific patient populations with either NVAF or VTE. Regulatory approvals of NOACs for each indication are based on these clinical data. Main outcomes of the pivotal clinical studies are summarized as follows:
Reduction of the risk of stroke in NVAF
Each NOAC was compared to warfarin in patients with NVAF in a confirmatory Phase III global clinical study (eg, RE-LY with dabigatran, ROCKET-AF with rivaroxaban, ARISTOTLE with apixaban, and ENGAGE AF-TIMI with edoxaban).Citation23–Citation28 NOACs were non-inferior or superior to warfarin with regard to prevention of stroke and SEE and had a comparable or significantly reduced major bleeding risk compared to warfarin in patients with NVAF. shows the design and main outcomes of those pivotal clinical studies.
Table 3 Comparison of the design and main outcomes of the pivotal clinical studies for reduction of stroke risk in NVAF
Treatment of VTE and reduction of the risk of recurrence of VTE
Each NOAC was compared to conventional anticoagulation therapy (eg, parenteral anticoagulant followed by VKA) for treatment of VTE in confirmatory Phase III global clinical studies (eg, RE-COVER and RE-COVER II with dabigatran EINSTEIN-DVT and EINSTEIN-PE with rivaroxaban, AMPLIFY with apixaban, HOKUSAI-VTE with edoxaban).Citation29,Citation30,Citation32–Citation34,Citation36 NOACs were as effective as the parenteral/VKA regimen and had comparable or significantly reduced major or clinically relevant non-major (CRNM) bleeding risk compared to the parenteral/VKA regimen for treatment of VTE. In particular, rivaroxaban and apixaban had efficacy and safety as a single oral drug without initial treatment with a parenteral anticoagulant. shows the design and main outcomes of those pivotal clinical studies.
Table 4 Comparison of the design and main outcomes of the pivotal clinical studies for the treatment of VTE
Three NOACs were evaluated for extended treatment of VTE in comparison with warfarin or placebo in patients already treated with anticoagulant for a specified period in separate extension clinical studies (eg, RE-MEDY and RE-SONATE with dabigatran, EINSTEIN-Extension with rivaroxaban, AMPLIFY-EXT with apixaban).Citation31,Citation32,Citation35 NOACs were as effective as warfarin or superior to placebo for secondary prevention of VTE after initial treatment. Although no extra extension clinical study with edoxaban existed, the HOKUSAI-VTE study with clinical-practice-based design (eg, patients with broad spectrum, various treatment duration for each patient, active comparator) and statistical analysis (eg, the primary efficacy outcome was confirmed in the modified intention-to-treat population for both of the overall study period and the on-treatment period) covered the evaluation of the extended treatment.Citation37,Citation52,Citation53 shows the design and main outcomes of those pivotal clinical studies.
Table 5 Comparison of the design and main outcomes of the pivotal clinical studies for secondary prevention of VTE
Challenges of using NOACs in specific patient populations
The optimal dose of NOACs in Asian patients with NVAF
Dabigatran
In the RE-LY study, both doses of dabigatran 110 mg and 150 mg showed non-inferior or superior efficacy and favorable safety compared to warfarin.Citation23–Citation25 The European Medicines Agency (EMA), the Korean Ministry of Food and Drug Safety (MFDS), and the Japanese Pharmaceuticals and Medical Devices Agency (PMDA) approved the 150-mg dose as a full-dose and the 110-mg dose as a reduced-dose for fragile patients (eg, geriatric, lower body weight, increased risk of bleeding, moderate renal impairment, and concomitant use of the P-glycoprotein 1 (P-gp) inhibitor).Citation41,Citation54,Citation55 Unlike the other medical regulatory agencies, the US Food and Drug Administration (FDA) did not approve the 110-mg dose.Citation37 The FDA emphasized the result of the RE-LY study that the dabigatran 150-mg dose was superior to warfarin for efficacy and similar for bleeding, but the 110-mg dose was non-inferior to warfarin for efficacy and caused less bleeding.Citation56 The events of ischemic stroke in the 110-mg group (1.3% per year) outnumbered the events in the warfarin group (1.1% per year).Citation56 Moreover, the post-hoc analysis showed that the 150-mg dose was statistically superior to the 110-mg dose in preventing stroke, and the exploratory analysis by reviewers in the US FDA confirmed subjects who experienced a major bleeding event and subsequently received the higher dabigatran dose were at no greater risk of experiencing a subsequent major bleeding event than those receiving the lower dose.Citation37,Citation56,Citation57 Therefore, the US FDA judged approval of the 110-mg dose provides the average patient with the option of taking a dose with reduced efficacy, leading to additional strokes and disability.Citation56 Finally, the US FDA approved only the 150-mg dose.Citation37,Citation57 In not approving the 110-mg dose, the US FDA limited the dosing options for fragile patients (eg, severe renal impairment, moderate renal impairment with concomitant use of the P-gp inhibitor). Therefore, the US FDA recommended a dose of 75 mg to provide these patient populations with access to dabigatran, based not on efficacy and safety data, but on pharmacokinetic and pharmacodynamic modeling.Citation37,Citation57
Recently, considerable data on the optimal dabigatran dose for real-world patients have been published. A post-hoc simulation analysis of the RE-LY study revealed that patients’ efficacy and safety outcomes using the EU label information would be more favorable compared to warfarin, and it supported the EU label information of dabigatran.Citation58 Furthermore, another prospective observational study in Canada supported approval of both dabigatran doses examined in the RE-LY study because drug exposures were observed to be similar in patients treated with either 110- or 150-mg dose as indicated in the label information.Citation59
On the other hand, patients treated with the 75-mg dose, approved only in the US for patients with severe renal impairment on the basis of pharmacokinetic modeling, had no significantly different outcomes (eg, effect on risk of ischemic stroke, major gastrointestinal bleeding, and mortality) compared to patients with warfarin except for a lower risk of intracranial hemorrhage in an observational study using the US Medicare database.Citation60 This result suggested that the 75-mg dose was suboptimal or patients were treated off-label and under-dosed with the 75-mg dose.Citation60 Moreover, there is a recommendation that the US FDA should reconsider approving the 110-mg dose to give flexible choices to patients who would benefit most by this intermediate dose.Citation61
Regarding the optimal dose in Asians, a retrospective study to determine the optimal dose of dabigatran in Korean patients with NVAF suggested a fixed dose of 110 mg might be sufficient regardless of dose-reduction criteria based on the comparable efficacy and favorable safety of the 110-mg dose to 150-mg dose.Citation62 This finding in Korea is consistent with the observational study of real-world data in Taiwan that did not show a benefit of the 150-mg dose over the 110-mg dose.Citation63 Also, those results support the real-world clinical practice for dabigatran in Asia that the 110-mg dose is preferred because of a concern about bleeding risk in Asian patients with a higher dose.Citation62,Citation63
The recommendation for dabigatran dose reduction to 110 mg in fragile patients is supported by real-world clinical data as well as the RE-LY study. However, clinical data on the optimal dabigatran dose in Asian patients with NVAF are not sufficient. More data are required to evaluate whether dabigatran 150 mg or 110 mg is a desirable dose in Asian patients. Additionally, we should also consider that subgroup analyses by Asian and non-Asian populations in the RE-LY study showed that favorable treatment effects of both doses of dabigatran compared to warfarin did not show a significant difference between Asian and non-Asian patients.Citation64 The US FDA has maintained the approved dabigatran regimen without a compromised intermediate dose and more intensive treatment is required for stroke prevention in Asia where the burden of AF is expected to increase.Citation6
