Abstract
Heart failure with preserved ejection fraction (HFpEF) is a major global public health problem. Diagnosis of HFpEF is still challenging and built based on the comprehensive echocardiographic analysis. Currently, there are no universally accepted therapies that alter the clinical course of HFpEF. This review attempts to summarize the current advances in the diagnosis of HFpEF and provide future directions of the patients´ management with this very widespread, heterogeneous clinical syndrome.
Introduction
Heart failure with preserved ejection fraction (HFpEF) is a major global public health issue.Citation1,Citation2 HFpEF can be defined as a clinical syndrome when the heart cannot pump the blood adequately without the cardiac filling pressures elevation.Citation3 Since 2007 when a consensus statement the Heart Failure Association (HFA) of the European Society of Cardiology (ESC)Citation4 was published, the terminology has evolved through HF with normal ejection fraction to the current definition as “HF with preserved ejection fraction (HFpEF)”.Citation5 Despite modern advances in the diagnosis and treatment of cardiovascular diseases, the prevalence of this disease is expected to increase worldwide. This is due to an increase in life expectancy, as well as an increase in the prevalence of obesity and diabetes.Citation6,Citation7 Currently, the hospitalization of patients in HFpEF accounts for more than half of all hospitalizations with decompensated heart failure.Citation8 HFpEF is heterogeneous in both aetiology and phenotypic manifestations, which significantly complicates the diagnosis of this condition in such patients. This disease develops more often in women, in elderly patients with risk factors and comorbidities such as obesity, hypertension, diabetes, chronic kidney disease, and chronic obstructive pulmonary disease.Citation9–Citation11 It was shown that the pathophysiology of HFpEF goes far beyond diastolic dysfunction and the essence of the pathophysiology of HFpEF is an increase of left ventricle (LV) filling pressure.Citation12 However, our understanding of the pathophysiology of HFpEF is incomplete, and drug development has proved very challenging.Citation13 Currently, there are no universally accepted therapies that alter the clinical course of HFpEF.Citation6 This review attempts to summarize the current advances in the diagnosis of HFpEF and provide future directions for the management of the patients with this very widespread and heterogeneous clinical syndrome.
Role of Imaging Modalities in the Diagnosis of Heart Failure with Preserved Ejection Fraction
Transthoracic Echocardiography
Current clinical trials in patients with heart failure use left ventricle ejection fraction (LVEF), measured using echocardiography as a cut-off for inclusion/exclusion criteria. According to ESC Guidelines, LVEF is still the main parameter to divide HF patients into 3 groups/categories based on LVEF: HFpEF patients considered those with LVEF ≥50%.Citation6 Although LVEF has several limitations, such as the quantification of LV function based on volumetric measures from a two-dimensional non-tomographic technique,Citation14 echocardiography remains the main non-invasive method in the diagnosis of patients with heart failure with preserved ejection fraction (HFpEF). Until last year, there were two cornerstone guidelines or expert recommendations concerning how to diagnose HFpEF by using transthoracic echocardiography. In effect, one has been elaborated by the American Society of Echocardiography and the European Association of Cardiovascular Imaging (ASE/EACVI) and another one by the European Society of Cardiology (ESC).Citation6,Citation12
The American Society of Echocardiography and European Association of Cardiovascular Imaging (ASE/EACVI) recommendations established an echocardiographic approach regarding how to determine elevated left ventricle filling pressures (LVFP) in patients with signs and symptoms of heart failure and with the myocardial disease. This approach is mainly based on the mitral E/A ratio. In this approach, patients with E/A ratio ≥ 2 are considered as having elevated LV filling pressures and thereby, the diagnosis of HFpEF could be established. In addition, in patients with mitral E/A ratio between 0.8 and 1.9 further three criteria should be considered for finally determining elevated LV filling pressures: i) left atrial volume index (LAVI) > 34 mL/m2; ii) peak velocity of tricuspid regurgitation (TR) > 2.8 m/s; iii) mitral average septal-lateral E/e´ ratio > 14. When two of these three criteria have been met, it confirms elevated LV filling pressures and accordingly the diagnosis of HFpEF (see ). On the other hand, in those patients when two of these parameters are negative and a mitral E/A ratio between 0.8–1.9 as well as in patients with mitral E/A ratio < 0.8 and mitral E-wave inflow < 50cm/s further evaluation by using a diastolic stress test should be considered to confirm the diagnosis of HFpEF or elevated LV filling pressures. Some clinical and methodological considerations should be taken into account regarding this approach recommended by the ASE/EACVI. In this respect, this approach is indicated for patients in sinus rhythm and thus, in patients with atrial fibrillation (AF), another workup should be used:Citation12
Peak TR velocity > 2.8 m/s is suggestive of elevated LAP.
In patients with depressed LVEFs, mitral DTs (≤ 160 msec) has reasonable accuracy for the prediction of increased LV diastolic pressures and adverse clinical outcomes.
In patients with incomplete TR jet other Doppler measurements can be applied, including peak acceleration rate of mitral E velocity ≥ 1.900 cm/sec2, IVRT ≤ 65 sec, DT of pulmonary venous diastolic velocity≤ 220 msec, E/Vp ratio ≥1.4, E/e´ ratio ≥ 11.
The variability of mitral inflow velocity with the RR cycle length is of value in patients with AF, as patients with increased filling pressure have less beat to beat variation.
Figure 1 Algorithm for estimation of LV filling pressures and grading LV diastolic function in patients with myocardial disease and normal LVEF after consideration of clinical and other 2D data.
Note: Data from Nagueh et al.Citation12
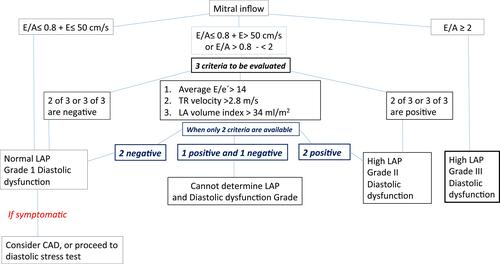
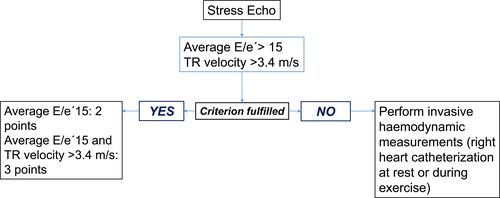
Figure 2 Functional tests in cases of diagnostic uncertainty. Advanced HFpEF workup. Echo stress test.
Note: Data from Pieske et al.Citation5
In addition, it is important to highlight that in patients with significant mitral valve disease such as calcification of the mitral annulus, any mitral stenosis or at least moderate mitral regurgitation, mitral valve repair or prosthetic mitral valve, left bundle branch block, ventricular paced rhythm or LV assist devices this approach should not be considered because of the inaccuracy of the mitral E/e´ ratio in this setting.
The ESC guidelines regarding how to diagnose HFpEF are based on the evidence of functional and structural cardiac alterations (see ). In this regard, key functional alterations are an average mitral E/e´ ratio ≥13 and an average septal-lateral e´ velocity < 9 cm/s. In addition, LV mass > 115/95 g/m2 men/women or LAVI > 34mL/m2 should be considered as key structural alterations. In effect, when functional and structural cardiac alterations, together with BNP/NT-pro-BNP values > 35/125 pg/mL have been found, the diagnosis of HFpEF can be done. Moreover, both guidelines also highlighted the potential role of diastolic stress test for those patients with suspected HFpEF despite negative functional or structural criteria.
