Abstract
Background: Avian AIV-H7N9 influenza progresses rapidly and has a high fatality rate. However, it lacks an early effective biomarker to predict disease severity and fatal outcomes successfully. Our study aimed to explore whether the neutrophil-to-lymphocyte ratio (NLR) taken within 24 h after admission can predict disease severity and fatality in AIV-H7N9-infected patients.
Methods: We retrospectively studied 237 AIV-H7N9-infected patients from multiple centers from 2013 to 2015. We used univariate analysis and multivariate analysis to compare clinical variables between the survival and fatal groups to evaluate the prognostic value.
Results: The NLR taken within 24 h after admission in the fatal group was significantly higher than that in the survival group (P<0.01). Our study found that NLR was independently associated with fatality. The area under the curve (AUC) of the NLR was 0.70, and moreover, when the NLR =19.94, the specificity was 100%, and the sensitivity was 28.4%. The fatality in the NLR ≥19.94 group was significantly increased relative to the patients with an NLR <19.94 (P<0.05).
Conclusion: The NLR is potentially a predictive prognostic biomarker in patients infected with the AIV-H7N9 influenza virus.
Background
The novel avian influenza virus (AIV) H7N9 subtype (AIV-H7N9) is characterized by rapid progression and with a high fatality rate of 39%.Citation1 Fatal outcome of AIV-H7N9 infections is often related to acute lung injury and acute respiratory distress syndrome (ARDS). Early biomarkers for predicting the progression of the disease and fatal outcomes are needed. Previous studies have shown that the level of angiotensin II and certain cytokines are biomarkers for the outcome of AIV-H7N9-infected patients.Citation2,Citation3 However, there is still a lack of clinically useful biomarkers to predict the disease progression and clinical outcome of a patient.
Recently, the neutrophil-to-lymphocyte ratio (NLR) was shown to be a useful indicator of systemic inflammation.Citation4 Studies have shown that the NLR was positively associated with serious diseases, such as acute pancreatitis, liver disease, certain rheumatic diseases, and ARDS.Citation5–Citation14 Moreover, the NLR was found to be associated with hospital fatality in critically ill patients.Citation15 Neutrophils and lymphocytes, as important components of the innate immune system, have vital functions in the development and recovery of influenza. The neutrophil count reflects mostly innate immune cell function.Citation16 Neutrophils release large amounts of cytokines and chemokines to promote the formation of cytokine storms. It has able been shown that neutrophils can serve as antigen-presenting cells to antiviral CD8+ T-cells.Citation17 Low levels of lymphocytes have been observed in severe AIV-H7N9 patients.Citation18 In our study, we monitored the proportion of cell types in the peripheral blood of patients infected with AIV-H7N9 viruses. Herein, we performed a retrospective study to evaluate the NLR within 24 h after admission in patients infected with AIV-H7N9. Our aim was to determine how changes in the differential leukocyte count in AIV-H7N9-infected patients correlated with the virulence of AIV.
Methods
Ethics statement
This study was approved by the First Affiliated Hospital of Zhejiang University ethics board.
Patient enrollment
There were initially a total of 282 patients included in the study, but 45 of them were excluded due to incomplete information. Thus, a total of 237 confirmed AIV-H7N9-infected patients were retrospectively enrolled from February 26, 2013 to June 30, 2015. These patients came from different hospitals in Zhejiang (110 patients), Guangzhou (47 patients), Shanghai (22 patients), Hunan (17 patients), Jiangsu (16 patients), Fujian (9 patients), Henan (4 patients), Anhui (3 patients), Beijing (2 patients), Guangdong (2 patients), Shandong (2 patients), Guangxi (2 patients), Guizhou (1 patient), and Yunnan (1 patient) provinces. Among them, 74 cases were from the First Affiliated Hospital of Zhejiang University Medical College. Informed written consents were obtained from all participants before enrollment in the study. Confirmed AIV-H7N9 patients were defined as patients with a positive laboratory diagnosis.Citation19
Data collection
Medical charts and electronic databases were reviewed, and standardized forms were used to gather information retrospectively. Clinical and laboratory information, including demographics, baseline clinical information, and follow-up clinical information, was collected systematically from admission to discharge for every patient. The laboratory examinations were all collected within 48 h after admission, and the routine blood tests were collected within 24 h after admission. The NLR was calculated by dividing the number of neutrophils (N) by the number of lymphocytes (L) from the blood test. The PaO2/FiO2 ratio was the partial pressure of arterial oxygen to the fraction of inspired oxygen. Secondary infection was defined as any bacterial or fungal infection having corresponding clinical manifestations, with one or more positive cultures. The cultures were obtained from blood, valid sputum, lower respiratory tract (endotracheal or bronchoalveolar lavage) samples, or other normally sterile fluids.