Rivaroxaban
The smaller body size and lower renal clearance in Asians compared to non-Asians, as well as ethnic differences in pharmacokinetic and pharmacodynamic features, render physicians to prefer reduced-dose NOACs for their patients.Citation62,Citation63,Citation65 Mirroring that tendency, especially among Asians, reduced-dose rivaroxaban has been used more frequently than the full-dose in patients without dose-reduction criteria (eg, renal dysfunction).Citation65,Citation66 However, concerns remain about the insufficient net clinical benefit of off-label underdosing and the optimal dose of rivaroxaban for Asian NVAF patients.Citation66,Citation67
Rivaroxaban was approved at a lower dose in Japan than in the US, Europe, and Korea as shown in . Distribution of rivaroxaban with the 15-mg dose in Japanese patients was predicted to be equivalent to that in Caucasian patients with the 20-mg dose in pharmacokinetic modeling data.Citation37,Citation68 Therefore, Japanese patients were not included in the global, pivotal ROCKET-AF study but were instead enrolled in a domestic Phase III clinical study with a lower dose because of the risk of bleeding complications with the full dose.Citation37,Citation39 Although the Japanese PMDA approved the lower dose based on the results of that local study, it was underpowered due to small sample size and showed borderline statistical significance for the primary efficacy endpoint; thus, it is uncertain that using a lower dose can be generalized to the entire Asian patient population.Citation66
A Taiwanese nationwide cohort study showed that almost 95% of patients received reduced-dose rivaroxaban (eg, 10–15 mg once daily) regardless of renal function and this reduced-dose had a significantly lower risk of stroke, major bleeding, intracranial hemorrhage, and mortality when compared with warfarin.Citation69 This study did not show a trend of insufficient efficacy in patients who took reduced-dose rivaroxaban. Furthermore, this result from reduced-dose rivaroxaban was comparable to the subgroup analysis of Asians from the ROCKET-AF study using full-dose rivaroxaban.Citation69 This study had a limit to determine whether those patients with reduced-dose rivaroxaban were correctly adjusted by dose-reduction criteria or were off-label underdosed.Citation69
Meanwhile, a retrospective, observational study using the Korean National Health Insurance Service (NHIS) database revealed that both on-label 20 mg and off-label 15 mg were associated with a lower risk of stroke, major bleeding, and all-cause death compared with warfarin in patients with NVAF and normal or mildly impaired renal function.Citation66 On-label 20 mg showed a nonsignificant trend toward lower risks of stroke, hospitalization for gastrointestinal bleeding or major bleeding, and all-cause death compared with off-label 15 mg.Citation66 Overall, on-label 20 mg showed significantly better results for the composite clinical outcome compared with off-label 15 mg.Citation66 On the other hand, another retrospective, observational study using the Korean NHIS database demonstrated that off-label use of reduced-dose rivaroxaban had comparable efficacy and safety outcomes compared to full-dose rivaroxaban in patients who were subject to full-dose rivaroxaban.Citation65 However, both studies should be interpreted cautiously due to patient selection bias (eg, elderly patients or patients with multimorbidity or high thromboembolic risk were not included).Citation65,Citation66
Although several studies were conducted in various Asian regions to examine the efficacy and safety of full- and reduced-dose rivaroxaban, the optimal dose for Asian patients with NVAF remains still uncertain. Considering the highest proportion of patients treated with rivaroxaban among patients treated with NOAC in Asia, issues related to the optimal dose of rivaroxaban for Asian patients are important.Citation65,Citation66,Citation69 For that reason, further studies are required to identify the desirable dose of rivaroxaban; careful evaluation of the individual’s thromboembolic and bleeding risk is needed at the dose selection level.Citation65,Citation66,Citation69
Apixaban
Among the four NOACs, only apixaban has an identical dosing regimen for patients with NVAF in both Western and Asian countries. Unlike dabigatran and rivaroxaban, recent studies indicated that off-label underdosing of apixaban was associated with less clinical benefit over warfarin in Asian patients.Citation65 In a retrospective, observational study using the Korean NHIS database, reduced-dose apixaban in patients whom the full-dose might be indicated showed a notable reduction in efficacy and loss of superiority over warfarin, although the safety profile was better in comparison with warfarin.Citation65 In addition, this finding in Korea is supported by the result from a study using the US administrative database that off-label underdosing of apixaban had a considerably higher risk of stroke but a similar risk of major bleeding compared with full-dose apixaban.Citation70 These findings suggest unjustified underdosing of apixaban causes insufficient stroke prevention without safety benefits.Citation65,Citation70
Edoxaban
Real-world clinical data are required to evaluate edoxaban in patients with NVAF with supranormal renal function to determine the optimal dose of edoxaban needed to protect patients against stroke. In the ENGAGE AF-TIMI study, the efficacy of edoxaban decreased with increasing CrCL as compared to well-managed warfarin and the medical regulatory agencies adopted different approaches regarding this issue.Citation37 The US FDA included a warning on the label information and mentioned that patients with CrCL >95 mL/min should not use edoxaban as a risk mitigation strategy.Citation37,Citation71 In contrast, the EMA did not prevent edoxaban use in patients with supranormal renal function but emphasized edoxaban should be used only after a careful evaluation of each individual’s thromboembolic and bleeding risk.Citation37,Citation47 An additional study entitled “Evaluation of Lixiana (edoxaban) in patients with non-valvular atrial fibrillation and high creatinine clearance” is ongoing in Europe to investigate whether a higher dose of edoxaban advances prevention against stroke in those patients as a measure of the risk management plan (RMP).Citation72
The Korean MFDS took a comparable stance with the EMA on this issue.Citation37,Citation53 However, Korea could not be included in that post-authorization study because the approval application for edoxaban was submitted prior to the introduction of the RMP system there. Recently, the first study was published reporting the efficacy and safety of edoxaban compared with warfarin, focusing on Korean patients with NVAF and good kidney function.Citation73 In this retrospective cohort study using the Korean NHIS data, edoxaban showed a statistically nonsignificant trend toward reduced stroke risk in both patients with CrCl >80–95 mL/min and >95 mL/min compared with warfarin, although the small sample size and short-term follow-up duration may have caused some statistical nonsignificance in the analysis results.Citation73 Nevertheless, the recent Korean AF Management Guideline prefers dabigatran, rivaroxaban, and apixaban to edoxaban in NVAF patients with CrCL >90mL/min.Citation74 Approved label information of edoxaban in Japan where it was first released for prevention of stroke in patients with NVAF has no precaution related to decreased efficacy with increasing CrCL.Citation75 Therefore, Korean and Japanese regulatory agencies should notice the upcoming results of that post-authorization study and evaluate the need to modify the label information and clinical guidelines of edoxaban.
