Abstract
In contrast to varied therapy approaches, mirror therapy (MT) can be used even in completely plegic stroke survivors, as it uses visual stimuli for producing a desired response in the affected limb. MT has been studied to have effects not just on motor impairments but also on sensations, visuospatial neglect, and pain after stroke. This paper attempts to systematically review and present the current perspectives on mirror therapy and its application in stroke rehabilitation, and dosage, feasibility and acceptability in stroke rehabilitation. An electronic database search across Google, PubMed, Web of Science, etc., generated 3871 results. After screening them based on the inclusion and exclusion criteria, we included 28 studies in this review. The data collected were divided on the basis of application in stroke rehabilitation, modes of intervention delivery, and types of control and outcome assessment. We found that most studies intervened for upper limb motor impairments post stroke. Studies were equally distributed between intervention in chronic and acute phases post stroke with therapy durations lasting between 1 and 8 weeks. MT showed definitive motor and sensory improvements although the extent of improvements in sensory impairments and hemineglect is limited. MT proves to be an effective and feasible approach to rehabilitate post-stroke survivors in the acute, sub-acute, and chronic phases of stroke, although its long-term effects and impact on activities of daily living need to be analysed extensively.
Introduction
Stroke is the 3rd leading cause of years of life lost (YLL) across the world: age-standardised YLL increased by 12.9% (10.6–15.2) from 1990 to 2007 and by 12.1% (9.9–14.1) from 2007 to 2017. Deaths from stroke increased from 5.29 million (5.22–5.40) to 6.17 million (6.04–6.33) across the globe between 2007 and 2017.Citation1 The rise of multi-morbidity and effects of longevity reported by the GBD (global burden of disease) thus increased DALYs (disability-adjusted life years) due to stroke from 3.54% to 9.66% from 1990 to 2013 and there were approximately 25.7 million stroke survivors in 2013, globally.Citation2 HIC (high-income countries) showed a 42% decrease in stroke while LMIC (low and middle-income countries) showed a 100% increase in the past four decades.Citation3 There are approximately 62 million stroke survivors across the world and one-third of them live with severe disabilities.Citation4 More than 80% of DALY occur in LMIC.Citation5,Citation6
In the post-stroke acute phase, approximately 60–80% of survivors present with upper or lower limb motor impairments.Citation7–Citation10 Only 20% of severely paretic survivors achieve full upper limb function as compared to 80% of mildly paretic stroke survivors.Citation10 Fifty per cent of stroke survivors with an initial presentation of plegic upper and lower limbs regain partial motor function.Citation7,Citation9 Painful upper limb (especially around the shoulders) and complex regional pain syndrome-type I (CRPS-type I) are experienced in approximately 50% of stroke survivors in the first year post stroke, affecting their activities of daily living (ADL).Citation11–Citation14 Around 40% with an acute right hemispheric stroke and 20% of people with a left hemispheric stroke present with hemineglect, especially visuospatial neglect, which reduces to 15% and 5% respectively at the 3rd month.Citation15 Spatial neglect has proven to be detrimental for functional recoveryCitation16,Citation17 and is associated with reduced quality of life.Citation18 Long-term functional recovery is also directly dependent on the initial severity of paresis.Citation19
Rehabilitation strategies are required to be repetitive, intensive, and task-specific for neuroplasticity to produce recovery.Citation20–Citation22 It is reported that when therapy begins within 16 hrs to 6 months post stroke, there is significant improvement in ADL performance with augmented exercise therapy.Citation23 In contrast to varied therapy approaches which require some degree of voluntary movement, mirror therapy (MT) can be used even in completely plegic, severely paretic stroke survivors, as MT uses visual rather than somatosensory stimuli for producing a desired response in the affected limb.Citation24 Mirror therapy is a type of rehabilitation approach where the reflection (visual input) of a moving non-affected limb gives the illusion of movement in the affected limb. This is achieved by placing a mirror between the arms or legs. MT has been studied to have effects not just on motor impairments but also on sensations, visuospatial neglect, and pain after stroke.Citation25
This paper attempts to systematically review and present the current perspectives on mirror therapy with respect to its:
Application in stroke rehabilitation
Dosage, feasibility, and acceptability in stroke rehabilitation
Methods
Inclusion criteria are as follows:
Study on mirror therapy for motor, sensory, and perceptual impairments after stroke
Rehabilitation in the acute, sub-acute, and chronic phases after stroke
Only randomised controlled trials
Articles published from January 2010 till June 2019
Exclusion criteria are as follows:
Studies written in languages other than English
Studies studying synergistic effects of mirror therapy with other forms of therapies
Studies on other forms of therapy targeting the mirror neuron system
Search Strategy
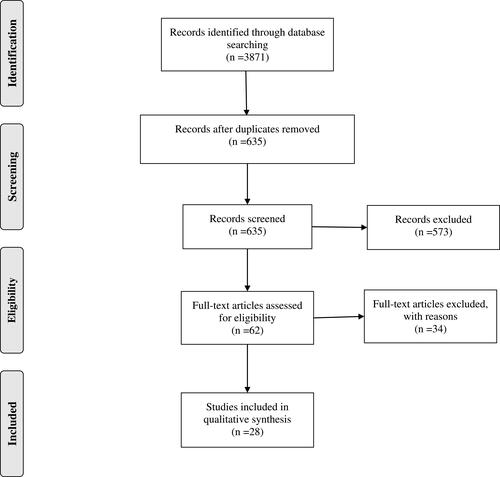
We conducted this review using PRISMA guidelines. An electronic database search was performed using the following databases: PubMed, Web of Science. The search strategy includes keywords combined with Boolean operators: mirror AND (therapy OR rehabilitation) AND (stroke OR poststroke OR post-stroke). The selection strategy of the studies is shown in the PRISMA flow chart ().Citation26
Review Process
The studies were screened by two independent reviewers based on their titles and abstracts. RCTs were identified and full articles were obtained for the selected RCTs. All of the full articles were then assessed in order to check the fulfilment of the inclusion criteria. In case of a disagreement between the reviewers, the decision was made by a third reviewer.