Table 1 Normal and Abnormal Values of Echocardiographic Indices of Diastolic Function of Left Ventricle at Rest According to Age Categories, Differentiated for Gender
Cardiac Magnetic Resonance
At the moment, cardiac magnetic resonance (CMR) stay the gold standard for measuring volumes, mass and ejection fraction both the left and right ventricles. It is the best alternative imaging modality of the heart in patients with non-diagnostic echocardiographic examinations due to suboptimal image quality.Citation6 CMR allows to assess myocardial fibrosis using late gadolinium enhancement (LGE) along with T1 mapping and therefore it is a unique technique to confirm HF aetiology.Citation6,Citation15,Citation16 Due to LGE CMR helps distinguish ischaemic vs non-ischaemic origins of HF and myocardial fibrosis vs scars. Moreover, CMR provides the myocardial tissue characterization in myocarditis, amyloidosis, sarcoidosis, Chagas disease, Fabry disease or non-compaction cardiomyopathy.Citation6,Citation15–Citation17 In some cardiac pathologies, especially in HFpEF, impairment in longitudinal function may precede a decrease in circumferential indices or global LVEF, that can lead to early LV dysfunction.Citation18 Therefore, cardiovascular magnetic resonance (CMR) feature tracking (FT-CMR) enables the reproducible and non-invasive assessment of global strain (or atrial strain) from cine CMR images and may provide better insight into myocardial dysfunction with incremental value beyond LVEF.Citation19–Citation22 Recently, a simple, automatic approach for assessing left ventricular longitudinal function with cine cardiovascular magnetic resonance - fast long-axis strain showed the effectiveness of the method to quantify long-axis LV function compared to conventional feature tracking and manual approaches in all HF phenotypes.Citation21 The fast approach–derived LV strain and strain rate parameters provide reproducible, consistent, and effective LV longitudinal function analysis. Moreover, the progressive reductions in left ventricular long-axis strain and strain rate measurements from HFpEF and heart failure with mid-range ejection fraction (HFmrEF) to heart failure with reduced ejection fraction (HFrEF) group was observed.Citation21
A New the HFA–PEFF Diagnostic Algorithm: HFpEF Consensus Recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC)
Recently, a new position paper from the Heart Failure Association was released, which offers an algorithm for diagnosing HFpEF HFA-PEFF, where P is a pre-test probability, E is a set of data from Echo and natriuretic peptides, F1-functional tests, in particular, diastolic stress test, F2-final conclusions on the aetiology of the pathological process.Citation5 This document provides current clarifications on the features of the echocardiographic diagnosis of HFpEF. This algorithm has been validated in two independent, well-phenotyped cohorts and demonstrated that the HFA-PEFF score is helpful in clinical practice for the diagnosis of HFpEF.Citation23
Step 1(P) should be performed in any patient who has the clinical suspicion (symptoms and/or signs) of HF. The detailed medical and demographic history should be described. Furthermore, the blood tests; electrocardiogram (ECG); standard echocardiography to exclude other causes such as heart failure with reduced ejection fraction (HFrEF) or heart valve disease; and investigations to exclude ischaemia, arrhythmias, anaemia, or pulmonary disease should be performed. Step P is intended to detect patients with a high probability of heart failure with preserved ejection fraction and rule-out or confirm other specific causes for their heart failure-like symptoms. Patients with a high probability of diagnosis HFpEF are frequently elderly female with comorbidities and preserved LVEF as well as elevated natriuretic peptides or atrial fibrillation. Coronary artery disease, significant valvular disease, pulmonary disease, and anaemia should be excluded during this initial workup as alternative causes. When Step P is positive, the second Step 2 (E) should be done: comprehensive echocardiography and brain natriuretic peptide/N-terminal natriuretic peptide (NP) levels analysis, if not already done on Step P.
According to the proposed classification, all criteria, including echocardiographic, were divided into major and minor. The score has functional, morphological, and biomarker domains. Within each domain, a major criterion scores 2 points or a minor criterion 1 point.
Functional Domain
In the functional domain septal and lateral mitral annular peak early diastolic velocity e´, age-specific, were used as Major criteria:
In subjects aged < 75 years: septal e´< 7 cm/s; or lateral e´ < 10 cm/s.
In subjects aged ≥ 75 years: septal e´< 5 cm/s; or lateral e´< 7 cm/s.
(II) Furthermore, the ratio of the peak velocity of mitral inflow during early diastole (E), recorded by pulsed Doppler on the tips of the mitral leaflets, over the average of septal and lateral mitral annular early diastolic peak velocities (e´) recorded by pulsed tissue Doppler was used as a second criterion in this domain:
Major criterion: average septal–lateral E/e´ ratio ≥ 15
Minor criterion: average septal–lateral E/e´ ratio 9 −14
(III) Pulmonary arterial systolic pressure (PASP), calculated from the modified Bernoulli equation using tricuspid regurgitation (TR) peak velocity > 2.8m/s (PASP > 35mmHg) as Major criterion and TR peak velocity < 2.8m/s as Minor criterion.
(IV) Left ventricular peak systolic global longitudinal strain (GLS), measured using speckle-tracking echocardiography as the average of systolic strain obtained from all segments in the apical 4-chamber, apical 2-chamber, and apical long-axis views were included for the first time, so far as a Minor criterion: GLS < 16%.
Morphological Domain
The maximal volume of the left atrium (LAV) from biplane or three-dimensional images indexed to body surface area [left atrial volume index (LAVI)], using separate cutoffs in sinus rhythm (SR) vs atrial fibrillation were suggested:
Major criterion: > 34mL/m2 for subjects in sinus rhythm
Major criterion: > 40mL/m2 for subjects in atrial fibrillation
Minor criterion: 29–34mL/m2 for subjects in sinus rhythm
Minor criterion: 34–40mL/m2 for subjects in atrial fibrillation
The absence of left ventricle hypertrophy (LVH) on echocardiography does not exclude HFpEF. Therefore, the finding of concentric hypertrophy (increased left ventricle mass index (LVMI) and increased RWT) as a Major criterion, or anyone of a lesser degree of LVH, relative wall thickness (RWT), and LV end-diastolic wall thickness as a minor criterion were recommended:
Major criterion: LVMI > 149 g/m2 in men or > 122 g/m2 in women and RWT > 0.42.
Minor criterion: LVMI >115 g/m2 in men or > 95 g/m2 in women or RWT > 0.42 or LV end-diastolic wall thickness > 12mm.
Finally, the natriuretic peptide levels should be interpreted in the context of underlying rhythm (SR or AF).
Calculating and Interpreting the HFA–PEFF Score
Each domain can add maximally 2 points if any major criterion from this domain is positive or 1 point if no major but any minor criterion is positive. If several major criteria within a single domain are positive, this domain adds 2 points; and if no major but several minor criteria are positive it gives still 1 point. Major and minor criteria are not additive in a single domain. Points can be added just when they originate from different domains.
A total score of ≥ 5 points is considered to confirm the HFpEF while a score of ≤ 1 point is considered to make a diagnosis of HFpEF very doubtful and to mandate further investigations for alternative causes. If the patient has an intermediate score (2–4 points) further evaluation should be done. [Step 3(F1)].
Summarizing the new data, echocardiographic criteria for HFpEF can be divided into structural and functional ().