Choice of NOAC in NVAF patients presenting with ACS and/or undergoing PCI
Up to one-third of all patients with AF eventually develop vascular disease, and up to one-fifth of all patients with AF are likely to undergo stenting at some point.Citation76 Therefore, it is important to carefully consider antithrombotic therapies by balancing their associated bleeding risk, stroke risk, and acute coronary syndrome (ACS) risk.Citation14 Conventional triple antithrombotic therapies, including warfarin, clopidogrel, and aspirin, are associated with high bleeding risk.Citation77–Citation79 The WOEST study evaluated the safety and efficacy of clopidogrel versus clopidogrel plus aspirin in patients receiving OACs and undergoing percutaneous coronary intervention (PCI) revealing that dual therapy is associated with a lower bleeding risk than triple therapy without an increase of thrombosis risk.Citation80 Although this study was too small to evaluate thrombotic outcomes, it supports the hypothesis that dual therapy with OAC and clopidogrel may be an alternative to triple therapy in AF patients undergoing PCI.Citation80
There was a subgroup analysis of RE-LY study with dabigatran suggesting that the benefits of NOACs are not affected regarding efficacy and safety compared to warfarin in the setting of triple therapy.Citation81 However, until recently most guidelines limited adding NOACs to antiplatelets in patients with NVAF with ACS and/or PCI with stenting due to limited clinical evidence.Citation14,Citation38,Citation82 Now, the most recently revised guidelines are recommending NOACs over a VKA based on the PIONEER AF-PCI and the RE-DUAL PCI study.Citation83–Citation85 Both studies showed lower bleeding risk among patients who received NOAC and a P2Y12 inhibitor than among those who received triple therapy, with no apparent increase in cardiovascular events, although the studies were not powered to evaluate them.Citation77,Citation78
Each of the four NOACs has been studied or is being assessed for combination therapy with antiplatelets in NVAF patients presenting with ACS and/or undergoing PCI. The PIONEER AF-PCI study with rivaroxaban showed that two rivaroxaban groups significantly lowered the rates of clinically significant bleeding than the triple therapy group (group 1: rivaroxaban 15 mg once daily + P2Y12 inhibitor for 12 months, group 2: rivaroxaban 2.5 mg twice daily + dual antiplatelet therapy for 1, 6, or 12 months, group 3: VKA + dual antiplatelet therapy for 1, 6, or 12 months).Citation77 It is important to note that both doses of rivaroxaban used in this study were lower than dose known to reduce the risk of stroke and SEE in NVAF.Citation77,Citation83
Two different dabigatran dose regimens (either 110 mg or 150 mg twice daily) plus a P2Y12 inhibitor significantly lowered the major or CRNM bleeding risk more than the triple therapy in the RE-DUAL PCI study.Citation78 Furthermore, the combined dual therapy group with dabigatran and a P2Y12 inhibitor was noninferior regarding the cardiovascular outcomes in the triple therapy group.Citation78 Although this study was not powered to access the risk of thrombosis by dabigatran dose, it revealed that both dabigatran doses balanced the risk of bleeding with the prevention of thromboembolic events in NVAF patients presenting with ACS and/or undergoing PCI and it proved the concept of the WOEST study with greater statistical power.Citation78
In the AUGUSTUS study, a dose of apixaban with proven efficacy for reducing stroke risk in NVAF patients significantly lowered the rates of major or CRNM bleeding without significant differences in ischemic events; this was compared to a regimen of a VKA, aspirin, or both in patients with AF and recent ACS or PCI treated with a P2Y12 inhibitor.Citation79 Moreover, this study directly evaluated the benefits and risks of omitting aspirin with both apixaban and VKA and revealed that the effect of dropping aspirin on the rate of bleeding events is greater than the benefit of using apixaban instead of a VKA.Citation79
The ongoing ENTRUST AF-PCI study of edoxaban (NCT02866175, EudraCT Number: 2016–002683-14) is expected to provide appropriate approaches for antithrombotic therapy in patients in whom both edoxaban and antiplatelet treatment are indicated.Citation86 The intensity of NOAC and antiplatelet treatment and the duration of antiplatelet treatment should be adjusted according to the bleeding and thrombotic risk profiles of the individual patient.Citation84
Use of NOACs in dialysis patients with NVAF
The risk of NVAF, ischemic stroke, and serious bleeding increases more in patients with chronic kidney disease (CKD) compared to people with normal renal function, and the relative risk of each of them increases progressively with advancing CKD, being greatest in dialysis patients.Citation87 Furthermore, NVAF in dialysis patients increases the risk of mortality and stroke.Citation88
However, although VKAs have been used as prophylaxis against embolic complications of AF in the general population and in early stage CKD, the efficacy is unclear and the bleeding risk increases significantly in severe CKD, particularly in dialysis patients.Citation87 Furthermore, there are no clinical data that demonstrate the efficacy and safety of NOACs in dialysis patients because these severe CKD patients were excluded in the premarketing pivotal clinical studies of NOACs.Citation23,Citation26–Citation28,Citation87 Increased bleeding risk and unclear efficacy of OACs in dialysis patients with AF should be considered when making decisions on whether to give OACs for these patients.Citation89
A recent “Kidney Disease: Improving Global Outcomes Controversies Conference” suggests that if dialysis patients with NVAF require OAC for stroke prevention, then an apixaban 2.5 mg twice-daily regimen could be considered while awaiting more clinical efficacy and safety data.Citation87,Citation90 NOACs may have advantages over VKAs in dialysis patients with NVAF, although two ongoing studies of apixaban versus VKA (AXADIA and RENAL-AF, NCT02933697, and NCT02942407) will evaluate the relative merits of NOAC vs VKA.Citation89,Citation90
Option to use reduced- or full-dose NOACs in the extended treatment of VTE
Extended anticoagulant therapy for reducing VTE recurrence is accompanied by bleeding risk and leading to hesitate for continuing anticoagulant therapy beyond 6–12 months.Citation91 Therefore, using lower-dose anticoagulant therapy can be considered to lower the bleeding risk when treatment is extended.Citation92 Several studies using reduced-dose NOACs revealed that they may effectively balance the benefit-to-risk profile with respect to recurrent VTE and bleeding when the risk of recurrent VTE decreases.Citation35,Citation91,Citation93
In the AMPLIFY-EXT study, the risk of recurrent VTE was significantly reduced with extended anticoagulation with apixaban at either a full-dose (5 mg twice daily) or a reduced-dose (2.5 mg twice daily) than with placebo, without a significant increase in bleeding rates.Citation35 In the EINSTEIN CHOICE study, extended anticoagulation with rivaroxaban at either a full-dose (20 mg once daily) for treatment and prophylaxis of VTE or a reduced-dose (10 mg once daily) significantly lowered the risk of recurrent VTE without increasing the bleeding rate.Citation91 Meta-analysis of these two similar extension studies suggests that reduced-dose NOACs are as effective as full-dose DOACs in reducing the risk of recurrent thrombotic events at 1 year without a significant increase in bleeding as compared with aspirin or placebo, unlike full-dose DOACs.Citation93 Therefore, reduced-dose NOACs may be considered as an attractive option for long-term therapy after VTE in patients in whom there is clinical equivalence for continuation or cessation of anticoagulant treatment.Citation93
However, there are several limitations related to the use of reduced-dose NOACs to patients with VTE. Both extension studies treated patients for up to 12 months; thus, the safety and efficacy of reduced-dose NOACs is unknown beyond 1 year.Citation35,Citation91,Citation93 Furthermore, patients requiring ongoing anticoagulant therapy with therapeutic doses (eg, active cancer or recurrent VTE) were excluded; thus, it is unknown whether reduced-dose NOACs would be sufficient to prevent recurrence in such patients.Citation35,Citation91,Citation93 Additionally, it is unclear whether the meta-analysis results are applicable to all NOACs as a class effect or only to apixaban and rivaroxaban.Citation93 Additional studies are needed to determine the utility of continuing treatment with reduced-dose NOACs for a longer period, and in patients with an indication for ongoing anticoagulant therapy.Citation35,Citation91,Citation93
Regarding the approved dose of rivaroxaban for preventing recurrent VTE, the maintenance dose after initial treatment in Japan is lower than in Korea as shown in . The Korean MFDS approved the maintenance dose based on the global, pivotal EINSTEIN-Extension study but the Japanese PMDA approved the lower dose based on a separate domestic study because the Japanese clinical guidelines recommended using lower-intensity anticoagulant therapy because of the bleeding risk.Citation37,Citation94,Citation95 Considering concerns about the bleeding risk with full-dose rivaroxaban in Asian patients, the optimal thromboprophylactic dose reflecting the characteristics of Korean VTE patients should be evaluated as in Japan.