Data Extraction
After the selection of studies, the data were extracted for information on the title, inclusion and exclusion criteria, type of intervention, sample size, study methodology, primary and secondary outcomes, study limitations, feasibility, and adherence. The data collected weremainly divided on the basis of application in stroke rehabilitation, modes of intervention delivery, and types of control and outcome assessment.
Risk of bias evaluation and methodological quality: the COCHRANE risk of bias tool was used to perform the risk of bias assessment. The methodological quality of each study was performed using the PEDro scale.Citation27
Results
Application in Stroke Rehabilitation
Motor — Upper Limb
Out of the 28 studies included () in this review, 78.6% of them studied the effects of MT on motor functions of upper extremities, in addition to sensory function in 6 studiesCitation28–Citation33 and ADL/QOL in 9 studies. We noted a reduction in impairment as recorded by FMA in almost all except for 5 studies. Improvement in upper limb motor function was reported in terms of either improved dexterity, gross and fine motor movements, grip force, decreased movement time, or proximal motor control in 10 studies.Citation28,Citation29,Citation32,Citation34–Citation40 Two other studies reported no significant difference with MT, in outcomes measuring motor, sensory, and ADL components.Citation41,Citation42 Only 4 studied the effects of MT on spasticity, out of which 3 reported no improvement as recorded on the Modified Ashworth scaleCitation28,Citation37,Citation43 and one showed improvement on the Ashworth scale,Citation44 with 6 weeks of MT along with conventional rehabilitation. Sensory impairments were measured in 6 studies, and 4 report improved response to either noxious, tactile, or temperature stimuli.Citation28–Citation31 Twelve of the studiesCitation28–Citation32,Citation36,Citation38–Citation40,Citation45–Citation47 intervened in the chronic phase of upper limb deficits after stroke (ie after 6 months) while the rest intervened within the acute and sub-acute phases. Duration of intervention ranged between 3 and 8 weeks with MT sessions lasting between 20 and 45 min; 4 studies provided no additional conventional rehabilitation.Citation32,Citation40,Citation45,Citation47
Table 1 Summary of Included Studies
Motor — Lower Limb/Gait/Balance
Six studies reported on effects of MT on lower extremity impairment/function, gait, and balance.Citation33,Citation39,Citation43,Citation48–Citation50 These studies showed improved motor recovery as recorded on Brunnstrom stages and improved lower extremity function through improved walking speed, single limb stance, step and stride lengths, static and dynamic balance, and decreased mediolateral and anteroposterior sway in standing. Two studies also reported a reduction in lower extremity impairment. Improved forward reach in standing and coordination was also reported but no improvement in cadence or stance or swing phase velocity was seen.
Activities of Daily Living and Quality of Life
Eight of the 22 studiesCitation28,Citation34,Citation36,Citation41,Citation47,Citation51–Citation53 reported on the effects of MT on ADLs and one on the quality of life through the Euro-QOL-5 Domain (EQ5D) scale.Citation32 ADLs showed no improvement with MT in 3 studies, and neither did quality of life. The rest of the studies did show improved performance in ADLs through mainly the Functional Independence Measure (FIM), otherwise through the Barthel Index and Repty’s Functional Index. All of these studies did not report on the long-term effects of MT on ADLS or QOL.
Sensory
We report paucity in the number of studies treating and recording change in sensory impairments after stroke. Six studies record changes in sensory impairments like pain, tactile discrimination, response to touch, temperature, etc. Only 1 studyCitation30 intervened for sensory issues by providing varied texture stimulus during MT sessions to the affected upper limbs. There was improved response to temperature and tactile sensation.Citation28 reported decrease in pain experienced post stroke. Another study intervening through task-based MTCitation46 reported that some of their patients experienced “certain perceptions“ in the affected upper limb like tingling, movement flicker, mild pain, pinprick, and associated movements after 6–8 weeks of MT, although this study did not actively intervene for sensory issues.
Unilateral Neglect
Studies by Pandian et al and Thieme et alCitation52,Citation54 reported recovery in visuospatial neglect in post-stroke patients with MT for 4 and 5 weeks respectively. The patients showed improvement in neglect in the near extrapersonal space and representational neglect. It is important to note that both of these studies intervened during the acute–sub-acute phases post stroke. The study by Thieme et alCitation52 studied effects of MT in visuospatial neglect only in a small percentage of their sample and the outcome was not blindly assessed. The MUST trial by Pandian et alCitation54 reports improved mean scores for star cancellation, line bisection, and picture identification tests at the 6th-month follow-up. This article also reports a 2-study meta-analysis concluding that MT was effective in treating unilateral neglect after stroke.