Statistical analysis
Categorical variables were calculated by frequency analysis. The numerical variables of normal distributions were represented by means ± standard deviations, and abnormal distributions were represented by medians (interquartile, IQR). The Student’s t-test and chi-square test were used to conduct univariate analysis. Multivariate analysis was performed by logistic regression. Receiver operator curves (ROC) were used to assess the accuracy of variables. Kaplan–Meier survival analysis was performed to compare the difference in the overall survival between different groups. A P-value <0.05 was considered significant. All analyses were conducted using SPSS for Windows (version 16.0, SPSS Inc., Chicago, IL, USA).
Results
Study population characteristics
The baseline characteristics of all of the 237 patients are shown in . The mean age of the 237 patients was 56.42 ±15.84 years, and 70.89% (168 patients) were male. There was a history of smoking in 23.20% (55 patients) of the patients, and 56.12% (133 patients) of the patients had one or more coexisting conditions, with hypertension, diabetes, and coronary heart disease as the most common coexisting conditions. The median value of the NLR taken within 24 h after admission was 10.29±12.16 for the entire study population. The overall fatality rate was 31.22% ().
Table 1 Univariate analyses of variables during the early stage of disease between the survival group and the fatal group.
NLR as an independent risk factor of fatality in AIV-H7N9-infected patients
The 237 patients were divided into two groups based on the survival of the patient. There were 163 patients in the survival group and 74 patients in the fatal group. The baseline characteristics of the survival and fatal group are listed in . There was a significant difference between the two groups in terms of age (P=0.00), PaO2/FiO2 (P=0.00), CRP (C-reactive protein; P=0.01), NLR (P=0.00), shock (P=0.00), mechanical ventilation (MV; P=0.00), intravenous immune globulin therapy (IVIG; P=0.04), continuous renal replacement therapy (CRRT; P=0.00), and extracorporeal membrane oxygenation (ECOM; P=0.00).
We used logistic regression to analyze the factors that had a P-value <0.05 in . In the multivariate regression analysis, age, NLR, shock, EV, and CRRT were statistically significant (). Moreover, the NLR, age, shock, EV, and CRRT were risk factors for the fatality of AIV-H7N9 infection. We constructed a ROC curve of the NLR to examine the predictive values of the NLR in AIV-H7N9-infected patients (). The area under the curve (AUC) of the NLR was 0.70. Our data suggest that the NLR value measured within 24 h after admission could potentially predict the fatal outcome of AIV-H7N9 infection. According to the ROC curve, the NLR was 1.54, with a sensitivity of 98.6% and specificity of 2.5%. An NLR of 19.94 showed 100% specificity and a sensitivity of 28.4%. We then used the NLR =19.94 as the cutoff value to divide the 237 patients into two groups (NLR ≥19.94 and NLR <19.94). There were 21 patients with an NLR ≥19.94, whereas 216 had an NLR <19.94. Furthermore, there was a statistically significant difference in fatality between the two groups (P=0.02). The Kaplan–Meier curve also demonstrated that an NLR ≥19.94 was associated with the survival time of AIV-H7N9-infected patients ().
Figure 1 Receiver operating characteristic (ROC) curves of the NLR taken within 24 h and other biomarkers for 180-day fatality.
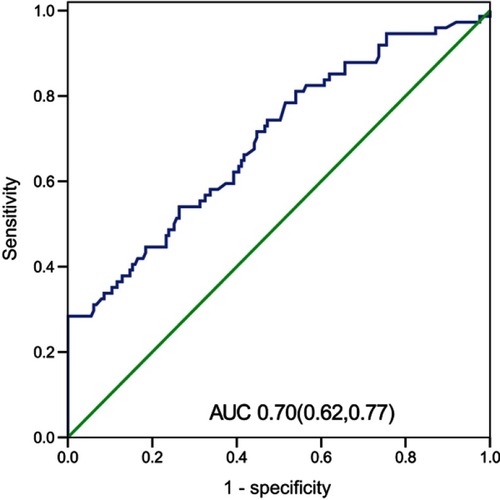
Figure 2 Kaplan–Meier analyses of the NLR ≥19.94 group and the NLR <19.94 group in patients infected with the AIV-H7N9 influenza virus.