Use of NOACs in patients with cancer-associated VTE
The risk for VTE is 4-fold higher in patients with cancer alone and the risk is higher more than 6-fold in cancer patients receiving chemotherapy as compared to healthy people.Citation96 Moreover, the risk of recurrent VTE is 2–5 times greater during anticoagulant treatment in patients with cancer than those without cancer and the risk of serious bleeding is similarly 2–6 times greater.Citation97 Therefore, treatment and secondary prevention of VTE with indefinite anticoagulant treatment are indispensable in patients with cancer-associated VTE (CAT).Citation16 Current clinical practice guidelines recommend administration of LMWH in the acute phase and the first 3–6 months as first-line therapy because indirect comparison suggests that NOACs are less effective than LMWH in patients with VTE and cancer.Citation15,Citation16,Citation98 However, the preferable treatment after the first 6 months is unclear, and no preference has been established for either LMWH or VKA or NOAC after the first 6 months.
NOACs have a number of advantages including oral administration, lack of interactions with foods or other medicines, and no need for monitoring drug levels, whereas injection of more expensive LMWH can cause pain and bruising at the injection site, and result in adverse effects like heparin-induced thrombocytopenia in patients with cancer.Citation99 However, limited evidence is available to confirm the efficacy and safety of NOACs in patients with VTE and cancer because these patients were excluded from the premarketing pivotal clinical studies and few cancer patients with a low risk of recurrent VTE and major bleeding were included in those clinical studies.Citation29,Citation30,Citation32–Citation34,Citation36 Therefore, it was unclear previously whether NOACs could be used safely in patients with CAT.Citation99 However, recently, several prospective clinical studies comparing NOACs with LMWH directly resolved uncertainties about using NOACs in these patients.
In the open-label ADAM VTE Trial, a fixed oral apixaban dose showed a very low rate of bleeding events with a significantly lower rate of VTE recurrence and better quality of life (eg, concern for excess bruising, stress, irritation, burden of delivery, and overall satisfaction with anticoagulant therapy) compared to subcutaneous injections of dalteparin at 6 months.Citation100 In the open-label Hokusai VTE Cancer study, a fixed oral edoxaban dose was noninferior to parenteral dalteparin with regard to the composite primary outcome of recurrent VTE or major bleeding for up to 12 months.Citation101 However, the rate of major bleeding as a secondary outcome was significantly higher with edoxaban compared to dalteparin due to the higher rate of upper gastrointestinal (GI) bleeding in patients with GI cancer.Citation101 In the open-label, pilot SELECT-D study, a fixed oral rivaroxaban dose was related not only to relatively low VTE recurrence within 6 months but to a 3-fold relative increase in CRNM bleeding compared with dalteparin.Citation102 Patients with esophageal or gastroesophageal cancer experienced more rivaroxaban-associated major bleeding because most of the major and CRNM bleeding occurred in GI.Citation102 Even though these studies had some limitations including their open-label designs, the relatively small number of enrolled patients, and not powered to evaluate the risk of recurrent VTE, they support the clinical use of NOACs for the acute treatment of VTE in patients with CAT.Citation100–Citation102 A recently published meta-analysis based on these studies showed that NOACs (especially direct Xa inhibitors) were more effective to reduce VTE recurrence for up to 6 months with better compliance when compared to LMWH; however, they had a significantly higher rate of major bleeding as well as a trend toward more CRNM bleeding.Citation103 Thus, NOACs can be potential alternatives to LMWH in treating CAT despite the increased risk of CRNM bleeding.Citation104
Although chemotherapy is a risk factor of VTE, routine thromboprophylaxis in ambulatory patients undergoing chemotherapy is not clinically recommended because LMWH or VKA are not effective for VTE risk reduction.Citation16,Citation105–Citation107 They are associated with an increased major bleeding risk, and parenteral thromboprophylaxis is not only expensive but also inconveniently requires daily injections.Citation16,Citation105–Citation107 However, two recently published studies evaluating oral direct Xa inhibitors may provide clinically valuable information for reducing the risk of VTE in ambulatory patients with cancer at intermediate-to-high risk for VTE.Citation107,Citation108
In the placebo-controlled, double-blind CASSINI study, rivaroxaban did not show a significantly lower rate of VTE or death due to VTE for up to 6 months although these events occurred in a lower percentage of patients in the rivaroxaban group.Citation108 However, during the intervention period, defined as the time from receipt of the first dose of rivaroxaban or placebo to the last dose plus 2 days, rivaroxaban resulted in a more favorable benefit than placebo with regard to VTE or VTE-related death, with a low incidence of major bleeding.Citation108 In the placebo-controlled, double-blind AVERT study, thromboprophylaxis with apixaban resulted in a significantly lower VTE rate than placebo in the 6-month trial period.Citation107 The major bleeding rate was significantly higher with apixaban than with placebo, but the rate of severe major bleeding events was similar in the apixaban group and the placebo group.Citation107 Although these studies had a small sample size and did not conduct subgroup analysis by individual tumor types or chemotherapy regimens, they support the benefit of antithrombotic prophylaxis against bleeding risk with oral direct Xa inhibitors in ambulatory cancer patients receiving chemotherapy at intermediate-to-high risk for VTE.Citation107,Citation108
Taken together, we have enough experiences in using NOACs in cancer patients showing the benefit of antithrombotic treatment counterbalanced the bleeding risk with NOACs. Therefore, NOACs can be considered in patients with cancer after careful evaluation of thrombotic and bleeding risk, patient’s underlying disease, and treatment preference. Currently, some challenges of CAT management exist due to differences in cancer types or chemotherapy regimens and comorbidities including renal impairment, GI problems, and thrombocytopenia; hence, more studies with various designs and a much larger sample are required to address these challenges.Citation104 Furthermore, all studies previously mentioned have evaluated oral direct Xa inhibitors; thus, findings and conclusions from these studies may not be extrapolated to a direct thrombin inhibitor.
Adherence and persistence of NOACs
Poor adherence and persistence (that is, adherence during the whole treatment period) to anticoagulant medication may result in increased rates of both thromboembolism and bleeding as well as worse morbidity and mortality.Citation109,Citation110 NOACs have an advantage of fixed oral dosing but also shorter half-lives than VKA and difficulty in checking adherence without blood level monitoring.Citation109,Citation111
Different dosing regimens among NOACs may impact on medication adherence; thus, individual patient preference should be considered in choosing a particular NOAC.Citation109 For VTE, rivaroxaban, and apixaban as a simple single-drug approach in initial treatment phase do not require previous treatment with parenteral anticoagulant, although dabigatran and edoxaban should be administered after parenteral anticoagulant for at least 5 days.Citation40–Citation47 Therefore, rivaroxaban and apixaban should be recommended for patients avoiding or hesitating parenteral therapy. For patients with NVAF or VTE, rivaroxaban should be taken with food, unlike dabigatran, apixaban, and edoxan, which can be taken with or without food.Citation40–Citation47 Rivaroxaban and edoxaban are administered once-daily (OD) but dabigatran and apixaban are dosed twice-daily (BID).Citation40–Citation47
However, reducing the dosing complexity and frequency from multiple dosing to OD dosing might not necessarily result in better adherence.Citation112–Citation114 Furthermore, OD dosing might be more disadvantageous than BID dosing regarding non-adherence because a single missed OD dose equals to 2–3 consecutively missed doses from a BID dosing regimen.Citation109 Therefore, the Korean AF Management Guideline recommends that OD vs BID regimens should not be considered as a primary factor in choosing a particular NOAC but considered with patient preference and situation (eg, on multiple medications).Citation74 In addition, a structured follow-up of patient adherence, evaluation of contributing risk factors for non-adherence, and sufficient patient education are necessary to enhance NOAC adherence in clinical practice.Citation74,Citation109
Switching to NOACs: focusing on the drug price and cost-effectiveness of NOACs
Although many patients have switched to NOACs, a significant proportion of patients remain on warfarin because of the high price of NOACs.Citation115 The drug price of each NOAC is much higher (23- to 70-fold) than that of warfarin in various countries as shown in .Citation116–Citation119 Additional cost considerations affecting the choice of warfarin over NOACs include variability in reimbursement coverage. This section would examine a case of Korea to investigate whether economic factors and cost-effectiveness affect the use of DOACs.