Yang et alCitation55 intervened in the sub-acute phase for Pusher syndrome post stroke. These patients showed improvement by decreased severity of the syndrome and lower extremity motor function improved on FMA. Another study reported the positive effects of MT in reducing pain and improving motor function in patients with complex regional pain syndrome after stroke. It is noteworthy that the intervention was in the chronic phase of stroke. One of the studies by Michielsen et alCitation32 describes improved activation within the affected motor cortex as an outcome measure with 6 weeks of MT training which included home-based sessions as well.
Intervention Details
Stage of Intervention
Thirteen of the studies studied the effects of MT in the chronic phase of stroke, ie after 6 months of the onset (those mentioned above and in 2016Citation48). The rest of the studies either intervened in the acute or sub-acute phase of stroke. The longest duration of intervention of 8 weeks was seen in chronic stroke in two studies, both intervening for upper extremity impairments.
Modes of Intervention Delivery
A majority of the studies (92.8%) used either mirror boxes or mirror frames to deliver MT training. A mirror box is a 3-D structure with the facility to place the affected limb within it to avoid direct viewing of it by the patient, whereas a 2-D mirror frame is placed between the 2 arms either vertically or inclined in such a way so that the patient is able to view the reflection of the normal arm in the mirror without viewing the affected arm. The dimensions varied based on which part of the body was being treated: upper or lower extremity. Among the studies using the above-mentioned mode of MT, 13 studies included bilateral symmetrical movements of the limbsCitation28–Citation30,Citation32,Citation35,Citation37,Citation39,Citation42,Citation44,Citation45,Citation51,Citation52,Citation54 as opposed to the remaining 13 studies which intervened with unilateral movement of the unaffected limb. The study by Harmsen et alCitation40 delivered a modified form of therapy using the participant-specific videos with reaching movements from the unaffected arm that were videotaped and mirrored, creating maximal postural familiarity and the illusion that the affected arm performed the reaching movements in a normal movement pattern. This form of action-observation mode showed improved speed of upper limb movements, although the long-term effect was not measured. A study by In et al used Virtual Reality Reflection Therapy (VRRT) in treating balance and gait after stroke. This is a technically enhanced version of MT training where the patients in a high sitting position placed their affected lower extremity into the VRRT box and observed the projected movement of the unaffected limb without visual asymmetry otherwise causing tilting of the head and trunk. The movements of the unaffected limb were captured through the camera and displayed over the affected limb as the virtual reality reflection. This study reported improved balance scores both in static and dynamic tests, decreased anteroposterior sway with eyes open, and decreased mediolateral sway with eyes open and closed, as well as improved walking speed on a 10-metre walk test.
Four studies used task-based activities during MT sessionsCitation30,Citation33,Citation46,Citation47 while the rest used reaching activities or simple graded movements of the limbs for therapy. Two studies also included home-based sessions of mirror therapy. It is important to note that no form of conventional therapy was provided to the intervention group in 4 studies. Out of these, 2 studies (Rodrigues LC 2015, Park Y 2015) showed definitive improvement in motor scores for the upper limb and functional activities. Although, 1 study for chronic stroke reported that these gains did not persist at the 6th-month follow-up.Citation32
Intensity
The total duration of intervention varied from 1 week to 8 weeks. Fifty per cent of the studies had an intervention period of 4 weeks with the frequency ranging from 3 to 5 sessions per week. Each session’s duration varied from 20 to 90 min; in some cases excluding 20–30 min of control/conventional therapy. One study reported the effects of 1 session of action-observation-based MT which was quite intensive with 70 repetitions within a 10 trial set of MT.Citation40 This study showed improvement in speed in upper limb movements.
Studies on upper extremities report positive effects with treatment sessions lasting between 20 and 60 min per day for 5 days a week, except for 2 studies that reported no difference in motor scores between the intervention and control groups. For the lower extremity, the treatment sessions lasted between 15 min and 1 h, for 5–6 days a week.
Types of Control
We found 2 broad categories of control arms, one providing sham mirror therapy/placebo (53.57% of studies) and the other providing conventional therapy (46.43%) to the control arm. Sham MT was provided either by using a non-reflecting surface placed between the limbs or by covering the mirror with a cloth or by displaying static images/interactive visual feedback or by placing no mirror between limbs. Conventional therapy varied from passive movements/strengthening of the affected limb to comprehensive treatment combining physical and occupational therapy along with speech and language therapy whenever needed. Functional and task-based activities were included in few of the studies as part of the control programme. All conventional rehabilitation sessions were tailor-made to patient needs and the duration lasted between 45 min and 5 hrs per day.
Types of Outcome Measures
The included studies present a varied range of outcomes measuring motor, sensory, and perceptual impairments along with balance/gait, ADLs, and QOL. We have categorised these scales based on the ICIDH, ie International Classification of Impairment, Disability (activity limitation) and Handicap (participation restriction), as presented in .