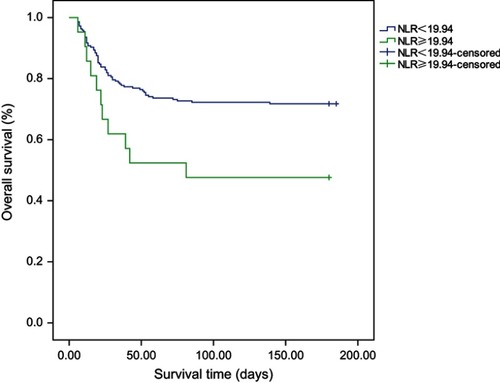
Table 2 Logistic regression analysis of independent outcomes predictor in AIV-H7N9-infected patients.
Discussion
Our retrospective study showed that the NLR levels in patients with AIV-H7N9 infection taken within 24 h after admission were higher in non-survivors than in survivors. The NLR might play a valuable role in predicting the in-hospital fatality of patients with AIV-H7N9 infection. To the best of our knowledge, this study is the first to show that the NLR is an independent predictor of fatality in patients infected with the AIV-H7N9 influenza virus.
Previous studies have described the relationship between NLR and severe, acute inflammatory diseases, such as sepsis and ARDS. An observational cohort study included 5,056 unselected patients in intensive care units (ICU), and concluded that the NLR was associated with hospital mortality.Citation15 However, in the subgroup analysis, there was no association between the NLR and mortality in sepsis patients.Citation15 Another study in patients with septic shock demonstrated an association between the NLR and mortality.Citation20 Furthermore, a retrospective study conducted in severe sepsis and septic shock patients in emergency departments found that the NLR taken within 24 h was associated with a 28-day mortality rate.Citation21 Li et al found that a high NLR was associated with a poor outcome in critically ill patients with ARDS.Citation9 In addition, Wang et al showed that the NLR in ARDS survivors was significantly lower than non-survivors, and an NLR >14 was associated with a shorter overall survival.Citation12 A prior study found an increased mean initial neutrophil count and a lower mean initial lymphocyte count in the patients who died of AIV-H7N9 infection compared to patients who recovered.Citation22 However, there is currently no research about the relationship between the NLR and the prognosis of influenza A patients.
Our data showed that the leukocytes in the peripheral blood within 24 h after admission were in the normal range in both groups, but the proportion of cell types was different. The neutrophils of non-survivors were higher, and the lymphocytes were lower than the survivors, which contributed to the higher NLR level in the non-survivors. The course of infection with the AIV-H7N9 virus varied according to the interaction between the virus and the immune system. In severe AIV-H7N9 infected patients, the immune system was overactivated, and substantial amounts of cytokines and chemokines were released.Citation3 Cytokine/chemokine storm and severe hyper-inflammatory responses are associated with pulmonary injury and ARDS, which ultimately results in fatal outcomes. Although several studies have reported on the role of neutrophils in the pathogenesis of influenza, there are still many unanswered questions.Citation23 Neutrophils are an important component of the innate immune system. They secrete large amounts of cytokines and chemokines to regulate the immune response.Citation24 Cytokines and chemokines are able to influence many aspects of the immune response, including antiviral defense, hematopoiesis, angiogenesis, and fibrogenesis.Citation25 Neutrophils can also act as antigen-presenting cells to antiviral CD8+ T-cells.Citation17 A previously published study reported that neutrophils release chromatin-based structures called neutrophil extracellular traps (NETs) to kill microbes when stimulated by various antigens, including viruses, bacteria, toxic factors, and cytokines, such as IL-8 and TNF-α.Citation26 However, excessive NET formation was shown to cause tissue damage.Citation26 This study also indicated that NETs had the potential to be used as a biomarker for predicting the prognosis of patients infected with influenza A virus. Furthermore, a high level of NETs was shown to cause lung injury and were related to the severity of disease.Citation12 Research has indicated that neutrophils can be infected by the AIV, and may act as vehicles transporting the virus to other organs.Citation27 Past studies have reported that neutrophils were implicated both in the development of ARDS and in recovery from ARDS.Citation28–Citation30 Some have even suggested that neutrophils should be a therapeutic target in severe influenza pneumonia.Citation31 In our study, the neutrophils of non-survivors were relatively higher than the survivors. In AIV-H7N9-infected patients, the neutrophils respond rapidly to inflammation, are continuously released from the bone marrow, and increase in numbers quickly.Citation3 Cytokines, especially serum IL-8 levels, were positively correlated with neutrophil counts in patients infected with the AIV-H7N9 virus.Citation12 The neutrophil count reflects mostly innate immune cell function, indicating systemic oxidative stress, inflammation, and tissue damage.Citation16 The level of neutrophils may represent the intensity of the immune response. A prior study found that severe influenza A virus infection caused an increase in the numbers of circulating neutrophils, as well as the serum IL-8 levels.Citation12 An early increase in the airway and circulating blood neutrophil levels is a feature of AIV infection in humans,Citation32,Citation33 and is common in experimental AIV infection in mice.Citation34–Citation36 In addition, the relatively higher neutrophil count in non-survivors may relate to secondary bacterial infection. Neutrophils respond to a variety of inflammatory conditions, especially those associated with bacterial infection.Citation28–Citation30 Studies have shown that one of the main causes of a fatal outcome when infected with the influenza virus is bacterial superinfection.Citation37 Our study showed that the proportion of patients with secondary infection was relatively high in non-survivors, which may be one of the reasons for the higher NLR in non-survivors.