Table 6 Drug prices and reimbursement of OACs for patients with NVAF in the US, UK, Korea, and Japan
In Korea, the high cost of NOACs contributed to strict reimbursement guidelines and preference of warfarin.Citation120 Before 2015, patients with NVAF with a history of thromboembolism or age ≥75 years or at least two of the following (heart failure, hypertension, diabetes, age 65–74 years, female gender, vascular disease) could take NOACs with reimbursement coverage only if warfarin was not suitable for these patients. In July 2015, reimbursement guidelines were revised to choose NOACs as the first therapy in these patients (Ministry of Health and Welfare notification No 2015–118, 29 June 2015). This drove a voluntarily decrease in the cost of NOACs and an increase in the prescription rate of NOACs between 2014 and 2015 (from 9% in 2014 to 38% in 2015).Citation121 Annual relative prescription rates of warfarin and LMWH for VTE decreased with NOACs with reimbursement coverage between 2012 and 2013 (warfarin, from 63.9% in 2012 to 42.6% in 2013; LMWH, from 25.8% in 2012 to 22.5% in 2013).Citation12 However, the reimbursement guidelines for VTE seem to remain strict in that patients with VTE can take NOACs only for 6 months with reimbursement coverage, and only the cost of warfarin can be reimbursed in Korea beyond 6 months (Ministry of Health and Welfare notification No 2012–173, 27 December 2012). Therefore, patients with a high risk of relapse, such as those with cancer, must pay the cost of NOACs by themselves after the first 6 months and the actual prescription rate of NOACs after the first 6 months cannot be identified using the Korean Health Insurance Review and Assessment Service (HIRA) databases.Citation12
Absolute benefits and harms of NOACs in NVAF patients using the number needed to treat (NNT) and/or number needed to harm (NNH) were addressed in several reports including a study conducted by Kumana et al.Citation122 Kumana et al showed absolute benefits of NOACs for primary-outcome prevention were modest in terms of NNT/year value (eg, 182–481, ) with greater acquisition costs compared to warfarin.Citation122 However, these findings should be considered with benefits from avoidance of INR monitoring and its impact on the quality of life, etc.Citation122 Therefore, we need to focus on the long-term cost-effectiveness of NOACs.
To our knowledge, three studies assessed the cost-effectiveness of NOACs compared to warfarin in Korean patients as shown in . Both studies conducted by Lee et al and Kim et al obtained data (eg, the baseline of patients, clinical event rates, costs, etc.) based on the results of pivotal clinical trials, literature, research data, and interviews with health care professionals.Citation123,Citation124 Another study conducted by Kim et al used specific real-world data from the Korean HIRA database.Citation125 All of these cost-effectiveness analyses demonstrate that NOACs, including other NOACs which are not evaluated in these studies, may be cost-effective alternatives to warfarin in Korean patients with NVAF.Citation123–Citation125
Table 7 Cost-effectiveness results of base case analyses for patients with NVAF receiving NOACs
It is expected that annual prescription rates of NOACs in Korea will steadily increase, therefore, that the drug price of NOACs will be dropped by the “prescription rate-drug price interoperation policy.”Citation121 Furthermore, generic drugs will emerge in the near future to expand the market of NOACs.Citation121 Cost-effective NOACs with lower drug prices will effectively reduce the burden of NVAF-related stroke in Asia where the burden of AF is expected to increase with a large elderly population and increased life expectancy.
Conclusion
NOACs have been approved for risk reduction of stroke and SEE in NVAF, treatment of VTE and risk reduction in the recurrence of VTE in various countries based on their comparable or favorable efficacy and safety profiles compared to the conventional anticoagulation therapy in NVAF or VTE patients.Citation37 Target-specific NOACs with predictable, reversible anticoagulant effects without a need for invasive monitoring are increasingly used as substitutes to conventional anticoagulation therapy and provide a wide range of patient treatment choices.Citation126 However, it is difficult to make a complete change to NOACs in specific patient populations due to the challenges discussed in this review. To compensate clinical-evidence and achieve optimal use of NOACs, we should pay attention to the outcomes of ongoing studies and evaluate more real-world data. Most of all, NOAC use should be regulated in individual patient to have a sufficient benefit for antithrombotic treatment or prophylaxis over bleeding risk.
Abbreviations
ACS, acute coronary syndrome; AF, atrial fibrillation; APTT, activated partial thromboplastin time; BID, twice-daily; CAT, cancer-associated VTE; CHMP, Committee for Medicinal Products for Human Use; CKD, chronic kidney disease; CrCL, creatinine clearance; CRNM, clinically relevant non-major; DVT, deep vein thrombosis; EMA, European Medicines Agency; FDA, Food and Drug Administration; FXa, factor Xa; HIRA, Health Insurance Review and Assessment Service; HR, hazard ratio; INR, international normalized ratio; LMWH, low-molecular-weight heparin; MFDS, Ministry of Food and Drug Safety; NI, non-inferiority; NOACs, non-vitamin K oral anticoagulants; NVAF, non-valvular atrial fibrillation; OACs, oral anticoagulants; OD, once-daily; PCI, percutaneous coronary intervention; PE, pulmonary embolism; P-gp, P-glycoprotein 1; PMDA, Pharmaceuticals and Medical Devices Agency; RMP, risk management plan; RR, relative risk; SEE, systemic embolic event; SUP, superiority; TTR, time in therapeutic range; UFH, unfractionated heparin; US, United States; VKAs, vitamin K antagonists; VTE, venous thromboembolism.
Disclosure
The author reports no conflicts of interest in this work.
References
- Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31(19):2369–2429. doi:10.1093/eurheartj/ehq27820802247
- What is atrial fibrillation? National institutes of health. Available from: https://www.nhlbi.nih.gov/health/health-topics/topics/af. Published 2014 Accessed 130, 2017.
- What are the signs and symptoms of atrial fibrillation? National institutes of health. Available from: https://www.nhlbi.nih.gov/health/health-topics/topics/af/signs. Published 2014 Accessed 130, 2017.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke. 1991;22(8):983–988.1866765
- Tse HF, Wang YJ, Ahmed Ai-Abdullah M, et al. Stroke prevention in atrial fibrillation – an Asian stroke perspective. Heart Rhythm. 2013;10(7):1082–1088. doi:10.1016/j.hrthm.2013.03.01723501173
- Chiang CE, Wang KL, Lip GY. Stroke prevention in atrial fibrillation: an Asian perspective. Thromb Haemost. 2014;111(5):789–797. doi:10.1160/TH13-11-094824500243
- Goldhaber SZ. Venous thromboembolism: epidemiology and magnitude of the problem. Best Pract Res Clin Haematol. 2012;25(3):235–242. doi:10.1016/j.beha.2012.06.00722959540
- What is deep vein thrombosis? National institutes of health. Available from: https://www.nhlbi.nih.gov/health/health-topics/topics/dvt/. Published 2011 Accessed 327, 2017.
- What is pulmonary embolism? National institutes of health. Available from: https://www.nhlbi.nih.gov/health/health-topics/topics/pe/. Published 2011 Accessed 327, 2017.