Table 2 Types of Outcome Measures
Feasibility and Acceptability/Adherence
Fourteen studies did not contain any information about feasibility or adherence at all.Citation28–Citation31,Citation36,Citation38,Citation40–Citation43,Citation46–Citation48,Citation51,Citation53 Apart from several occasional and short-lived episodes of fatigue, soreness, or swelling in the paretic limb,Citation33,Citation42 the MT was generally well tolerated and no serious adverse effects were reported.Citation33,Citation34,Citation37,Citation39,Citation42,Citation44,Citation50,Citation54,Citation55 Overall, adherence to the MT is assumed to be high, regarding that the majority of trials employ directly supervised intervention;Citation33 two studies even explicitly stated a 100% participation rate.Citation32,Citation49 The highest drop-out rate of 18.6% was recorded in a study comparing effectiveness of MT in a group vs individual setting; this study, however, concluded that the compliance and retention rates in the group intervention are comparable to the individual approach, and thus the MT group seems to be possible even for severely disabled stroke patients.Citation52
A study comparing two patient-led therapies (MT vs lower limb exercises) reported that both interventions were feasible, with 90% adherence. Nevertheless, both groups did less therapy than recommended; and participants receiving MT inclined to do less practice than those undergoing lower limb exercises. Furthermore, those with neglect performed 69% less MT than those without, which was not observed in the exercise group.Citation33 In another study on adding object-related bilateral symmetrical training to MT in chronic stroke, the physiotherapist conducted the rehabilitation programme at the patient's home in order to increase participation. Despite 16% of sessions not being performed, all subjects obtained an identical number of sessions and finished the treatment.Citation45 A 14.6% drop-out rate was calculated in stroke patients with a severely impaired arm.Citation42 At last, patients experiencing MT demonstrated higher levels of motivation compared to those receiving sham therapy.Citation35
Limitations
A small sample size was the prevailing limitation among the included studies.Citation29,Citation31,Citation33,Citation37,Citation38,Citation40,Citation41,Citation43,Citation45,Citation46,Citation49,Citation52,Citation53,Citation55 Additionally, the absence of follow-up did not allow assessment of long-term retention of functional improvement in patients after rehabilitation.Citation28,Citation31,Citation34–Citation37,Citation41,Citation43,Citation44,Citation46,Citation47,Citation49,Citation53,Citation55 A number of studies reported difficulties with generalisation of the outcomes due to specific pre-selection criteriaCitation32,Citation36,Citation38,Citation47,Citation48,Citation51 or because they included patients with a distinct level of functional impairment and time post ictus.Citation29,Citation31,Citation32,Citation39,Citation41,Citation52 Another complication to the interpretation of the MT effectiveness was that three studies observed a difference in baseline measurements between the experimental and control groups.Citation37,Citation50,Citation54 The impact of MT on changes in cortical reorganisation and neural activation pre and post therapy could not be examined as only one study implemented fMRI in its protocol.Citation32
Besides the aforementioned, there were some other limitations declared by individual research teams which may be pertaining to the remainder of studies as well. First, the interactive character of the experimental condition excluded the blinding of both the therapists and the participants.Citation37,Citation52 Second, the design of the mirror box precludes movements such as shoulder overhead motion and rotation, which might be the cause of less pronounced improvement in the upper arm movement compared to the wrist and hand in the intervention group.Citation46 Third, several authors expressed the lack of quality of movementCitation46,Citation49 or the active range of motionCitation43 assessment as one of their limitations. When applying attention-dependent rehabilitation techniques, a comprehensive cognition and depression evaluation both before and during or after the treatment would be of value.Citation28,Citation41,Citation44 The role of MT in patients presenting with an additional effect of the presence of cognitive impairments (seen commonly in lacunar strokes)Citation56,Citation57 needs to be analysed. Our study does not evaluate the effect of MT in those with cognitive impairments. Extending our results to such a group of patients needs to be extensively researched.
Future studies should involve a larger sample size and more homogeneous distribution in relation to sensory impairment or motor paresis.Citation38 Further on, new studies ought to be executed on optimal duration, intensity, and contentCitation38 while also focusing on ADL.Citation53
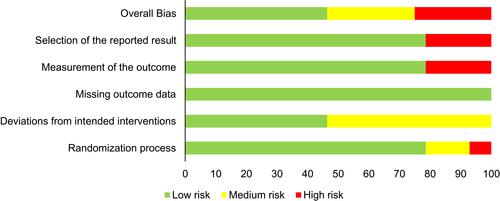
Risk of bias and methodological quality: the average PEDro score was 7±0.93 and no study showed a poor score (score <4), 2 (7.1%) of the studies showed fair quality (score 4–5), and most studies (89.3%) had a good methodological quality. Only 1 study (3.6%) showed excellent quality (score 9–10). The risk of bias scored on the Cochrane tool is depicted in .