Table 2 Step 2 (E): Echocardiographic and Natriuretic Peptide Heart Failure with Preserved Ejection Fraction Workup and Scoring System (Diagnostic Workup)
H2FPEF Score
A group of experts from Mayo clinic evaluated 414 consecutive patients undergoing evaluation for unexplained dyspnea (267 patients with HFpEF and 147 control patients with non-cardiac causes of dyspnea) for the discrimination of HFpEF from non‐cardiac causes of dyspnea.Citation24 Notably, all patients underwent the gold standard test to confirm the diagnosis of HFpEF using invasive haemodynamic exercise testing.Citation24 Using simple clinical characteristics and conventional echocardiographic information they have proposed the H2FPEF score (). The six clinical and echocardiographic variables that constitute the H2FPEF score include the following: (i) obesity (H); (ii) the use of ≥2 antihypertensive drugs (H); (iii) atrial fibrillation (F); (iv) pulmonary hypertension (P); (v) an age > 60 years (E); and (vi) E/e´ > 9 (F). A score was assigned to these six variables based on strength of association in logistic regression with HFpEF [atrial fibrillation-3 points, obesity-2 points, others-1 each], creating the H2FPEF score ranging from 0–9.Citation24 The probability of HFpEF increased with increasing H2FPEF score:
Table 3 Description of the H2FPEF Score and Point Allocations for Each Clinical Characteristic (Top Box), with Associated Probability of Having HFpEF Based Upon the Total Score as Estimated from the Model (Lower Box)
- H2FPEF score of 0–1: low probability (<20%), unlikely HFpEF
- H2FPEF score of 2–5: intermediate probability
- H2FPEF score of 6–9: High probability (>90%), HFpEF is likely
The generalizability and prognostic value of the score have been demonstrated in several independent analyses.Citation25–Citation28 The authors showed that H2FPEF score provided strong discrimination of HFpEF from controls [AUC 0.841, 95% CI:0.802–0.881]. The H2FPEF score better discriminated HFpEF from non-cardiac causes of dyspnea compared to widely used diagnostic algorithms based upon expert consensusCitation4,Citation6 (AUC comparison +0.169 [95% CI +0.120 to +0.217] vs 2016 ESC guidelines and +0.173 [95% CI +0.132 to +0.215] vs 2007 ESC guidelines, both p<0.0001).Citation24 Interestingly, the use of NTproBNP levels did not incrementally add the diagnostic ability to the H2FPEF score.Citation24 A major advantage of the H2FPEF score is that the probability that HFpEF is the cause of symptoms can be estimated accurately, which helps to guide further evaluation.Citation29 As an HFA–PEFF score, it gives a central role of exercise testing in both algorithms among patients with diagnostic uncertainty. Patients with an intermediate score (2–5) require further evaluation to reach a definitive diagnosis. It can be either echocardiographic or invasive hemodynamic exercise stress tests. On the one hand, the invasive exercise testing can directly assess the parameters that define HFpEF and serves as the gold standard test to prove (or refute) that HFpEF is the cause of symptoms.Citation3 On the other hand, invasive exercise testing is associated with high costs and can be performed only in centres with high expertise in this field. Furthermore, the invasive cardiopulmonary exercise testing (CPET) has emerged as the gold standard test to define causes of dyspnea and exertional limitation in patients with unexplained exertional dyspnea.Citation30
Diastolic Stress Test
Echocardiography at rest remains an important and the most commonly used method to characterize the underlying functional and structural changes in HFpEF.Citation5,Citation6,Citation12,Citation31 However, in some patients with HFpEF who have symptoms such as dyspnea only during exercise, and often echocardiographic analyses at rest such mitral E/e´ ratio and TR velocity can be normal.Citation5,Citation6,Citation12,Citation32 Symptoms compatible with HF can be confirmed to originate from the heart if invasive testing demonstrates a high LV filling pressure [left ventricular end-diastolic pressure (LVEDP) ≥ 16mmHg, pulmonary capillary wedge pressure (PCWP) ≥ 15mmHg] at rest, or PCWP ≥ 25 mmHg during exercise.Citation5
In line, several studies demonstrated that in some patients with HFpEF LV diastolic abnormalities occur only during exercise.Citation32–Citation38 Thus, adding diastolic analysis during exercise can increase the sensibility to diagnose HFpEF.Citation32,Citation36,Citation39
In effect, as currently recommended by all guidelines, a diastolic stress test should be added to the resting echocardiographic approach in the setting of suspicion of HFpEF and inconclusive criteria by using diastolic measurements at rest.Citation5,Citation6,Citation12,Citation24
Some methodological considerations should be taken into account before to perform a diastolic stress test. The most validated and recommended protocol for a diastolic stress test is the semi-supine bicycle.Citation40 Normally, the exercise begins with a 25 W load, increasing every 3 min 25 W at 50–60 rpm until the patient reaches a maximal predicted workload, a maximal predicted heart rate (220-age), or until the patient is not able to continue the cycling due to fatigue, dyspnea, chest pain, blood pressure increase or drop or other reasons for termination. In older patients, exercise can begin with a lower workload level (e.g.20W) and/or workload increase step can be set at 10 W every 2 minutes.Citation41
It is well known, that in some patients E and A peaks from the mitral inflow and e´ and a´ TDI peaks can be fused during exercise. Therefore, in such patients, the measurements should be done immediately after the termination of the test during the recovery phase (1–2 minutes after exercise).
In line with this, treadmill exercise by measuring the mitral E/e´ ratio and TR velocity within 2 min after the test is an alternative to the recommended bicycle protocol.Citation40
Furthermore, it is important to highlight that some patients can be exhausted earlier and can reach only 25 or 50 W level. In such patients measurements of the mitral E/e′ ratio and TR velocity preferably should be analyzed in each stage.Citation32,Citation35
On the other hand, in many patients at the peak exercise large variations of the mitral E/e′ ratio can occur (due to respiratory efforts and/or poor echocardiographic window) and thus, the maximal value of the mitral E/e′ ratio from at least 3 cardiac cycles should be measured to get the averaged E/e´ values during exercise.
In case of indeterminate results when average mitral E/e′ septal-lateral ratio > 14 (or mitral E/e′ septal ratio > 15), but a TR velocity ≤ 2.8 m/s (or not detectable) other additional echocardiographic parameters (such as early diastolic strain rate or global longitudinal strain or left atrial strain or strain rate) should be further tested during exercise in the larger studies or the results should be interpreted according to the clinical scenario or probability of HFpEF.Citation42,Citation43 In effect, an isolated elevation of the mitral E/e′ ratio with normal values or not detectable TR velocity are more probable to indicate elevated LV filling pressures than an isolated elevation of TR velocity with normal values of the mitral E/e′ ratio.Citation12,Citation32,Citation40
According to the HFA consensus, diastolic stress test (DST) should be considered abnormal if average E/e′ ratio at peak stress increases to ≥15, with or without a peak TR velocity ≥ 3.4m/s.5 An increase in TR velocity alone should not be used to diagnose HFpEF because it could be caused just by a normal hyperdynamic response to exercise (increased pulmonary blood flow) in the absence of LV diastolic dysfunction.Citation44
An average E/e′ ratio during exercise ≥ 15 gives 2 points to the HFA–PEFF score. An average E/e′ ratio ≥ 15 with a peak TR velocity >3.4 m/s adds 3 points to the previous score from Step 2 (E). If the combined score from Step 2 (E) and Step 3 (F1) is ≥ 5 points, then the diagnosis of HFpEF can be confirmed (Figure 2).Citation5
Cardiopulmonary exercise testing (CPET) is another useful tool that can be used together with DST helping to identify HFpEF patients with indeterminate echocardiographic parameters at rest.Citation45
Furthermore, it should be noted, that the level of evidence or amount of published studies by using diastolic stress test to estimate elevated LV filling pressures in patients with atrial fibrillation is low.Citation46 Hence, we considered that the previous cutoff of the mitral E/e′ ratio and TR velocity during exercise should not be taken as conclusive to diagnose or exclude HFpEF in patients with atrial fibrillation.
LV Systolic Dysfunction in HFpEF
Along with diastolic dysfunction, it was shown that the symptoms of patients with HFpEF are related not only with LV diastolic dysfunction but also with an impaired LV longitudinal systolic functionCitation47,Citation48 and impaired ventricular contractility.Citation49,Citation50 Moreover, other multiple non-diastolic abnormalities such as left atrial impairment, relative pericardial restraint, abnormal right ventricular-pulmonary artery coupling, pulmonary vascular disease, systemic vascular stiffening, coronary and peripheral microvascular dysfunction, and chronotropic incompetence are also contributed to the disease progression.Citation51 A speckle-tracking analysis is a developing modality with additive value to standard echocardiography.Citation52 LV global longitudinal strain is relatively independent of traditional diastolic parameters such as E/e′ and e′Citation18 and has confirmed that the longitudinal systolic function of the LV is significantly altered in a high proportion of patients with HFpEF.Citation48 Moreover, impaired LV systolic mechanics in HFpEF also predict an increased risk of adverse outcomes.Citation51,Citation53,Citation54
Left Atrial Dysfunction in HFpEF
The left atrium (LA) plays a key role in HFpEF pathophysiology, and indices of LA mechanics have diagnostic and prognostic utility in HFpEF.Citation55–Citation58 Moreover, LA remodelling and dysfunction secondary to increased LV filling pressure are common in HFpEF and are associated with worse symptoms, more pulmonary vascular disease, greater RV dysfunction, depressed exercise capacity, and adverse outcomes, suggesting that patients with relatively greater “atrial myopathy” may also constitute a different phenotype within the HFpEF spectrum.Citation3,Citation51,Citation59–Citation62 Our results demonstrated that abnormal LA strain (< 23%) is significantly associated with worse New York Heart Association functional classification (NYHA) class and with the risk of HF hospitalization at 2 years independently from age and sex.Citation57 Mandoli et al suggested that LA strain (≤ 20%) can help classify patients with diastolic dysfunction in the indeterminate range according to standard criteria.Citation63 Recently, Khan et al performed a systematic review and meta-analysis to evaluate the association of impaired LA function with outcomes in HFpEF.Citation64 Multiple databases were searched for original studies measuring different phases of LA function in HFpEF patients. Comparative LA function between HFpEF patients and healthy controls was assessed by pooling weighted mean differences (WMD). LA reservoir [WMD = −13.38% (−16.07, −10.68); P < 0.001], conduit [WMD = −4.09% (−6.77, −1.42); P = 0.003], and pump [WMD = −3.53% (−4.47, −2.59); P < 0.001] strains were also significantly lower in HFpEF patients. Decreased LA reservoir strain [HR 1.24 (1.02, 1.50); P = 0.03] was significantly associated with the risk of composite all-cause mortality or heart failure hospitalization.Citation64 Hence, based on these studies, LA strain could be of potential usefulness and clinical relevance in the evaluation of patients with HFpEF.