Previous studies have shown that AIV-H7N9-infected patients have a low percentage of peripheral blood T lymphocytes.Citation3 Moreover, it was reported that T cell levels in patients that succumbed to AIV-H7N9 infection were much lower on the day of admission compared to the survivors of AIV-H7N9 infection.Citation3 Shen et al investigated 18 AIV-H7N9 patients and found that all the patients had lymphopenia (<1.5 x 109 lymphocytes/L).Citation18 Yu et al investigated six fatal cases of AIV-H7N9 infection and found that five had lymphopenia.Citation38 Lymphopenia was observed not only in AIV-H7N9 infection, but also other types of influenza A infections, such as H5N1 and H1N1.Citation18 Our study showed lower lymphocytes in non-survivors. Previous studies have shown that infection with several virulent influenza A viruses (such as H5N1) causes hypoplastic marrow with histiocytic hyperplasia, with these histiocytes showing hemophagocytosis.Citation39–Citation42 An H5N1 murine infection model demonstrated that the number of CD4+ and CD8+ T cells decreased in mice infected with the H5N1 virus. It was also shown that after exposure to the influenza A virus, the lymphocytes underwent apoptosis through Fas–Fas ligand signaling.Citation43 Furthermore, a previous study found that in fatal cases of AIV-H7N9 infected patients, the number of cytokines and chemokines were high on the day of admission and even higher on the day of death, while the lymphocyte count was just the opposite.Citation3 Hence, cytokine-driven conditions may be associated with this lymphopenia.Citation42,Citation44 A decrease in the levels of lymphocytes results in the absence of cellular immunity, delayed viral clearance, and an increased death rate. Lymphopenia has been reported as an early reliable biomarker of influenza A H1N1 infection.Citation43 This study also concluded that low lymphocyte counts (<0.5 x 109 cells/L) were a risk factor for an adverse clinical outcome of AIV-H7N9 infection.Citation18 Thus, T lymphocyte counts were shown to be a potential indicator that can be utilized to assess the severity of diseases, in addition to serving as an early predictor of the clinical outcome. Furthermore, since the lymphocytes decreased and the neutrophils increased in fatal cases, the NLR would be high, which indicates that the NLR may also serve as a potential indicator of disease progression and outcome.
Avian AIV-H7N9 influenza was characterized with high fatality. There were extensive amounts of cytokines and chemokines produced by the immune system of patients in attempts to clear the virus from the body; however, this also resulted in systemic inflammation and the progression of ARDS. More than 70% of AIV-H7N9-infected patients developed ARDS,Citation45 which was associated with high fatality in AIV-H7N9 patients.Citation46,Citation47 Furthermore, two retrospective studies showed that the NLR was a predictive prognostic biomarker in ARDS patients.Citation9,Citation12 Since ARDS was associated with the high fatality rate of AIV-H7N9-infected patients, and the NLR was a predictive prognostic biomarker of ARDS, then the NLR may also predict the clinical outcome of AIV-H7N9 patients.