Discussion
Various hypotheses have been postulated on the neurophysiological basis of MT. The first hypothesis suggests the presence of a mirror neuron system (MNS) in the frontotemporal region and superior temporal gyrus (STG)Citation58,Citation59 which discharges with a goal-oriented hand action or through observation of a similar action by another person.Citation60,Citation61 This action-observation facilitates the corticospinal pathway; in turn improving motor function by eliciting mental imageryCitation62 and inducing motor learning.Citation63 Observation of biological motion also is thought to aid in recovery from neglect by activation of the STG.Citation64,Citation65 The second hypothesis suggests potential mechanisms like increased self-awareness and spatial attention by activation of the STG, precuneus, and posterior cingulate cortex (PCC). MT increases activity in primary and secondary visual and somatosensory areas, thus enhancing attention, conscious awareness of sensory feedback, and avoidance of learned non-use of the affected limb.Citation66–Citation69 The third hypothesis describes the role of MT in activation and recruitment of the otherwise dormant, ipsilateral motor pathways originating in the unaffected hemisphere and projecting ipsilaterally to the paretic side of the body.Citation70–Citation72 The role of MT in promoting normalisation of balance within the hemispheres post stroke by modulating the excitability of the primary motor cortex (M1) has also been hypothesised.Citation24,Citation73 During MT, both the affected limb movement and the passive observation of movement of the unaffected limb as reflected in the mirror influence M1 excitability.Citation24 Bhasin et alCitation74 observed an increase in the activation of primary motor area Brodmann area 4 post MT (restitution principle of neuroplasticity).
Our review has reported effects of MT in rehabilitation post stroke. More than half of the studies intervened and recorded improvements in the acute phase of stroke. This can potentially change clinical practice as MT can intervene for a completely flaccid limb, unlike other rehabilitation approaches (Constraint Induced Movement Therapy (CIMT), therapy with computer games, virtual reality, etc.) where a minimal amount of voluntary movement is a pre-requisite for initiating therapy.
Few studies have previously reported that MT, when combined with bilateral arm training, increases the visual or mental imagery feedback, which in turn facilitates upper limb motor function.Citation75 Our review supports this finding and additionally reports that bilateral arm training shows positive results in both sub-acute and chronic motor impairments of the upper limb and for hemineglect.
A future scope for MT would be to identify its relation to the differing presentations of stroke among men and women. The differing risk factors, stroke severity, and neurological outcomes between men and women may demand a modified application of MT for rehabilitation in individual genders.Citation76 Research is also needed into the effect of MT in different subtypes of stroke, be it pure motor strokes or those with sensory and other components. The role of MT in rehabilitating acute and chronic lacunar strokes (which show better functional prognosis), its long-term effects, and associated improvement in quality of life can be investigated to set the stage.Citation77
Conclusion
MT is a feasible method for training post-stroke impairments (motor, sensory, perceptual deficits) in acute, sub-acute, and chronic phases. Inclusion of bilateral arm training improves patient response to MT. The required dosage of MT, long-term effects, and impact on ADLs and QOL on various subtypes of stroke need to be analysed extensively in larger populations.
Disclosure
Dr Dorcas BC Gandhi reports grants from Wellcome Trust Research Training Fellowship, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788.
- Feigin VL, Krishnamurthi RV, Parmar P, et al. Update on the global burden of ischemic and haemorrhagic stroke in 1990–2013: the GBD 2013 study. Neuroepidemiology. 2015;45:161–176. doi:10.1159/000441085
- Feigin VL, Norrving B. A new paradigm for primary prevention strategy in people with elevated risk of stroke. Int J Stroke. 2014;9:624–626. doi:10.1111/ijs.12300
- Ferri CP, Schoenborn C, Kalra L, et al. Prevalence of stroke and related burden among older people living in Latin America, India and China. J Neurol Neurosurg Psychiatry. 2011;82:1074–1082. doi:10.1136/jnnp.2010.234153
- Feigin VL, Roth GA, Naghavi M, et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016;15:913–924. doi:10.1016/S1474-4422(16)30073-4
- Hata J, Kiyohara Y. Epidemiology of stroke and coronary artery disease in Asia. Circ J. 2013;77:1923–1932. doi:10.1253/circj.CJ-13-0786
- Kwakkel G, Boudewijn J, Jeroen V, et al. Probability of regaining dexterity in the flaccid upper limb impact of severity of paresis and time since onset in acute stroke. Stroke. 2003;34:2181–2186. doi:10.1161/01.STR.0000087172.16305.CD
- Barker WH, Mullooly JP. Stroke in a defined elderly population, 1967-1985. A less lethal and disabling but no less common disease. Stroke. 1997;28(2):284–290. doi:10.1161/01.STR.28.2.284
- Jorgensen HS, Nakayama H, Raaschou HO, et al. Recovery of walking function in stroke patients: the copenhagen stroke study. Arch Phys Med Rehabil. 1995;76(1):27–32. doi:10.1016/S0003-9993(95)80038-7
- Nakayama H, Jorgensen HS, Raaschou HO, et al. Recovery of upper extremity function in stroke patients: the copenhagen stroke study. Arch Phys Med Rehabil. 1994;75(4):394–398. doi:10.1016/0003-9993(94)90161-9
- Jönsson AC, Lindgren I, Hallström B, et al. Prevalence and intensity of pain after stroke: a population based study focusing on patients’ perspectives. J Neurol Neurosurg Psychiatry. 2006;77(5):590–595. doi:10.1136/jnnp.2005.079145
- Kocabas H, Levendoglu F, Ozerbil OM, et al. Complex regional pain syndrome in stroke patients. Int J Rehabil Res. 2007;30(1):33–38. doi:10.1097/MRR.0b013e3280146f57
- Lundström E, Smits A, Terént A, et al. Risk factors for stroke-related pain 1 year after first-ever stroke. Eur J Neurol. 2009;16(2):188–193. doi:10.1111/ene.2009.16.issue-2
- Sackley C, Brittle N, Patel S, et al. The prevalence of joint contractures, pressure sores, painful shoulder, other pain, falls, and depression in the year after a severely disabling stroke. Stroke. 2008;39(12):3329–3334. doi:10.1161/STROKEAHA.108.518563
- Ringman JM, Saver JL, Woolson RF, et al. Frequency, risk factors, anatomy, and course of unilateral neglect in an acute stroke cohort. Neurology. 2004;63(3):468–474. doi:10.1212/01.WNL.0000133011.10689.CE
- Buxbaum LJ, Ferraro MK, Veramonti T, et al. Hemispatial neglect subtypes, neuroanatomy, and disability. Neurol. 2004;62:749–756. doi:10.1212/01.WNL.0000113730.73031.F4
- Farne` A, Buxbaum LJ, Ferraro M, et al. Patterns of spontaneous recovery of neglect and associated disorders in acute right brain-damaged patients. J Neurol Neurosurg Psychiatry. 2004;75:1401–1410. doi:10.1136/jnnp.2002.003095
- Franceschini M, La Porta F, Agosti M, et al. Is health-related-quality of life of stroke patients influenced by neurological impairments at one year after stroke? Eur J Phys Rehabil Med. 2010;46(3):389–399.