- Raskob GE, Angchaisuksiri P, Blanco AN, et al. Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol. 2014;34(11):2363–2371.
- Jang MJ, Bang SM, Oh D. Incidence of venous thromboembolism in Korea: from the health insurance review and assessment service database. J Thromb Haemost. 2011;9(1):85–91. doi:10.1111/j.1538-7836.2010.04108.x20942850
- Hong J, Lee JH, Yhim HY, et al. Incidence of venous thromboembolism in Korea from 2009 to 2013. PLoS One. 2018;13(1):e0191897. doi:10.1371/journal.pone.019189729370290
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the heart rhythm society. Circulation. 2014;130(23):2071–2104. doi:10.1161/CIR.000000000000004024682348
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Rev Esp Cardiol (Engl Ed). 2017;70(1):50. doi:10.1016/j.rec.2016.11.03328038729
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315–352. doi:10.1016/j.chest.2015.11.02626867832
- Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033–3069, 3069a-3069k. doi:10.1093/eurheartj/ehu283
- Label information of COUMADIN. U.S. food and drug administration. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/009218s116lbl.pdf. Published 2016 Accessed 21, 2017.
- Label information of LOVENOX. U.S. food and drug administration. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020164s102lbl.pdf. Published 2013 Accessed 527, 2017.
- Label information of INNOHEP. U.S. food and drug administration. Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process. Published 2010 Accessed 527, 2017.
- Label information of FRAGMIN. U.S. food and drug administration. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020287s069lbl.pdf. Published 2017 Accessed 527, 2017.
- Sinauridze EI, Panteleev MA, Ataullakhanov FI. Anticoagulant therapy: basic principles, classic approaches and recent developments. Blood Coagul Fibrinolysis. 2012;23(6):482–493. doi:10.1097/MBC.0b013e328355c9cb22732252
- Label information of ARIXTRA. U.S. food and drug administration. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021345s032lbl.pdf. Published 2014 Accessed 527, 2017.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. doi:10.1056/NEJMoa090556119717844
- Connolly SJ, Ezekowitz MD, Yusuf S, Reilly PA, Wallentin L. Newly identified events in the RE-LY trial. N Engl J Med. 2010;363(19):1875–1876. doi:10.1056/NEJMc100737821047252
- Connolly SJ, Wallentin L, Yusuf S. Additional events in the RE-LY trial. N Engl J Med. 2014;371(15):1464–1465. doi:10.1056/NEJMc140790825251519
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. doi:10.1056/NEJMoa100963821830957
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. doi:10.1056/NEJMoa110703921870978
- Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–2104. doi:10.1056/NEJMoa131090724251359
- Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342–2352. doi:10.1056/NEJMoa090659819966341
- Schulman S, Kakkar AK, Goldhaber SZ, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129(7):764–772. doi:10.1161/CIRCULATIONAHA.113.00445024344086
- Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368(8):709–718. doi:10.1056/NEJMoa111369723425163
- Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499–2510. doi:10.1056/NEJMoa100790321128814
- Buller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366(14):1287–1297. doi:10.1056/NEJMoa111357222449293
- Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799–808. doi:10.1056/NEJMoa130250723808982
- Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368(8):699–708. doi:10.1056/NEJMoa120754123216615
- Buller HR, Decousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369(15):1406–1415. doi:10.1056/NEJMoa130663823991658
- Cho IY, Choi KH, Sheen YY. How Does “Regulatory Practice” Create Discrepancies in Drug Label Information Between Asian and Western Countries? Different Label Information for Direct Oral Anticoagulants Approved in the United States, Europe, Korea, and Japan. Ther Innov Regul Sci. 2019;53(2):233–242.
- Macle L, Cairns J, Leblanc K, et al. 2016 focused update of the Canadian cardiovascular society guidelines for the management of atrial fibrillation. Can J Cardiol. 2016;32(10):1170–1185. doi:10.1016/j.cjca.2016.07.59127609430
- JCS Joint Working Group. Guidelines for pharmacotherapy of atrial fibrillation (JCS 2008): digest version. Circ J. 2010;74(11):2479–2500.20962419
- Label information of PRADAXA. U.S. Food and Drug Administration. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022512s028lbl.pdf. Published 2015 Accessed 28, 2017.
- PRADAXA: EPAR-Product Information. European medicines agency. Available from: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000829/WC500041059.pdf. Published 2017 Accessed 28, 2017.
- Label information of Xarelto. U.S. food and drug administration. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/022406s019s020lbl.pdf. Published 2016 Accessed 28, 2017.
- XARELTO: EPAR-Product Information. European medicines agency. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000944/WC500057108.pdf. Published 2016 Accessed 28, 2017.
- Label information of ELIQUIS. U.S. Food And Drug Administration. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/202155s012lbl.pdf. Published 2016 Accessed 28, 2017.
- Eliquis : EPAR – product information European medicines agency. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002148/WC500107728.pdf. Published 2016 Accessed 28, 2017.
- Label information of SAVAYSA. U.S. food and drug administration. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/206316s004lbl.pdf. Published 2016 Accessed 28, 2017.
- Lixiana : EPAR – Product Information European Medicines Agency. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002629/WC500189045.pdf. Published 2016 Accessed 28, 2017.
- Yeh CH, Gross PL, Weitz JI. Evolving use of new oral anticoagulants for treatment of venous thromboembolism. Blood. 2014;124(7):1020–1028. doi:10.1182/blood-2014-03-56305624923298
- Baglin T, Hillarp A, Tripodi A, Elalamy I, Buller H, Ageno W. Measuring oral direct inhibitors of thrombin and factor Xa: a recommendation from the subcommittee on control of anticoagulation of the scientific and standardization committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2013;11(4):756–760. doi:10.1111/jth.2013.11.issue-4
- Pollack CV Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med. 2015;373(6):511–520. doi:10.1056/NEJMoa150200026095746
- ANDEXXA: Summary Basis for Regulatory Action. U.S. food and drug administration. Available from: https://www.fda.gov/downloads/biologicsbloodvaccines/cellulargenetherapyproducts/approvedproducts/ucm610006.pdf. Published 2018 Accessed 66, 2018.
- Edoxaban: CHMP assessment report. European medicines agency. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002629/WC500189047.pdf. Published 2015 Accessed 45, 2017.
- New drug approval report of LIXIANA. Korea ministry of food and drug safety. Available from: http://www.mfds.go.kr/index.do?x=0&searchkey=product_nm&mid=1176&searchword=릭시아나&cd=191&y=0&pageNo=1&seq=24202&cmd=v. Published 2016 Accessed 45, 2017.
- Approved label of pradaxa in Korea – Korean Ministry of Food and Drug Administration. Available from: http://www.health.kr/images/insert_pdf/IN_2011022100001_01.pdf. Published 2017 Accessed 721, 2017.
- Approved label of pradaxa in Japan pharmaceuticals and medical devices agency. Available from: http://www.info.pmda.go.jp/go/pack/3339001M1024_1_11/. Published 2016 Accessed 721, 2017.
- Summary review of PRADAXA. U.S. food and drug administration. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022512Orig1s000SumR.pdf. Published 2010 Accessed 212, 2017.