Previous studies had found several indicators that predict the prognosis of AIV-H7N9 patients. Huang et al found that angiotensin II was a biomarker for lethality in influenza infections.Citation2 Guo et al reported that the levels of MIF, SCF, MCP-1, HGF, and SCGF-β were positively correlated with AIV-H7N9 disease severity, and the mediators MIF, SCF, MCP-1, HGF, SCGF-β, IP-10, IL-18, and IFN-γ were independent prognostic factors.Citation48 Additionally, Zhu et al found that high levels of NETs correlated with the poor prognosis of severe influenza A infection.Citation49 However, there is still a lack of biomarkers that are not only easily collected from the patient, but that can also be used to predict disease progression and fatal outcomes. The neutrophil and lymphocyte count is easily obtained, as routine blood testing is available and affordable for each patient; thus, the NLR may serve as a potential biomarker.
In our study, we took the median NLR (=19.94) as the cutoff point to divide the 237 patients into two groups, and we used 180-day fatality as an outcome. The Kaplan–Meier curve showed that patients with an NLR >19.94 had a higher fatality rate (P<0.01), indicating that patients with an NLR >19.94 have an increased chance of dying of AIV-H7N9 infection, and thus, a poor prognosis. Since AIV-H7N9 is characterized by rapid progression and high fatality, it is essential to identify the patients who may potentially have a poor prognosis. Therefore, when a confirmed AIV-H7N9 patient is admitted to the hospital, analysis of the NLR value may be useful for risk stratification. And it can help clinicians comprehensively evaluate the condition and progress of AIV-H7N9-infected patients to determine the initial treatment (including outpatient service, hospitalization, or ICU admittance) and select the appropriate treatment. Furthermore, it helps predict the outcome of AIV-H7N9 patients. It may also be used to determine the timing of some special treatments and discharge time. However, further studies are needed in order to fully understand the NLR value and fatal outcomes in AIV-infected patients.
Our study also showed that compared with survivors, patients in the fatal group were older, exhibited increased CRP levels, had a worse PaO2/FiO2, suffered more occurrences of shock, and needed more MV, IVIG, CRRT, and ECOM therapy. Our findings agree with previous studies.Citation50–Citation53 Elderly people had a worse prognosis, probably because they have an increased risk of coexisting illnesses and are more susceptible to severe forms of disease than younger persons.Citation54 CRP is not only a systemic marker of inflammation, but also a mediator of inflammatory factors.Citation55 Studies have found that high levels of CRP are corrected with a high fatality rate in AIV-H7N9-infected patients.Citation56,Citation57 Another study showed that a peak CRP of 120 mg/L or higher was significantly associated with case fatality in AIV-H7N9 infection.Citation56 In fatal cases, more patients developed clinical complications, such as ARDS, heart failure, and septic shock,Citation51 and as such, they needed more MV, IVIG, CRRT, and ECOM therapy.
There are, however, several limitations to the current study. First, it was a retrospective observational study, and as such, subject selection bias was avoidable. Second, the NLR value used in this study was collected within 24 h after admission and not on the day of the onset of disease. Thus, the NLR value might be influenced by drugs already given to the patient and comorbidities. The dynamic changes of the NLR at different time points during disease have not been explored in this paper; therefore, the best time for the NLR to predict death has not been determined. Third, although our study included 237 patients from several different regions, a prospective study with larger sample size is required in order to definitively confirm the NLR as a biomarker of AIV-H7N9 disease progression and clinical outcome.
In conclusion, our study demonstrates that the NLR is a simple and useful biomarker for predicting in-hospital fatality in patients infected with the AIV-H7N9 influenza virus. A high NLR value within 24 h after admission was associated with an overall poor prognosis. Furthermore, since the NLR value is calculated from the neutrophil and lymphocyte counts, which are easily obtained from a routine blood draw, this value could be widely in used as a clinical biomarker for disease progression and clinical outcomes in patients infected with the AIV-H7N9 influenza virus.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
We thank the National Health and Family Planning Commission of China for coordinating data collection and analysis. This study was funded by the National Key Research and Development Program of China (grant number 2016YFC1200204), the National Natural Science Foundation of China (31500138), and the National Science and Technology Major Project (2017ZX10204401001002).
Availability of data and materials
The dataset used and analyzed during this study is available from the corresponding author upon request.
Disclosure
The authors report no conflicts of interest in this work.