Race Differences in HFpEF
So far, limited epidemiologic data are available regarding racial and ethnic differences in HFpEF. Data from the multiregional cross-sectional Identification of patients with heart failure and PREserved systolic Function: an Epidemiological Regional (I-PREFER) study demonstrated that HFpEF also accounts for a significant proportion of HF in non-Western countries.Citation65 Interestingly, substantial regional variation with a higher incidence of HFpEF in Latin American (69%) and North Africa (75%) compared to the Middle East (41%) was observed.Citation65,Citation66 Compared to whites, African Americans have a 50% higher prevalenceCitation67 and ~80% higher incidence of HF.Citation68–Citation70 Additionally, they have worse outcomes once HF develops regardless of LVEF.Citation71,Citation72 Besides, HFpEF accounts for up to 70% of prevalent HF in African Americans.Citation66,Citation73 A recent study from the Asian Sudden Cardiac Death in Heart Failure (ASIAN-HF) registryCitation74 showed that Asian patients with HFpEF were relatively young (with more than a third under the age of 65 years) and lean (with only a fifth being obese) compared to those from Western populations, yet they carried a high comorbidity burden (70% of patients had ≥2 co‐morbidities).Citation74 Additionally, there were striking regional differences in types of co‐morbidities, cardiac remodelling and outcomes of HFpEF across Asia. These regional and ethnic differences should have important implications for public health measures and should be considered in the global HFpEF trial design.Citation74
Sex Differences in HFpEF
Studies published in the last few years have revealed that cardiovascular disease (CVD) is the main cause of death among women, and more women than men die from CVD.Citation75,Citation76 There is clear evidence of important differences between the sexes: the ratio between women and men with HFpEF is 2:1.Citation77 Women with HFpEF were more likely to have concentric LV remodelling, more severe diastolic dysfunction and higher LV filling pressures, compared to men with HFpEF.Citation78 Moreover, women had higher pulmonary capillary wedge pressures adjusted to workload, greater LV end-systolic and diastolic elastance, and higher LV filling pressures both at rest and peak exercise than men.Citation79 Furthermore, higher exercise PCWP, poorer stroke volume recruitment and larger body mass index (BMI) factors associated with exercise intolerance in HFpEF.Citation79,Citation80 When compared with men, women are at greater risk of the systemic inflammatory and metabolic disorders that are linked to HFpEF as they experience exaggerated cardiovascular responses to the hemodynamic and inflammatory stresses that predispose to HFpEF. Additionally, the inflammatory-metabolic phenotype of HFpEF, which is characterized by biomarkers of inflammation, an expanded epicardial adipose tissue mass, microvascular endothelial dysfunction, increased left ventricular volumes and systolic blood pressures, and possibly, altered activity of adipocyte-associated inflammatory mediators is primarily seen in women.Citation81 Therefore, sex differences in cardiac structure, function and metabolism, vascular ageing, and immune system biology are considerable. Future research initiatives of potential sex-specific mechanisms in HFpEF may provide important insights for the optimal prevention and management of HFpEF in both women and men.Citation11,Citation79
Role of Echocardiography for the Risk Stratification of HFpEF Patients
While validated parameters and recommendations have been established to diagnose HFpEF, the evidence regarding how to stratify the risk of HFpEF by using echocardiography is lower. The ASE/EACVI recommendations addressed this important issue and stated that patients with criteria for elevated LV filling pressures should be considered as patients at high risk since several studies have shown the poor outcomes that having this group of patients has had. In addition, the ASE/EACVI addressed the role of new parameters such as left ventricle global longitudinal systolic strain to stratify the risk of HFpEF patients. Recently, a meta-analysis confirmed that the longitudinal systolic function of the LV is altered in a high proportion of patients with HFpEF.Citation48 Besides, two large multicenter studies demonstrated that an abnormal LV longitudinal systolic function is significantly linked to cardiovascular mortality and heart failure hospitalization in HFpEF patients.Citation54,Citation82 In effect, the current data suggest that patients with low GLS have worse cardiovascular prognosis than those without this myocardial alteration.
Management of Patients with Heart Failure with Preserved Ejection Fraction
Pharmacological Therapy
Current European guidelinesCitation6 for the management of HFpEF focus on the treatment of underlying conditions (eg hypertension or atrial fibrillation) that may contribute to the natural course of the disease. Only the use of diuretics is recommended for the relief of symptoms (class 1b recommendation).
There is inconsistent evidence in symptoms relief in patients receiving angiotensin receptor blockers (ARBs). In the Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: CHARM-Preserved trial, 3023 patients with NYHA class II–IV HF, a prior cardiac hospitalization, and a left ventricle ejection fraction>40% were randomized to the group of candesartan versus placebo.Citation83 The primary endpoint, a composite of cardiovascular death or HF hospitalization, occurred in 22% and 24% of participants in the candesartan and placebo arms respectively at a median follow-up of 36.6 months (hazard ratio [HR], 0.89; [95% CI, 0.77–1.03]; P = 0.118). There was no apparent impact on cardiovascular death (adjusted HR 0.95 (0.76–1.18), P = 0.635 and the benefit was chiefly in preventing admissions to hospital for chronic heart failure (adjusted HR 0.84; [0.70–1.00]; P = 0.047). These data led to an IIb recommendation for consideration of the use of ARBs to decrease hospitalizations in HFpEF in the American College of Cardiology/American Heart Association (ACCF/AHA) guideline for the management of heart failure.Citation31 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure say that candesartanCitation83,Citation84 and angiotensin-converting enzyme inhibitorCitation85 showed an improvement in NYHA class. Evidence, that beta-blockers and mineralocorticoid receptor antagonist improve symptoms in these patients, was lacking.Citation6
For patients in sinus rhythm, there is some evidence that nebivolol,Citation86–Citation88 digoxin,Citation94 spironolactoneCitation95 might reduce HF hospitalizations. In older patients with heart failure with reduced, preserved or mid-range ejection fraction, nebivolol reduced the combined endpoint of death or cardiovascular hospitalization,Citation86,Citation87 with no significant interaction between treatment effect and baseline LVEF.Citation88
Moreover, for a long time, different cut-offs of LVEF for HFpEF were used. Therefore, until now, no treatment has yet been shown, convincingly, to reduce morbidity or mortality in patients with HFpEF.
Non-Pharmacological Therapy
Atrial fibrillation is the most common arrhythmia in HF regardless of concomitant LVEF. AF in patients with HFpEF is associated with more severe symptoms, worse quality of life and higher morbidity and mortality.Citation89 Cryoballoon pulmonary vein isolation is a promising treatment option in patients with AF and HFpEF.Citation90 Although Catheter Ablation vs Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) trial, the largest trial to date, showed no significant difference for its clinical primary endpoint between the strategies of ablation or rate- or rhythm-control meds alone in patients with atrial fibrillation.Citation91 Therefore, larger prospective trials testing the efficacy of ablation are urgently needed in this patient cohort.