- Hendricks HT, Limbeek JV, Geurts AC, et al. Motor recovery after stroke: a systematic review of the literature. Arch Phys Med Rehabil. 2002;83:1629–1637. doi:10.1053/apmr.2002.35473
- Barreca S, Wolf SL, Fasoli S, et al. Treatment interventions for the paretic upper limb of stroke survivors: a critical review neurorehabil neural repair. Neurorehabilitation Neural Repair. 2003;17(4):220–226. doi:10.1177/0888439003259415
- Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51(1):225–239. doi:10.1044/1092-4388(2008/018)
- Van Peppen RP, Kwakkel G, Wood-Dauphinee S, et al. The impact of physical therapy on functional outcomes after stroke: what’s the evidence? Clin Rehabil. 2004;18(8):833–862. doi:10.1191/0269215504cr843oa
- Kwakkel G, van PR, Wagenaar RC, et al. Effects of augmented exercise therapy time after stroke: a meta-analysis. Stroke. 2004;35(11):2529–2539.
- Garry MI, Loftus A, Summers JJ. Mirror, mirror on the wall: viewing a mirror reflection of unilateral hand movements facilitates ipsilateral M1 excitability. Exp Brain Res. 2005;163(1):118–122. doi:10.1007/s00221-005-2226-9
- Thieme H, Morkisch N, Mehrholz J, et al. Mirror therapy for improving motor function after stroke (Review). Cochrane Database Syst Rev. 2018;7.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:7. doi:10.1371/journal.pmed.1000100
- Foley NC, Teasell RW, Bhogal SK, et al. Stroke rehabilitation evidence-based review: methodology. Top Stroke Rehabil. 2003;10(1):1–7.
- Vural SP, Yuzer GFN, Ozcan DS, Ozbudak SD, Ozgirgin N. Effects of mirror therapy in stroke patients with complex regional pain syndrome type 1: a randomized controlled study. Arch Phys Med Rehabil. 2016;97(4):575–581. doi:10.1016/j.apmr.2015.12.008
- Wu CY, Huang PC, Chen YT, Lin KC, Yang HW. Effects of mirror therapy on motor and sensory recovery in chronic stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2013;94(6):1023–1030. doi:10.1016/j.apmr.2013.02.007
- Arya KN, Pandian S, Puri V. Mirror illusion for sensori-motor training in stroke: a randomized controlled trial. J Stroke Cerebrovascular Dis. 2018;27(11):3236–3246. doi:10.1016/j.jstrokecerebrovasdis.2018.07.012
- Colomer C, Noe E, Llorens Rodríguez R. Mirror therapy in chronic stroke survivors with severely impaired upper limb function: a randomized controlled trial. Eur J Phys Rehabil Med. 2016;52(3):271–278.
- Michielsen ME, Selles RW, van der Geest JN, et al. Motor recovery and cortical reorganization after mirror therapy in chronic stroke patients: a Phase II randomized controlled trial. Neurorehabil Neural Repair. 2011;25(3):223–233. doi:10.1177/1545968310385127
- Tyson S, Wilkinson J, Thomas N, et al. Phase II pragmatic randomized controlled trial of patient-led therapies (mirror therapy and lower-limb exercises) during inpatient stroke rehabilitation. Neurorehabil Neural Repair. 2015;29(9):818–826. doi:10.1177/1545968314565513
- Invernizzi M, Negrini S, Carda S, Lanzotti L, Cisari C, Baricich A. The value of adding mirror therapy for upper limb motor recovery of subacute stroke patients: a randomized controlled trial. Eur J Phys Rehabil Med. 2013;49(3):311–317.