References
- Kile JC, Ren R, Liu L, et al. Update: increase in human infections with novel Asian lineage avian influenza A(H7N9) viruses during the fifth epidemic - China, October 1, 2016-August 7, 2017. MMWR Morb Mortal Wkly Rep. 2017;66(35):928–932. doi:10.15585/mmwr.mm6635a228880856
- Huang F, Guo J, Zou Z, et al. Angiotensin II plasma levels are linked to disease severity and predict fatal outcomes in H7N9-infected patients. Nat Commun. 2014;5:3595. doi:10.1038/ncomms597224800963
- Chen Y, Li X, Tian L, et al. Dynamic behavior of lymphocyte subgroups correlates with clinical outcomes in human H7N9 infection. J Infect. 2014;69(4):358–365. doi:10.1016/j.jinf.2014.05.00624841136
- Zahorec R. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102(1):5–14.11723675
- Fan Z, EnQiang C, Yao DL, et al. Neutrophil-lymphocyte ratio predicts short term mortality in patients with hepatitis B virus-related acute-on-chronic liver failure treated with an artificial liver support system. PLoS One. 2017;12(4):e0175332. doi:10.1371/journal.pone.017533228426800
- Hao X, Li D, Wu D, Zhang N. The relationship between hematological indices and autoimmune rheumatic diseases (ARDs), a meta-analysis. Sci Rep. 2017;7(1):10833. doi:10.1038/s41598-017-11398-428883472
- Jeon TJ, Park JY. Clinical significance of the neutrophil-lymphocyte ratio as an early predictive marker for adverse outcomes in patients with acute pancreatitis. World J Gastroenterol. 2017;23(21):3883–3889. doi:10.3748/wjg.v23.i21.388328638228
- Kumar P, Law S, Sriram KB. Evaluation of platelet lymphocyte ratio and 90-day mortality in patients with acute exacerbation of chronic obstructive pulmo7ary disease. J Thorac Dis. 2017;9(6):1509–1516. doi:10.21037/jtd.2017.05.7728740663
- Li W, Ai X, Ni Y, Ye Z, The Association Between the Neutrophil-to-Lymphocyte Ratio and Mortality in Patients With Acute Respiratory Distress Syndrome: A Retrospective Cohort Study. Shock 2019;51(2):161–167.
- Liu X, He L, Han J, et al. Association of neutrophil-lymphocyte ratio and T lymphocytes with the pathogenesis and progression of HBV-associated primary liver cancer. PLoS One. 2017;12(2):e0170605. doi:10.1371/journal.pone.017060528231294
- Suppiah A, Malde D, Arab T, et al. The prognostic value of the neutrophil-lymphocyte ratio (NLR) in acute pancreatitis: identification of an optimal NLR. J Gastrointest Surg. 2013;17(4):675–681. doi:10.1007/s11605-012-2121-123371356
- Wang Y, Ju M, Chen C, et al. Neutrophil-to-lymphocyte ratio as a prognostic marker in acute respiratory distress syndrome patients: a retrospective study. J Thorac Dis. 2018;10(1):273–282. doi:10.21037/jtd.2017.12.13129600057
- Yang W, Wang X, Zhang W, et al. Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio are 2 new inflammatory markers associated with pulmonary involvement and disease activity in patients with dermatomyositis. Clin Chim Acta. 2017;465:11–16. doi:10.1016/j.cca.2016.12.00727965019
- Yao C, Liu X, Tang Z. Prognostic role of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio for hospital mortality in patients with AECOPD. Int J Chron Obstruct Pulmon Dis. 2017;12:2285–2290. doi:10.2147/COPD.S14176028814856
- Salciccioli JD, Marshall DC, Pimentel MA, et al. The association between the neutrophil-to-lymphocyte ratio and mortality in critical illness: an observational cohort study. Crit Care. 2015;19:13. doi:10.1186/s13054-014-0731-625598149
- Xiang F, Chen R, Cao X, et al. Monocyte/lymphocyte ratio as a better predictor of cardiovascular and all-cause mortality in hemodialysis patients: a prospective cohort study. Hemodial Int Symp Home Hemodial. 2018;22(1):82–92. doi:10.1111/hdi.12549
- Hufford MM, Richardson G, Zhou H, et al. Influenza-infected neutrophils within the infected lungs act as antigen presenting cells for anti-viral CD8(+) T cells. PLoS One. 2012;7(10):e46581. doi:10.1371/journal.pone.004658123056353