Coronary artery disease is often in many patients with HFpEF. It should be mentioned that subendocardial ischemia might also develop in the absence of epicardial coronary stenosis in HFpEF, due to the combination of coronary microvascular dysfunction and hemodynamic imbalances that compromise subendocardial perfusion.Citation92 Consequently, it has been shown that stress imaging, including echocardiography, was less precise in patients with HFpEF, with high rates of false positive and false negative tests.Citation93 When angina persists despite treatment with antianginal drugs, myocardial revascularization is recommended (class IA).Citation6 But the choice between coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI) should be made by the Heart Team after careful evaluation of the patient’s clinical status, coronary anatomy, expected completeness of revascularization, coexisting valvular disease and co-morbidities.Citation6
Future Directions
Pharmacological Therapy
While HFpEF leads to about 50% of hospital admission for HF and there is no proven benefit from pharmacotherapy in this group of patients, investigation of exercise training was a potentially beneficial intervention.Citation96 In the evaluation of outcomes for HFpEF, a meta-analysis that included 276 patients with well-compensated heart failure in six randomized controlled trials demonstrated no major adverse effects of exercise training, although it was suggested that exercise training improved cardiorespiratory fitness by an increase in the peak VO2 and the quality of life. These improvements were noted to be unrelated to a significant change in the diastolic LV function.Citation97
According to the effectiveness of neprilysin inhibition and ARB in HFrEF an attempt has been made to prove efficacy in patients with HFpEF in the PARAGON-HF trial (Prospective Comparison of Angiotensin Receptor Neprilysin Inhibitor with Angiotensin Receptor Blocker Global Outcomes in HFpEF). This was a Phase III trial with an end-point of cardiovascular death or HF hospitalization. PARAGON-HF investigated the efficacy of sacubitril-valsartan compared with valsartan alone among 4822 patients with NYHA II–IV HF, LVEF ≥45%, with evidence of cardiac structural remodelling and either a prior HF hospitalization or elevated natriuretic peptide level. In the sacubitril–valsartan group there were 894 primary events in 526 patients and 1009 primary events in 557 patients in the valsartan group (rate ratio, 0.87; 95% confidence interval [CI], 0.75 to 1.01; P=0.06). Sacubitril–valsartan group had 8.5% incidence of death from cardiovascular causes and 8.9% in the valsartan group (hazard ratio, 0.95; 95% CI, 0.79 to 1.16); there were 690 and 797 total hospitalizations for heart failure, respectively (rate ratio, 0.85; 95% CI, 0.72 to 1.00). Moreover, NYHA class improvement in 15.0% of the patients in the sacubitril–valsartan group and 12.6% in the valsartan group were observed (odds ratio, 1.45; 95% CI, 1.13 to 1.86). The worsening of renal function was 1.4% and 2.7% respectively (hazard ratio, 0.50; 95% CI, 0.33 to 0.77).The results of the Phase III PARAGON-HF study showed a 13% relative reduction in the primary composite endpoint of cardiovascular death and total (first and recurrent) heart failure hospitalizations, but narrowly missed statistical significance.Citation98
Sodium-glucose co-transporter-2 inhibitors (SGLT-2is) have significantly improved HF outcomes in patients with diabetes mellitus type 2 (T2D) and may represent a new therapeutic alternative for patients with T2D at risk for or with HF. They have a novel and unique mechanism of action. By inhibiting sodium and glucose reabsorption in the proximal tubule, SGLT-2i result in a number of downstream effects, including glucosuria, weight loss, osmotic diuresis and natriuresis, which should theoretically be beneficial in HF.Citation99
Moreover, the treatment with SGLT2 inhibitors is poised to ameliorate many of the pathophysiological abnormalities seen in HFpEF. The ongoing EMPEROR‐Preserved Trial designed to specifically prove the effect of empagliflozin on the risk for cardiovascular death or hospitalization for heart failure in patients with HFpEF, as well as the drug’s effect on the heart failure hospitalizations.Citation100 The trial will also examine the ability of empagliflozin to prevent the time‐dependent deterioration of glomerular filtration, which characterizes patients with HFpEF. The authors hope that the results of this trial will shed more light on potential beneficial CV and renal effects of SGLT2 inhibitors in HFpEF patients, including those without T2DM.
Device-Based Therapies
Remote Monitoring of Heart Failure
Fluid retention in patients with HFpEF cause dyspnea and peripheral oedema and can lead to hospitalization due to cardiac decompensation and in sum worse the prognosis. Since every hospitalization in patients with HF make the prognosis worse and associated with higher mortality riskCitation101,Citation102 remote monitoring strategies have been developed to improve ambulatory care of heart failure patients and reduce heart failure hospitalizations.Citation103 The most significant advancement in the arena of implantable hemodynamic monitoring capabilities was taken with a novel, wireless, battery-free, pulmonary artery pressure monitoring system called the CardioMEMS HF System (CardioMEMS).Citation104 This sensor implants using a transvenous delivery catheter into the pulmonary artery and continuously monitors pulmonary artery pressure. The usage of CardioMEMS in the CHAMPION trial in patients with HF (NYHA III), irrespective of their LVEF and previous hospital admission for heart failure was able to reduce HF-related hospitalizations.Citation105,Citation106 An important pre-specified subgroup analysis of the CHAMPION trial demonstrated significant efficacy in patients with HFpEF. The primary efficacy endpoint of heart failure hospitalization rate at 6 months for patients with preserved ejection fraction was 46% lower in the treatment group compared with the control group (p < 0.0001).Citation107 Therefore, the CardioMEMS device was added to the European Society of Cardiology Heart Failure Treatment Guidelines as a consideration in patients with symptomatic heart failure with a previous heart failure hospitalization (IIb recommendation and Level of Evidence Class B).Citation6 Currently, GUIDE-HF trial (NCT03387813) recruit symptomatic HF patients in order to confirm the previous achievements. It is the largest clinical trial of hemodynamic-guided HF management across a broad population of HF patients, with study design and sample size adequate to examine survival, cumulative HF events, quality of life, and functional capacity.Citation108
Interatrial Shunt Device
Exertional dyspnea is the main symptom in HFpEF is mainly due to high filling pressure. Therefore, an attempt was done to evaluate the ability of device-based approaches to reduce cardiac filling pressures. The REDUCe Elevated Left Atrial Pressure in Patients with Heart Failure (REDUCE LAP-HF) study was designed to assess the device performance and safety of a transcatheter, transvenous interatrial shunt device in symptomatic patients with HFPEF. Citation109 In this study a transvenous interatrial shunt device was used to reduce left atrial pressure by creating a small left-to-right shunt (shunt fraction of 25%). Overall, the results of this open-label non-randomised study showed that transcatheter transvenous placement of an interatrial shunt device (IASD) is feasible and might be associated with improvements in exercise hemodynamics, functional capacity, and quality of life.109 The first randomized clinical trial (RCT) REDUCE LAP-HF I was conducted in 22 centres in the United States, Europe, and Australia on patients with NYHA class III or ambulatory class IV, LVEF≥40%, exercise PCWP≥25 mm Hg, and PCWP-right atrial pressure gradient≥5 mmHg.Citation110 Safety was assessed by major adverse cardiac, cerebrovascular, or renal events (MACCRE). Exploratory outcomes evaluated at 1 year were hospitalizations for HF, NYHA class, quality of life, a 6-minute walk test, and device patency. The REDUCE LAP-HF I Phase 2, sham-controlled RCT confirmed the longer-term patency of the IASD. Through 1 year of follow-up, IASD treatment appeared safe, with no significant differences in MACCRE in patients received IASD compared with those who received sham control treatment.Citation111 In the pooled analysis from these 2 trials (n=79) assessing the effects of the IASD on resting and exercise hemodynamics in HF patients with LVEF≥40% and no significant pulmonary vascular disease or RV dysfunction with baseline and repeated hemodynamic evaluation between 1 and 6 months the creation of a therapeutic left to right shunt was associated with improvements in pulmonary vascular function at rest and during exercise.Citation112 Recently, patients from REDUCE LAP-HF trial were followed for a median duration of 739 days. It was shown that IASD implantation may be associated with a reduction in mortality in HFpEF.Citation113 Currently, a randomized controlled study, REDUCE LAP-HF II is currently underway to definitely determine the clinical utility of this procedure.Citation114