- Lee MM, Cho HY, Song CH. The mirror therapy program enhances upper-limb motor recovery and motor function in acute stroke patients. Am j Phys Med Rehabil. 2012;91(8):689–700. doi:10.1097/PHM.0b013e31824fa86d
- Park JY, Chang M, Kim KM, Kim HJ. The effect of mirror therapy on upper-extremity function and activities of daily living in stroke patients. J Phys Ther Sci. 2015;27(6):1681–1683. doi:10.1589/jpts.27.1681
- Samuelkamaleshkumar S, Reethajanetsureka S, Pauljebaraj P, Benshamir B, Padankatti SM, David JA. Mirror therapy enhances motor performance in the paretic upper limb after stroke: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2014;95(11):2000–2005. doi:10.1016/j.apmr.2014.06.020
- Amasyali SY, Yaliman A. Comparison of the effects of mirror therapy and electromyography-triggered neuromuscular stimulation on hand functions in stroke patients: a pilot study. Int J Rehabil Res. 2016;39(4):302–307. doi:10.1097/MRR.0000000000000186
- Lin KC, Huang PC, Chen YT, Wu CY, Huang WL. Combining afferent stimulation and mirror therapy for rehabilitating motor function, motor control, ambulation, and daily functions after stroke. Neurorehabil Neural Repair. 2014;28(2):153–162. doi:10.1177/1545968313508468
- Harmsen WJ, Bussmann JB, Selles RW, Hurkmans HL, Ribbers GM. A mirror therapy–based action observation protocol to improve motor learning after stroke. Neurorehabil Neural Repair. 2015;29(6):509–516. doi:10.1177/1545968314558598
- Antoniotti P, Veronelli L, Caronni A, et al. No evidence of effectiveness of mirror therapy early after stroke: an assessor-blinded randomized controlled trial. Clin Rehabil. 2019;33(5):885–893. doi:10.1177/0269215518824737
- Chan WC, Au-Yeung SS. Recovery in the severely impaired arm post-stroke after mirror therapy: a randomized controlled study. Am j Phys Med Rehabil. 2018;97(8):572–577. doi:10.1097/PHM.0000000000000919
- Xu Q, Guo F, Salem HM, Chen H, Huang X. Effects of mirror therapy combined with neuromuscular electrical stimulation on motor recovery of lower limbs and walking ability of patients with stroke: a randomized controlled study. Clin Rehabil. 2017;31(12):1583–1591. doi:10.1177/0269215517705689
- Cristina LM, Matei D, Ignat B, Popescu CD. Mirror therapy enhances upper extremity motor recovery in stroke patients. Acta Neurologicabelgica. 2015;115(4):597–603.
- Rodrigues LC, Farias NC, Gomes RP, Michaelsen SM. Feasibility and effectiveness of adding object-related bilateral symmetrical training to mirror therapy in chronic stroke: a randomized controlled pilot study. Physiother Theory Pract. 2016;32(2):83–91. doi:10.3109/09593985.2015.1091872
- Arya KN, Pandian S, Kumar D, Puri V. Task-based mirror therapy augmenting motor recovery in poststroke hemiparesis: a randomized controlled trial. J Stroke Cerebrovascular Dis. 2015;24(8):1738–1748. doi:10.1016/j.jstrokecerebrovasdis.2015.03.026
- Park Y, Chang M, Kim KM, An DH. The effects of mirror therapy with tasks on upper extremity function and self-care in stroke patients. J Phys Ther Sci. 2015;27(5):1499–1501. doi:10.1589/jpts.27.1499
- In T, Lee K, Song C. Virtual reality reflection therapy improves balance and gait in patients with chronic stroke: randomized controlled trials. Med Sci Monitor. 2016;22:4046. doi:10.12659/MSM.898157
- Ji SG, Kim MK. The effects of mirror therapy on the gait of subacute stroke patients: a randomized controlled trial. Clin Rehabil. 2015;29(4):348–354. doi:10.1177/0269215514542356
- Mohan U. Effectiveness of mirror therapy on lower extremity motor recovery, balance and mobility in patients with acute stroke: a randomized sham-controlled pilot trial. Ann Indian Acad Neurol. 2013;16(4):634. doi:10.4103/0972-2327.120496
- Radajewska A, Opara JA, Kucio C, Blaszczyszyn M, Mehlich K, Szczygiel J. The effects of mirror therapy on arm and hand function in subacute stroke in patients. Int J Rehabil Res. 2013;36(3):268–274. doi:10.1097/MRR.0b013e3283606218
- Thieme H, Bayn M, Wurg M, Zange C, Pohl M, Behrens J. Mirror therapy for patients with severe arm paresis after stroke–a randomized controlled trial. Clin Rehabil. 2013;27(4):314–324. doi:10.1177/0269215512455651
- Gurbuz N, Afsar SI, Ayaş S, Cosar SN. Effect of mirror therapy on upper extremity motor function in stroke patients: a randomized controlled trial. J Phys Ther Sci. 2016;28(9):2501–2506. doi:10.1589/jpts.28.2501
- Pandian JD, Arora R, Kaur P, Sharma D, Vishwambaran DK, Arima H. Mirror therapy in unilateral neglect after stroke (MUST trial): a randomized controlled trial. Neurology. 2014;83(11):1012–1017. doi:10.1212/WNL.0000000000000773