- Shen Z, Chen Z, Li X, et al. Host immunological response and factors associated with clinical outcome in patients with the novel influenza A H7N9 infection. Clin Microbiol Infect. 2014;20(8):O493–500. doi:10.1111/1469-0691.1250524350809
- Li Q, Zhou L, Zhou M, et al. Epidemiology of human infections with avian influenza A(H7N9) virus in China. N Engl J Med. 2014;370(6):520–532. doi:10.1056/NEJMoa130461723614499
- Riche F, Gayat E, Barthelemy R, Le Dorze M, Mateo J, Payen D. Reversal of neutrophil-to-lymphocyte count ratio in early versus late death from septic shock. Critical Care. 2015;19:439. doi:10.1186/s13054-015-1144-x26671018
- Hwang SY, Shin TG, Jo IJ, et al. Neutrophil-to-lymphocyte ratio as a prognostic marker in critically-ill septic patients. Am J Emerg Med. 2017;35(2):234–239. doi:10.1016/j.ajem.2016.10.05527806894
- Cheng Q-L, Ding H, Sun Z, et al. Retrospective study of risk factors for mortality in human avian influenza A(H7N9) cases in Zhejiang Province, China, March 2013 to June 2014. Int J Infect Dis. 2015;39:95–101. doi:10.1016/j.ijid.2015.09.00826376223
- Tate MD, Ioannidis LJ, Brooks AG, Croker B, Brown LE, Reading PC. The role of neutrophils during mild and severe influenza virus infections of mice. PLoS One. 2011;6(3):e17618. doi:10.1371/journal.pone.001761821423798
- Tecchio C, Micheletti A, Cassatella MA. Neutrophil-derived cytokines: facts beyond expression. Front Immunol. 2014;5:508. doi:10.3389/fimmu.2014.0050825374568
- Cassatella MA. Neutrophil-derived proteins: selling cytokines by the pound. Adv Immunol. 1999;73:369–509.10399011
- Cheng OZ, Palaniyar N. NET balancing: a problem in inflammatory lung diseases. Front Immunol. 2013;4:1. doi:10.3389/fimmu.2013.0000123355837
- Camp JV, Bagci U, Chu YK, et al. Lower respiratory tract infection of the ferret by 2009 H1N1 pandemic influenza a virus triggers biphasic, systemic, and local recruitment of neutrophils. J Virol. 2015;89(17):8733–8748. doi:10.1128/JVI.00817-1526063430
- Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31(4 Suppl):S195–199. doi:10.1097/01.CCM.0000057843.47705.E812682440
- Craig A, Mai J, Cai S, Jeyaseelan S. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect Immun. 2009;77(2):568–575. doi:10.1128/IAI.00832-0819015252
- Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011;17(3–4):293–307. doi:10.2119/molmed.2010.0013821046059
- Narasaraju T, Harshini A. Neutrophils as possible therapeutic targets in severe influenza pneumonia. J Infect Pulm Dis 2016;2(2). doi:10.16966/2470-3176.115
- Kuiken T, Taubenberger JK. Pathology of human influenza revisited. Vaccine. 2008;26(Suppl 4):D59–66.19230162
- Taubenberger JK, Morens DM. The pathology of influenza virus infections. Annu Rev Pathol. 2008;3:499–522. doi:10.1146/annurev.pathmechdis.3.121806.15431618039138
- Perrone LA, Plowden JK, Garcia-Sastre A, Katz JM, Tumpey TM. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog. 2008;4(8):e1000115. doi:10.1371/journal.ppat.100011518670648
- Long JP, Kotur MS, Stark GV, et al. Accumulation of CD11b(+)Gr-1(+) cells in the lung, blood and bone marrow of mice infected with highly pathogenic H5N1 and H1N1 influenza viruses. Arch Virol. 2013;158(6):1305–1322. doi:10.1007/s00705-012-1593-323397329
- Camp JV, Chu YK, Chung DH, et al. Phenotypic differences in virulence and immune response in closely related clinical isolates of influenza A 2009 H1N1 pandemic viruses in mice. PLoS One. 2013;8(2):e56602. doi:10.1371/journal.pone.005660223441208
- Rynda-Apple A, Robinson KM, Alcorn JF. Influenza and bacterial superinfection: illuminating the immunologic mechanisms of disease. Infect Immun. 2015;83(10):3764–3770. doi:10.1128/IAI.00298-1526216421
- Yu L, Wang Z, Chen Y, et al. Clinical, virological, and histopathological manifestations of fatal human infections by avian influenza A(H7N9) virus. Clin Infect Dis. 2013;57(10):1449–1457. doi:10.1093/cid/cit54123943822
- To KF, Chan PK, Chan KF, et al. Pathology of fatal human infection associated with avian influenza A H5N1 virus. J Med Virol 2001;63(3):242–246.