Renal Denervation
Renal denervation (RDN) can be considered as a therapeutic option in patients with resistant hypertension, whose blood pressure cannot be controlled by a combination of lifestyle modification and pharmacological therapy according to current guidelines.Citation115 Renal denervation (RDN), a catheter-based, radiofrequency ablation of the renal sympathetic nerves, which has been shown to effectively lower blood pressure.Citation116 Moreover, reduction of cardiac sympathetic activity occurs independently from blood pressure reduction leading to LV mass reducing and improving diastolic function suggesting direct effects on the heart.Citation117–Citation121 Results RDN in HFpEF was tested only in one underpowered trial including 25 patients with HFpEF and did not confirm a favorable effect of RDN on diastolic parameters and quality of life.Citation122 Therefore, further studies are needed to establish the therapeutic value of RDN in HFpEF. Moreover, the first randomized sham-controlled trial, SYMPLICITY-HTN-3, did not lower significantly office or 24-h ambulatory systolic blood pressure (BP) compared with sham treatment.Citation123 Nevertheless, two recent randomized sham-controlled trials in patients not taking antihypertensive drugs (SPYRAL HTN-OFF MED) or continuing to take drugs (SPYRAL HTN-ON MED) performed RDN with the second-generation radiofrequency ablation system showed that RDN significantly reduced office and 24-h ambulatory BP compared with sham treatment.Citation124,Citation125 Some authors believe that these trials have renewed clinical and scientific interest in determining the appropriate role of RDN in the treatment of hypertension.Citation126
HFpEF Phenotyping
As heart failure with preserved ejection fraction is a heterogeneous syndrome for which effective therapies is still lacking, understanding which factors determine this heterogeneity may be helped by better phenotyping. Accordingly, echocardiography may be a very useful tool to classifying various phenotypes in a wider range of HFpEF into pathophysiologically homogenous groups. Consequently, our understanding of the phenotypic heterogeneity of HFpEF, which comprises the etiologic and pathophysiologic heterogeneity of the syndrome, might help to conduct targeted (and more successful) clinical trials in HFpEF.Citation127 Last years, several candidate phenotypes that might be used for deeper characterization by echocardiography in HFpEF were proposed.Citation128 Since obesity is common in HFpEF and has multiple adverse cardiovascular effects it was suggested as an important candidate for phenotyping in HFpEF.Citation129 Obokata et al performed the detailed clinical assessment, echocardiography and invasive hemodynamic exercise testing in subjects with obese HFpEF (BMI≥35kg/m2, n=99), non-obese HFpEF (BMI<30kg/m2, n=96), and non-obese controls free of HF (n=71). They found that patients with obesity-related HFpEF had unique pathophysiologic characteristics that include greater biventricular remodelling, volume overload, more right ventricular dysfunction, greater ventricular interaction and pericardial restraint, worse exercise capacity, more profound hemodynamic derangements, and impaired pulmonary vasodilation.Citation130
Pulmonary hypertension (PH) is another important comorbidity which common in patients with HFpEF and is associated with a worse prognosis.Citation128,Citation131 PH is a common complication in patients with HFpEF in response to a passive increase in left-sided filling pressures, more specifically left atrial pressure.Citation132,Citation133 However, some patients developed pulmonary vascular disease (PVD) with elevation in pulmonary vascular resistance and reduction in pulmonary arterial compliance.Citation134,Citation135 It was shown that patients with HFpEF and pulmonary vascular disease have reduced exercise capacity, impaired RV systolic reserve and worse outcomes.Citation136 Consequently, Borlaug et al suggested that such patients have a different phenotype in the HFpEF spectrum.Citation137 Moreover, some HFpEF patients with PVD demonstrated distinctive haemodynamic limitations only during exercise that restrict aerobic capacity and lead to impaired recruitment of LV preload due to excessive right heart congestion and blunted RV systolic reserve.Citation138
Conclusion
HFpEF is a multifactorial, clinically heterogeneous, and prognostically unfavorable disease. Diagnosis of HFpEF syndrome based on symptoms most of which are associated with the clinical manifestation of high filling pressure. Thus, current consensus and guidelines emphasize the importance of the non-invasive assessment of left ventricle filling pressure to confirm the HFpEF diagnosis. Regardless of LVEF, HFpEF is associated with reduced quality of life and adverse outcomes. The results of large clinical trials demonstrate different approaches to the treatment of HF depending on LVEF. Although LVEF does not reflect the full range of disorders of intracardiac hemodynamics, its definition is very important for the choice of therapeutic tactics. Most of the clinical studies and randomized trials have demonstrated the benefits of certain types of medical treatment for patients with HFrEF, but not in HFpEF. Therefore, further study of HFpEF phenotypes, current diagnostic principles, and new opportunities for treatment and prevention of complications is an important and urgent task that opens up prospects and opportunities for managing this complex problem.
Disclosure
The authors report no conflicts of interest in this work.
References
- Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi:10.1056/NEJMoa052256
- Oren O, Goldberg S. Heart failure with preserved ejection fraction: diagnosis and management. Am J Med. 2017;130:510–516. doi:10.1016/j.amjmed.2016.12.031
- Borlaug BA. Evaluation and management of heart failure with preserved ejection fraction [published online ahead of print, 2020 Mar 30]. Nat Rev Cardiol. 2020. doi:10.1038/s41569-020-0363-2
- Paulus WJ, Tschope C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi:10.1093/eurheartj/ehm037
- Pieske B, Tschöpe C, de Boer RA, et al. How to diagnose heart failure with preserved ejection fraction: the HFA–PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019;40:3297–3317. doi:10.1093/eurheartj/ehz641
- Ponikowski P, Voors AA, Anker SD, et al. Authors/Task Force Members; Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi:10.1002/ejhf.592
- Seferovic PM, Petrie MC, Filippatos GS, et al. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the EuropeanSociety of Cardiology. Eur J Heart Fail. 2018;20:853–872. doi:10.1002/ejhf.1170
- Fonarow GC, Stough WG, Abraham WT, OPTIMIZE‐HF Investigators and Hospitals, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure. J Am Coll Cardiol. 2007;50:768–777. doi:10.1016/j.jacc.2007.04.064
- Chioncel O, Lainscak M, Seferovic PM, et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC heart failure long-term registry. Eur J Heart Fail. 2017;19(12):1574–1585. doi:10.1002/ejhf.813
- Triposkiadis F, Giamouzis G, Parissis J, et al. Reframing the association and significance of comorbiditiesin heart failure. Eur J Heart Fail. 2016;18:744–758. doi:10.1016/j.ijcard.2018.04.001
- Beale AL, Meyer P, Marwick TH, et al. Sex differences in cardiovascular pathophysiology: why women are overrepresented in heart failure with preserved ejection fraction. Circulation. 2018;138:198–205. doi:10.1161/CIRCULATIONAHA.118.034271
- Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the american society of echocardiography and the european association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321–1360. doi:10.1016/j.echo.2016.01.011
- Upadhya B, Kitzman DW. Heart failure with preserved ejection fraction: new approaches to diagnosis and management. Clin Cardiol. 2019. doi:10.1002/clc.23321
- Cikes M, Solomon SD. Beyond ejection fraction: an integrative approach for assessment of cardiac structure and function in heart failure. Eur Heart J. 2016;37:1642‐1650. doi:10.1093/eurheartj/ehv510
- Gonzalez JA, Kramer CM. Role of imaging techniques for diagnosis, prognosis and management of heart failure patients: cardiac magnetic resonance. Curr Heart Fail Rep. 2015;12(4):276–283. doi:10.1007/s11897-015-0261-9
- Moon JC, Messroghli DR, Kellman P, et al. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR working group of the european society of cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15:92. doi:10.1186/1532-429X-15-92
- Hundley WG, Bluemke DA, Finn JP, et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the american college of cardiology foundation task force on expert consensus documents. Circulation. 2010;121(22):2462–2508. doi:10.1161/CIR.0b013e3181d44a8f
- Kraigher-Krainer E, Shah AM, Gupta DK, et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63(5):447‐456. doi:10.1016/j.jacc.2013.09.052
- Ito H, Ishida M, Makino W, et al. Cardiovascular magnetic resonance feature tracking for characterization of patients with heart failure with preserved ejection fraction: correlation of global longitudinal strain with invasive diastolic functional indices. J Cardiovasc Magn Reson. 2020;22(1):42. doi:10.1186/s12968-020-00636-w
- Leng S, Tan RS, Zhao X, Allen JC, Koh AS, Zhong L. Validation of a rapid semi-automated method to assess left atrial longitudinal phasic strains on cine cardiovascular magnetic resonance imaging. J Cardiovasc Magn Reson. 2018;20(1):71. doi:10.1186/s12968-018-0496-1
- Leng S, Tan RS, Zhao X, Allen JC, Koh AS, Zhong L. Fast long-axis strain: a simple, automatic approach for assessing left ventricular longitudinal function with cine cardiovascular magnetic resonance. Eur Radiol. 2020. doi:10.1007/s00330-020-06744-6
- Schuster A, Hor KN, Kowallick JT, Beerbaum P, Kutty S. Cardiovascular magnetic resonance myocardial feature tracking: concepts and clinical applications. Circ Cardiovasc Imaging. 2016;9(4):e004077. doi:10.1161/CIRCIMAGING.115.004077
- Barandiarán Aizpurua A, Sanders-van Wijk S, Brunner-la Rocca HP, et al. Validation of the HFA-PEFF score for the diagnosis of heart failure with preserved ejection fraction. Eur J Heart Fail. 2020;22(3):413–421. doi:10.1002/ejhf.1614
- Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA, Simple A. Evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138(9):861‐870. doi:10.1161/CIRCULATIONAHA.118.034646
- Segar MW, Patel KV, Berry JD, Grodin JL, Pandey A. Generalizability and implications of the H2FPEF score in a cohort of patients with heart failure with preserved ejection fraction. Circulation. 2019;139(15):1851‐1853. doi:10.1161/CIRCULATIONAHA.118.039051
- Myhre PL, Vaduganathan M, Claggett BL, et al. Application of the H2 FPEF score to a global clinical trial of patients with heart failure with preserved ejection fraction: the TOPCAT trial. Eur J Heart Fail. 2019;21(10):1288‐1291. doi:10.1002/ejhf.1542
- Suzuki S, Kaikita K, Yamamoto E, et al. H2 FPEF score for predicting future heart failure in stable outpatients with cardiovascular risk factors. ESC Heart Fail. 2020;7(1):65–74. doi:10.1002/ehf2.12570
- Takahari K, Hidaka T, Ueda Y, et al. H2FPEF score for the prediction of exercise intolerance and abnormal hemodynamics in japanese- evaluation by exercise stress echocardiography combined with cardiopulmonary exercise testing. Circ J. 2019;83(12):2487–2493. doi:10.1253/circj.CJ-19-0699
- Del Buono MG, Iannaccone G, Scacciavillani R, et al. Heart failure with preserved ejection fraction diagnosis and treatment: an updated review of the evidence. Prog Cardiovasc Dis. 2020;S0033-0620(20):30083–30089. doi:10.1016/j.pcad.2020.04.011
- Jain CC, Borlaug BA. Performance and interpretation of invasive hemodynamic exercise testing. Chest. 2020;S0012–3692(20):31617–31620. doi:10.1016/j.chest.2020.05.552
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi:10.1016/j.jacc.2013.05.019
- Burgess MI, Jenkins C, Sharman JE, et al. Diastolic stress echocardiography: hemodynamic validation and clinical significance of estimation of ventricular filling pressure with exercise. J Am Coll Cardiol. 2006;47(9):1891–1900. doi:10.1016/j.jacc.2006.02.042
- Borlaug BA, Nishimura RA, Sorajja P, et al. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. doi:10.1161/CIRCHEARTFAILURE.109.930701
- Flachskampf F, Biering-Sørensen T, Solomon S, et al. Cardiac imaging to evaluate left ventricular diastolic function. JACC Cardiovasc Imaging. 2015;8:1071–1093. doi:10.1016/j.jcmg.2015.07.004
- Hammoudi N, Laveau F, Helft G, et al. Low level exercise echocardiography helps diagnose early stage heart failure with preserved ejection fraction: a study of echocardiography versus catheterization. Clin Res Cardiol. 2017;106(3):192–201. doi:10.1007/s00392-016-1039-0
- Obokata M, Kane GC, Reddy YN, et al. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasive-echocardiographic study. Circulation. 2017;135:825–838. doi:10.1161/CIRCULATIONAHA.116.024822
- Reddy YNV, Carter RE, Obokata M, et al. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138:861–870. doi:10.1161/CIRCULATIONAHA.118.034646
- Lundberg A, Johnson J, Hage C, et al. Left atrial strain improves estimation of filling pressures in heart failure: a simultaneous echocardiographic and invasive haemodynamic study. Clin Res Cardiol. 2018. doi:10.1007/s00392-018-1399-8
- Kasner M, Sinning D, Lober J, et al. Heterogeneous responses of systolic and diastolic left ventricular function to exercise in patients with heart failure and preserved ejection fraction. ESC Heart Failure. 2015;2:121–132. doi:10.1002/ehf2.12049
- Lancellotti P, Pellikka PA, Budts W, et al. The clinical use of stress echocardiography in non-ischaemic heart disease: recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2016;17:1191–1229. doi:10.1093/ehjci/jew190
- Erdei T, Smiseth OA, Marino P, et al. A systematic review of diastolic stress test in heart failure with preserved ejection fraction, with proposals from EU-FP7 MEDIA study group. Eur J Heart Fail. 2014;16:1345–1361. doi:10.1002/ejhf.184
- Sugimoto T, Bandera F, Generati G, et al. Left atrial dynamics during exercise in mitral regurgitation of primary and secondary origin: pathophysiological insights by exercise echocardiography combined with gas exchange analysis. JACC Cardiovasc Imaging. 2019;S1936-878X(19):30162–30167. doi:10.1016/j.jcmg.2018.12.031
- Morris DA, Takeuchi M, Nakatani S, et al. Lower limit of normality and clinical relevance of left ventricular early diastolic strain rate for the detection of left ventricular diastolic dysfunction. Eur Heart J Cardiovasc Imaging. 2018;19(8):905–915. doi:10.1093/ehjci/jex185
- Nagueh SF, Chang SM, Nabi F, et al. Cardiac imaging in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging. 2017;10:e006547.
- Belyavskiy E, Morris DA, Url-Michitsch M, et al. Diastolic stress test echocardiography in patients with suspected heart failure with preserved ejection fraction: a pilot study. ESC Heart Fail. 2019;6(1):146–153. doi:10.1002/ehf2.12375
- Chen SM, He R, Li WH, et al. Relationship between exercise induced elevation of left ventricular filling pressure and exercise intolerance in patients with atrial fibrillation. J Geriatr Cardiol. 2016;13:546–551. doi:10.11909/j.issn.1671-5411.2016.06.016
- Morris DA, Boldt LH, Eichstädt H, Ozcelik C, Haverkamp W. Myocardial systolic and diastolic performance derived by 2-dimensional speckle tracking echocardiography in heart failure with normal left ventricular ejection fraction. Circ Heart Fail. 2012;5(5):610‐620. doi:10.1161/CIRCHEARTFAILURE.112.966564
- Morris DA, Ma XX, Belyavskiy E, et al. Left ventricular longitudinal systolic function analysed by 2D speckle-tracking echocardiography in heart failure with preserved ejection fraction: a meta-analysis. Open Heart. 2017;4(2):e000630. doi:10.1136/openhrt-2017000630
- Zhong L, Poh KK, Lee LC, Le TT, Tan RS. Attenuation of stress-based ventricular contractility in patients with heart failure and normal ejection fraction. Ann Acad Med Singapore. 2011;40:179‐185.