- Beasley BN, Unger EF, Temple R. Anticoagulant options – why the FDA approved a higher but not a lower dose of dabigatran. N Engl J Med. 2011;364(19):1788–1790. doi:10.1056/NEJMp110305021488759
- Lip GY, Clemens A, Noack H, Ferreira J, Connolly SJ, Yusuf S. Patient outcomes using the European label for dabigatran. A post-hoc analysis from the RE-LY database. Thromb Haemost. 2014;111(5):933–942. doi:10.1160/TH13-09-073424326736
- Chan NC, Coppens M, Hirsh J, et al. Real-world variability in dabigatran levels in patients with atrial fibrillation. J Thromb Haemost. 2015;13(3):353–359. doi:10.1111/jth.1282325523236
- Graham DJ, Reichman ME, Wernecke M, et al. Cardiovascular, bleeding, and mortality risks in elderly medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation. 2015;131(2):157–164. doi:10.1161/CIRCULATIONAHA.114.01206125359164
- Hernandez I. Time to reconsider dabigatran 110 mg in the USA. Am J Cardiovasc Drugs. 2015;15(5):307–309. doi:10.1007/s40256-015-0137-026068887
- Lee KH, Park HW, Lee N, et al. Optimal dose of dabigatran for the prevention of thromboembolism with minimal bleeding risk in Korean patients with atrial fibrillation. Europace. 2017;19(suppl_4):iv1–iv9. doi:10.1093/europace/eux24729220421
- Chan YH, Yen KC, See LC, et al. Cardiovascular, bleeding, and mortality risks of dabigatran in Asians with nonvalvular atrial fibrillation. Stroke. 2016;47(2):441–449. doi:10.1161/STROKEAHA.115.01147626732563
- Hori M, Connolly SJ, Zhu J, et al. Dabigatran versus warfarin: effects on ischemic and hemorrhagic strokes and bleeding in Asians and non-Asians with atrial fibrillation. Stroke. 2013;44(7):1891–1896. doi:10.1161/STROKEAHA.113.00099023743976
- Cho MS, Yun JE, Park JJ, et al. Outcomes after use of standard- and low-dose non-vitamin k oral anticoagulants in Asian patients with atrial fibrillation. Stroke. 2019;50:110–118. Strokeaha118023093.
- Lee SR, Choi EK, Han KD, Jung JH, Oh S, Lip GYH. Optimal rivaroxaban dose in Asian patients with atrial fibrillation and normal or mildly impaired renal function. Stroke. 2019;50(5):1140–1148. doi:10.1161/STROKEAHA.118.02421030913984
- Steinberg BA, Shrader P, Thomas L, et al. Off-label dosing of non-vitamin K antagonist oral anticoagulants and adverse outcomes: the ORBIT-AF II registry. J Am Coll Cardiol. 2016;68(24):2597–2604. doi:10.1016/j.jacc.2016.09.96627978942
- Hori M, Matsumoto M, Tanahashi N, et al. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation – the J-ROCKET AF study. Circ J. 2012;76(9):2104–2111.22664783
- Chan YH, See LC, Tu HT, et al. Efficacy and safety of apixaban, dabigatran, rivaroxaban, and warfarin in Asians with nonvalvular atrial fibrillation. J Am Heart Assoc. 2018;7:8. doi:10.1161/JAHA.118.008528
- Yao X, Shah ND, Sangaralingham LR, Gersh BJ, Noseworthy PA. Non-Vitamin K antagonist oral anticoagulant dosing in patients with atrial fibrillation and renal dysfunction. J Am Coll Cardiol. 2017;69(23):2779–2790. doi:10.1016/j.jacc.2017.03.60028595692
- Risk assessment and risk mitigation review. U.S. Food and Drug Administration. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/206316Orig1Orig2s000RiskR.pdf. Published 2015 Accessed 216, 2017.
- Summary of the risk management plan (RMP) for Lixiana (edoxaban). European medicines agency. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Risk-management-plan_summary/human/002629/WC500186050.pdf. Published 2015 Accessed 227, 2017.
- Lee SR, Choi EK, Han KD, et al. Non-vitamin K antagonist oral anticoagulants in Asian patients with supranormal renal function. Stroke. 2019;50(6):1480–1489. doi:10.1161/STROKEAHA.118.02426431084339
- Lee KH, Joung B, Lee S-R, et al. 2018 KHRS expert consensus recommendation for oral anticoagulants choice and appropriate doses: specific situation and high risk patients. Korean J Intern Med. 2018;93(2):110–132. doi:10.3904/kjm.2018.93.2.110
- Approved label of lixiana in Japan pharmaceuticals and medical devices agency. Available from: http://www.info.pmda.go.jp/go/pack/3339002F1020_1_08/. Published 2016 Accessed 721, 2017.
- Lip GY, Windecker S, Huber K, et al. Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary or valve interventions: a joint consensus document of the European Society of Cardiology working group on thrombosis, European Heart Rhythm Association (EHRA), European Association of Percutaneous Cardiovascular Interventions (EAPCI) and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS) and Asia-Pacific Heart Rhythm Society (APHRS). Eur Heart J. 2014;35(45):3155–3179. doi:10.1093/eurheartj/ehu29825154388
- Gibson CM, Mehran R, Bode C, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375(25):2423–2434. doi:10.1056/NEJMoa161159427959713
- Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377(16):1513–1524. doi:10.1056/NEJMoa170845428844193
- Lopes RD, Heizer G, Aronson R, et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019;380(16):1509–1524. doi:10.1056/NEJMoa181708330883055
- Dewilde WJ, Oirbans T, Verheugt FW, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381(9872):1107–1115. doi:10.1016/S0140-6736(12)62177-123415013
- Dans AL, Connolly SJ, Wallentin L, et al. Concomitant use of antiplatelet therapy with dabigatran or warfarin in the randomized evaluation of long-term anticoagulation therapy (RE-LY) trial. Circulation. 2013;127(5):634–640. doi:10.1161/CIRCULATIONAHA.112.11538623271794
- Chen CH, Chen MC, Gibbs H, et al. Antithrombotic treatment for stroke prevention in atrial fibrillation: the Asian agenda. Int J Cardiol. 2015;191:244–253. doi:10.1016/j.ijcard.2015.03.36925978611
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation. 2019 Cir0000000000000665. doi:10.1161/CIR.0000000000000665
- Angiolillo DJ, Goodman SG, Bhatt DL, et al. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention. Circulation. 2018;138(5):527–536. doi:10.1161/CIRCULATIONAHA.118.03472230571525
- Lip GYH, Collet JP, Haude M, Huber K. Management of antithrombotic therapy in AF patients presenting with ACS and/or undergoing PCI: a summary of the joint consensus document of the European Heart Rhythm Association (EHRA), European Society of Cardiology working group on thrombosis, European Association of Percutaneous Cardiovascular Interventions (EAPCI) and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), Latin America Heart Rhythm Society (LAHRS), and Cardiac Arrhythmia Society of Southern Africa (CASSA). Eur Heart J. 2018;39(31):2847–2850. doi:10.1093/eurheartj/ehy39630137444
- Edoxaban Treatment Versus Vitamin K Antagonist in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention (ENTRUST-AF-PCI, NCT02866175). Available from: ClinicalTrials.gov.https://clinicaltrials.gov/ct2/show/NCT02866175?term=NCT02866175&rank=1. Accessed 428, 2019.