- Liu S, Pang L, Ruan S, Zhang X. Global dynamics of avian influenza epidemic models with psychological effect. Comput Math Methods Med. 2015;2015:913726. doi:10.1155/2015/91372625861378
- Tumpey TM, Lu X, Morken T, Zaki SR, Katz JM. Depletion of lymphocytes and diminished cytokine production in mice infected with a highly virulent influenza A (H5N1) virus isolated from humans. J Virol. 2000;74(13):6105–6116. doi:10.1128/jvi.74.13.6105-6116.200010846094
- To KF, Chan PK, Chan KF, et al. Pathology of fatal human infection associated with avian influenza A H5N1 virus. J Med Virol. 2001;63(3):242–246.11170064
- Banoei MM, Vogel HJ, Weljie AM, et al. Plasma metabolomics for the diagnosis and prognosis of H1N1 influenza pneumonia. Critical Care. 2017;21(1):97. doi:10.1186/s13054-017-1686-128424077
- Akashi K, Hayashi S, Gondo H, et al. Involvement of interferon-gamma and macrophage colony-stimulating factor in pathogenesis of haemophagocytic lymphohistiocytosis in adults. Br J Haematol. 1994;87(2):243–250.7947264
- Alkhouri N, Morris-Stiff G, Campbell C, et al. Neutrophil to lymphocyte ratio: a new marker for predicting steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 2012;32(2):297–302. doi:10.1111/j.1478-3231.2011.02639.x22097893
- Ichikawa A, Kuba K, Morita M, et al. CXCL10-CXCR3 enhances the development of neutrophil-mediated fulminant lung injury of viral and nonviral origin. Am J Respir Crit Care Med. 2013;187(1):65–77. doi:10.1164/rccm.201203-0508OC23144331
- Morita M, Kuba K, Ichikawa A, et al. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell. 2013;153(1):112–125. doi:10.1016/j.cell.2013.02.02723477864
- Guo J, Huang F, Liu J, et al. The serum profile of hypercytokinemia factors identified in H7N9-infected patients can predict fatal outcomes. Sci Rep. 2015;5:10942. doi:10.1038/srep1094226028236
- Zhu L, Liu L, Zhang Y, et al. High level of neutrophil extracellular traps correlates with poor prognosis of severe influenza a infection. J Infect Dis. 2018;217(3):428–437. doi:10.1093/infdis/jix47529325098
- Zheng S, Tang L, Gao H, et al. Benefit of early initiation of neuraminidase inhibitor treatment to hospitalized patients with avian influenza A(H7N9) virus. Clin Infect Dis. 2018;66(7):1054–1060. doi:10.1093/cid/cix93029077848
- Ma W, Huang H, Chen J, et al. Predictors for fatal human infections with avian H7N9 influenza, evidence from four epidemic waves in Jiangsu Province, Eastern China, 2013–2016. Influenza Other Resp Virus. 2017;11(5):418–424. doi:10.1111/irv.12461
- Yang Y, Guo F, Zhao W, et al. Novel avian-origin influenza A (H7N9) in critically ill patients in China*. Crit Care Med. 2015;43(2):339–345. doi:10.1097/CCM.000000000000069525365721
- Gao R, Wang L, Bai T, Zhang Y, Bo H, Shu Y. C-reactive protein mediating immunopathological lesions: a potential treatment option for severe influenza a diseases. EBioMedicine. 2017;22:133–142. doi:10.1016/j.ebiom.2017.07.01028734805
- Hanshaoworakul W, Simmerman JM, Narueponjirakul U, et al. Severe human influenza infections in Thailand: oseltamivir treatment and risk factors for fatal outcome. PLoS One. 2009;4(6):e6051. doi:10.1371/journal.pone.000605119557130
- Thiele JR, Zeller J, Bannasch H, Stark GB, Peter K, Eisenhardt SU. Targeting C-reactive protein in inflammatory disease by preventing conformational changes. Mediators Inflamm. 2015;2015:372432. doi:10.1155/2015/12538026089599
- Cheng QL, Ding H, Sun Z, et al. Retrospective study of risk factors for mortality in human avian influenza A(H7N9) cases in Zhejiang Province, China, March 2013 to June 2014. Int J Infect Dis. 2015;39:95–101. doi:10.1016/j.ijid.2015.09.00826376223
- Wu W, Shi D, Fang D, et al. A new perspective on C-reactive protein in H7N9 infections. Int J Infect Dis. 2016;44:31–36. doi:10.1016/j.ijid.2016.01.00926809124