- Yang YR, Chen YH, Chang HC, Chan RC, Wei SH, Wang RY. Effects of interactive visual feedback training on post-stroke pusher syndrome: a pilot randomized controlled study. Clin Rehabil. 2015;29(10):987–993. doi:10.1177/0269215514564898
- Jacova C, Pearce LA, Costello R, et al. Cognitive impairment in lacunar strokes: the SPS3 trial. Ann Neurol. 2012;72(3):351–362. doi:10.1002/ana.v72.3
- Blanco-Rojas L, Arboix A, Canovas D, et al. Cognitive profile in patients with a first-ever lacunar infarct with and without silent lacunes: a comparative study. BMC Neurol. 2013;16(13):203. doi:10.1186/1471-2377-13-203
- Lamont K, Chin M, Kogan M. Mirror box therapy: seeing is believing. Explore. 2011;7(6):369–372. doi:10.1016/j.explore.2011.08.002
- Luigi C, Giacomo R. The mirror neuron system. Arch Neurol. 2009;66(5):557–560. doi:10.1001/archneurol.2009.41
- Buccino G, Binkofski F, Riggio L. The mirror neuron system and action recognition. Brain Lang. 2004;89(2):370–376. doi:10.1016/S0093-934X(03)00356-0
- Giacomo R. Maddalena FD, Luigi C. Mirror neurons and their clinical relevance. Nature Clin Pract Neurol. 2009;5(1):24. doi:10.1038/ncpneuro0990
- Pomeroy VM, Clark CA, Miller JS, et al. The potential for utilizing the “mirror neurone system” to enhance recovery of the severely affected upper limb early after stroke: a review and hypothesis. Neurorehabil Neural Repair. 2005;19(1):4–13. doi:10.1177/1545968304274351
- Buccino G, Solodkin A, Small SL. Functions of the mirror neuron system: implications for neurorehabilitation. Cognit Behav Neurol. 2006;19(1):55–63. doi:10.1097/00146965-200603000-00007
- Karnath HO. New insights into the functions of the superior temporal cortex. Nature Rev Neurosci. 2001;2(8):568. doi:10.1038/35086057
- Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends Cogn Sci. 2000;4(7):267–278. doi:10.1016/S1364-6613(00)01501-1
- Rothgangel A, de Bie RA, Bastiaenen CH, et al. The role of the mirror neuron system in rehabilitation with mirror therapy following middle cerebral artery infarction: a pilot fMRI study-51. Annual Meeting of the German Society of Medical Informatics, Biometry and Epidemiology (GMDS); 2006; 34; Leipzig.
- Matthys K, Smits M, Van der Geest JN, et al. Mirror-induced visual illusion of hand movements: a functional magnetic resonance imaging study. Arch Phys Med Rehabil. 2009;90(4):675–681. doi:10.1016/j.apmr.2008.09.571
- Michielsen ME, Smits M, Ribbers GM, et al. The neuronal correlates of mirror therapy: an fMRI study on mirror induced visual illusions in patients with stroke. J Neurol Neurosurg Psychiatry. 2011;82(4):393–398. doi:10.1136/jnnp.2009.194134
- Deconinck FJ, Smorenburg AR, Benham A, et al. Reflections on mirror therapy: a systematic review of the effect of mirror visual feedback on the brain. Neurorehabil Neural Repair. 2015;29(4):349–361. doi:10.1177/1545968314546134
- Ezendam D, Bongers R, Jannink M. Systematic review of the effectiveness of mirror therapy in upper extremity function. DisabilRehabil. 2009;31:2135–2149.
- Staudt M, Grodd W, Gerloff C, et al. Two types of ipsilateral reorganization in congenital hemiparesis: a TMS and fMRI study. Brain. 2002;125(10):2222–2237. doi:10.1093/brain/awf227
- Schwerin S, Dewald JPA, Hatzi M, et al. Ipsilateral versus contralateral cortical motor projections to a shoulder adductor in chronic hemiparetic stroke: implications for the expression of arm synergies. Exp Brain Res. 2008;185:509–519. doi:10.1007/s00221-007-1169-8
- Dong Y, Winstein CJ, Albistegui-DuBois R, et al. Evolution of FMRI activation in the perilesional primary motor cortex and cerebellum with rehabilitation training-related motor gains after stroke: a pilot study. Neurorehabil Neural Repair. 2007;21(5):412–428. doi:10.1177/1545968306298598
- Bhasin A, Srivastava PMV, Kumaran SS, et al. Neural interface of mirror therapy in chronic stroke patients: a functional magnetic resonance imaging study. Neurol India. 2012;60(6):570–576. doi:10.4103/0028-3886.105188
- Yavuzer G, Selles R, Sezer N, et al. Mirror therapy improves hand function in subacute stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2008;89:393–398. doi:10.1016/j.apmr.2007.08.162
- Arboix A, Cartanyà A, Lowak M, et al. Gender differences and woman-specific trends in acute stroke: results from a hospital-based registry (1986–2009). Clin Neurol Neurosurg. 2014;127:19–24. doi:10.1016/j.clineuro.2014.09.024
- Arboix A, Blanco-Rojas L, Martí-Vilalta JL. Advancements in understanding the mechanisms of symptomatic lacunar ischemic stroke: translation of knowledge to prevention strategies. Expert Rev Neurother. 2014;14(3):261–276. doi:10.1586/14737175.2014.884926