- Kalra PA, Burlacu A, Ferro CJ, Covic A. Which anticoagulants should be used for stroke prevention in non-valvular atrial fibrillation and severe chronic kidney disease? Curr Opin Nephrol Hypertens. 2018;27(6):420–425. doi:10.1097/MNH.000000000000044330020140
- Zimmerman D, Sood MM, Rigatto C, Holden RM, Hiremath S, Clase CM. Systematic review and meta-analysis of incidence, prevalence and outcomes of atrial fibrillation in patients on dialysis. Nephrol Dial Transplant. 2012;27(10):3816–3822. doi:10.1093/ndt/gfs41623114904
- Heine GH, Brandenburg V, Schirmer SH. Oral anticoagulation in chronic kidney disease and atrial fibrillation. Dtsch Arztebl Int. 2018;115(17):287–294. doi:10.3238/arztebl.2018.028729789105
- Turakhia MP, Blankestijn PJ, Carrero JJ, et al. Chronic kidney disease and arrhythmias: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Eur Heart J. 2018;39(24):2314–2325. doi:10.1093/eurheartj/ehy06029522134
- Weitz JI, Lensing AWA, Prins MH, et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. 2017;376(13):1211–1222. doi:10.1056/NEJMoa170051828316279
- Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med. 2003;348(15):1425–1434. doi:10.1056/NEJMoa03502912601075
- Vasanthamohan L, Boonyawat K, Chai-Adisaksopha C, Crowther M. Reduced-dose direct oral anticoagulants in the extended treatment of venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost. 2018;16(7):1288–1295. doi:10.1111/jth.1415629772108
- Yamada N, Hirayama A, Maeda H, et al. Oral rivaroxaban for Japanese patients with symptomatic venous thromboembolism – the J-EINSTEIN DVT and PE program. Thromb J. 2015;13:2. doi:10.1186/s12959-015-0035-325717286
- JCS Joint Working Group. Guidelines for the diagnosis, treatment and prevention of pulmonary thromboembolism and deep vein thrombosis (JCS 2009). Circ J. 2011;75(5):1258–1281.21441695
- Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160(6):809–815.10737280
- Halamkova J, Penka M. [Current recommendations for the prevention and treatment of venous thromboembolism in cancer patients]. Klin Onkol. 2017;30(2):100–105. doi:10.14735/amko201710028397505
- Carrier M, Cameron C, Delluc A, Castellucci L, Khorana AA, Lee AY. Efficacy and safety of anticoagulant therapy for the treatment of acute cancer-associated thrombosis: a systematic review and meta-analysis. Thromb Res. 2014;134(6):1214–1219. doi:10.1016/j.thromres.2014.09.03925457583
- ASH 2018: Apixaban for the Treatment of Cancer-Associated VTE. The ASCO post. Available from: https://www.ascopost.com/News/59535. Published 2018 Accessed 530, 2019.
- McBane RD, Wysokinski WE, Le-Rademacher J, et al. Apixaban, dalteparin, in active cancer associated venous thromboembolism, the ADAM VTE trial. Blood. 2018;132(Suppl 1):421. doi: https://doi.org/10.1182/blood-2018-99-118808
- Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615–624.29231094
- Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol. 2018;36(20):2017–2023.29746227
- Li A, Garcia DA, Lyman GH, Carrier M. Direct oral anticoagulant (DOAC) versus low-molecular-weight heparin (LMWH) for treatment of cancer associated thrombosis (CAT): a systematic review and meta-analysis. Thromb Res. 2019;173:158–163. doi:10.1016/j.thromres.2018.02.14429506866
- Kim S-A, Yhim H-Y, Bang S-M. Current management of cancer-associated venous thromboembolism: focus on direct oral anticoagulants. J Korean Med Sci. 2019;34:6.
- Lyman GH, Bohlke K, Khorana AA, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update 2014. J Clin Oncol. 2015;33(6):654–656. doi:10.1200/JCO.2014.59.735125605844
- Di Nisio M, Porreca E, Candeloro M, De Tursi M, Russi I, Rutjes AW. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst Rev. 2016;12:Cd008500. doi:10.1002/14651858.CD003091.pub427906452
- Carrier M, Abou-Nassar K, Mallick R, et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380(8):711–719. doi:10.1056/NEJMoa181446830511879
- Khorana AA, Soff GA, Kakkar AK, et al. Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N Engl J Med. 2019;380(8):720–728. doi:10.1056/NEJMoa181463030786186
- Potpara TS, Lane DA, Lip GY. Optimizing stroke prevention in atrial fibrillation: better adherence and compliance from patients and physicians leads to better outcomes. Europace. 2015;17(4):507–508. doi:10.1093/europace/euv04125833879
- Kneeland PP, Fang MC. Current issues in patient adherence and persistence: focus on anticoagulants for the treatment and prevention of thromboembolism. Patient Prefer Adherence. 2010;4:51–60.20361065
- Rodriguez RA, Carrier M, Wells PS. Non-adherence to new oral anticoagulants: a reason for concern during long-term anticoagulation? J Thromb Haemost. 2013;11(2):390–394. doi:10.1111/jth.1208623206117
- Srivastava K, Arora A, Kataria A, Cappelleri JC, Sadosky A, Peterson AM. Impact of reducing dosing frequency on adherence to oral therapies: a literature review and meta-analysis. Patient Prefer Adherence. 2013;7:419–434. doi:10.2147/PPA.S4464623737662
- Laliberte F, Nelson WW, Lefebvre P, Schein JR, Rondeau-Leclaire J, Duh MS. Impact of daily dosing frequency on adherence to chronic medications among nonvalvular atrial fibrillation patients. Adv Ther. 2012;29(8):675–690. doi:10.1007/s12325-012-0040-x22898791
- Laliberte F, Bookhart BK, Nelson WW, et al. Impact of once-daily versus twice-daily dosing frequency on adherence to chronic medications among patients with venous thromboembolism. Patient. 2013;6(3):213–224. doi:10.1007/s40271-013-0020-523857628
- Ikeda T, Yasaka M, Kida M, Imura M. A survey of reasons for continuing warfarin therapy in the era of direct oral anticoagulants in Japanese patients with atrial fibrillation: the SELECT study. Patient Prefer Adherence. 2018;12:135–143. doi:10.2147/PPA.S15258429398909
- GoodRx. Available from: https://www.goodrx.com/. Accessed 1210, 2018.
- British national formulary March - September 2017. Available from: https://vnras.com/wp-content/uploads/2017/06/BNF-73-2017.pdf. Accessed 1210, 2018.
- KIMS. Available from: http://www.kimsonline.co.kr/. Accessed 1210, 2018.
- yakka-search. Available from: https://yakka-search.com/. Accessed 16, 2019.
- Bang OY, Hong KS, Heo JH, et al. New oral anticoagulants may be particularly useful for Asian stroke patients. J Stroke. 2014;16(2):73–80. doi:10.5853/jos.2014.16.2.7324949312
- Kim IY. Analysis of Anticoagulants Drug Expenditure and Usage: Focused on Stroke Prevention in Non-valvular Atrial Fibrillation in Korea. Seoul: The Graduate School, Sungkyunkwan University; 2018.
- Kumana CR, Cheung BM, Siu DC, Tse HF, Lauder IJ. Non-vitamin K oral anticoagulants versus warfarin for patients with atrial fibrillation: absolute benefit and harm assessments yield novel insights. Cardiovasc Ther. 2016;34(2):100–106. doi:10.1111/1755-5922.1217326727005
- Kyung-Ah Lee M, Han-Ra Park M. Cost-effectiveness of rivaroxaban versus warfarin for stroke prevention in non-valvular atrial fibrillation patients in Korea. J Health Tech Assess. 2014;2(2):105–113.
- Kim JS, Oh YH, Kim JD. Cost-effectiveness of dabigatran etexilate versus warfarin for the prevention of stroke and systemic embolism in Korea patients with non-valvular atrial fibrillation. J Health Technol Assess. 2014;2(2):124–129.
- Kim H, Kim H, Cho SK, Kim JB, Joung B, Kim C. Cost-effectiveness of rivaroxaban compared to warfarin for stroke prevention in atrial fibrillation. Korean Circ J. 2019;49(3):252–263.30468041
- Ko Y-J, Kim S, Park K, et al. Impact of the health insurance coverage policy on oral anticoagulant prescription among patients with atrial fibrillation in Korea from 2014 to 2016. J Korean Med Sci. 2018;33:23. doi:10.3346/jkms.2018.33.e101