- Zhong L, Ng KK, Sim LL, et al. Myocardial contractile dysfunction associated with increased 3-month and 1-year mortality in hospitalized patients with heart failure and preserved ejection fraction. Int J Cardiol. 2013;168(3):1975‐1983. doi:10.1016/j.ijcard.2012.12.084
- Obokata M, Reddy YNV, Borlaug BA. Diastolic dysfunction and heart failure with preserved ejection fraction: understanding mechanisms by using noninvasive methods. JACC Cardiovasc Imaging. 2020;13(1 Pt 2):245‐257. doi:10.1016/j.jcmg.2018.12.034
- Telles F, Marwick TH. Imaging and management of heart failure and preserved ejection fraction. Curr Treat Options Cardiovasc Med. 2018;20(11):90. doi:10.1007/s11936-018-0689-9
- Borlaug BA, Lam CS, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54(5):410‐418. doi:10.1016/j.jacc.2009.05.013
- Shah AM, Claggett B, Sweitzer NK, et al. Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation. 2015;132:402–414. doi:10.1161/CIRCULATIONAHA.115.015884
- Morris DA, Gailani M, Vaz Pérez A, et al. Left atrial systolic and diastolic dysfunction in heart failure with normal left ventricular ejection fraction. J Am Soc Echocardiogr. 2011;24(6):651–662. doi:10.1016/j.echo.2011.02.004
- Freed BH, Daruwalla V, Cheng JY, et al. Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction: importance of left atrial strain. Circ Cardiovasc Imaging. 2016;9(3):e003754. doi:10.1161/CIRCIMAGING.115.003754
- Morris DA, Belyavskiy E, Aravind-Kumar R, et al. Potential usefulness and clinical relevance of adding left atrial strain to left atrial volume index in the detection of left ventricular diastolic dysfunction. JACC Cardiovasc Imaging. 2018;11(10):1405–1415. doi:10.1016/j.jcmg.2017.07.029
- Braunauer K, Pieske-Kraigher E, Belyavskiy E, et al. Early detection of cardiac alterations by left atrial strain in patients with risk for cardiac abnormalities with preserved left ventricular systolic and diastolic function. Int J Cardiovasc Imaging. 2018;34(5):701–711. doi:10.1007/s10554-017-1280-2
- Santos AB, Kraigher-Krainer E, Gupta DK, et al. Impaired left atrial function in heart failure with preserved ejection fraction. Eur J Heart Fail. 2014;16(10):1096–1103. doi:10.1002/ejhf.147
- Santos AB, Roca GQ, Claggett B, et al. Prognostic relevance of left atrial dysfunction in heart failure with preserved ejection fraction. Circ Heart Fail. 2016;9(4):e002763. doi:10.1161/CIRCHEARTFAILURE.115.002763
- Melenovsky V, Borlaug BA, Rosen B, et al. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49(2):198–207. doi:10.1016/j.jacc.2006.08.050
- Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. 2015;8(2):295‐303. doi:10.1161/CIRCHEARTFAILURE.114.001667
- Mandoli GE, Sisti N, Mondillo S, Cameli M. Left atrial strain in left ventricular diastolic dysfunction: have we finally found the missing piece of the puzzle? Heart Fail Rev. 2020;25(3):409–417. doi:10.1007/s10741-019-09889-9
- Khan MS, Memon MM, Murad MH, et al. Left atrial function in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Eur J Heart Fail. 2020;22(3):472‐485. doi:10.1002/ejhf.1643
- Magaña-Serrano JA, Almahmeed W, Gomez E, et al. Prevalence of heart failure with preserved ejection fraction in Latin American, Middle Eastern, and North African Regions in the I PREFER study (Identification of Patients With Heart Failure and PREserved Systolic Function: an epidemiological regional study). Am J Cardiol. 2011;108(9):1289‐1296. doi:10.1016/j.amjcard.2011.06.044
- Pfeffer MA, Shah AM, Borlaug BA. Heart failure with preserved ejection fraction in perspective. Circ Res. 2019;124(11):1598‐1617. doi:10.1161/CIRCRESAHA.119.313572
- Brown DW, Haldeman GA, Croft JB, Giles WH, Mensah GA. Racial or ethnic differences in hospitalization for heart failure among elderly adults: medicare, 1990 to 2000. Am Heart J. 2005;150(3):448–454. doi:10.1016/j.ahj.2004.11.010
- Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168(19):2138‐2145. doi:10.1001/archinte.168.19.2138
- Okin PM, Kjeldsen SE, Dahlöf B, Devereux RB. Racial differences in incident heart failure during antihypertensive therapy. Circ Cardiovasc Qual Outcomes. 2011;4(2):157‐164. doi:10.1161/CIRCOUTCOMES.110.960112
- Eaton CB, Abdulbaki AM, Margolis KL, et al. Racial and ethnic differences in incident hospitalized heart failure in postmenopausal women: the Women’s Health Initiative. Circulation. 2012;126(6):688‐696. doi:10.1161/CIRCULATIONAHA.111.066688
- Dries DL, Exner DV, Gersh BJ, Cooper HA, Carson PE, Domanski MJ. Racial differences in the outcome of left ventricular dysfunction. N Engl J Med. 1999;340(8):609‐616. doi:10.1056/NEJM199902253400804
- East MA, Peterson ED, Shaw LK, Gattis WA, O’Connor CM. Racial differences in the outcomes of patients with diastolic heart failure. Am Heart J. 2004;148(1):151‐156. doi:10.1016/j.ahj.2004.01.017
- Gupta DK, Shah AM, Castagno D, et al. Heart failure with preserved ejection fraction in African Americans: the ARIC (Atherosclerosis Risk In Communities) study. JACC Heart Fail. 2013;1(2):156‐163. doi:10.1016/j.jchf.2013.01.003
- Tromp J, Teng TH, Tay WT, et al. Heart failure with preserved ejection fraction in Asia. Eur J Heart Fail. 2019;21(1):23‐36. doi:10.1002/ejhf.1227
- Garcia M, Mulvagh SL, Merz CN, Buring JE, Manson JE. Cardiovascular disease in women: clinical perspectives. Circ Res. 2016;118(8):1273‐1293. doi:10.1161/CIRCRESAHA.116.307547
- Tadic M, Cuspidi C, Plein S, Belyavskiy E, Heinzel F, Galderisi M. Sex and heart failure with preserved ejection fraction: from pathophysiology to clinical studies. J Clin Med. 2019;8(6):792. doi:10.3390/jcm8060792
- Devereux RB, Roman MJ, Liu JE, et al. Congestive heart failure despite normal left ventricular systolic function in a population-based sample: the Strong Heart Study. Am J Cardiol. 2000;86(10):1090‐1096. doi:10.1016/s0002-9149(00)01165-6
- Lundorff IJ, Sengeløv M, Godsk Jørgensen P, et al. Echocardiographic predictors of mortality in women with heart failure with reduced ejection fraction. Circ Cardiovasc Imaging. 2018;11(11):e008031. doi:10.1161/CIRCIMAGING.118.008031
- Lam CSP, Arnott C, Beale AL, et al. Sex differences in heart failure. Eur Heart J. 2019;40(47):3859‐3868c. doi:10.1093/eurheartj/ehz835
- Wolsk E, Kaye D, Komtebedde J, et al. Central and peripheral determinants of exercise capacity in heart failure patients with preserved ejection fraction. JACC Heart Fail. 2019;7(4):321‐332. doi:10.1016/j.jchf.2019.01.006
- Packer M, Lam CSP, Lund LH, Maurer MS, Borlaug BA. Characterization of the inflammatory-metabolic phenotype of heart failure and a preserved ejection fraction: a hypothesis to explain influence of sex on the evolution and potential treatment of the disease. Eur J Heart Fail. 2020. doi:10.1002/ejhf.1902
- Donal E, Lund LH, Oger E; KaRen Investigators, et al. Importance of combined left atrial size and estimated pulmonary pressure for clinical outcome in patients presenting with heart failure with preserved ejection fraction. Eur Heart J Cardiovasc Imaging. 2017;18:629–635. doi:10.1093/ehjci/jex005
- Yusuf S, Pfeffer MA, Swedberg K; CHARM Investigators and Committees, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-preserved trial. Lancet. 2003;362:777–781. doi:10.1016/S0140-6736(03)14285-7
- Massie BM, Carson PE, McMurray JJ, et al. I-PRESERVE Investigators Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359(23):2456–2467. doi:10.1056/NEJMoa0805450
- Cleland JGF, Tendera M, Adamus J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345. doi:10.1056/NEJMoa0805450
- Flather MD, Shibata MC, Coats AJS; SENIORS Investigators, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005;26:215–225. doi:10.1093/eurheartj/ehi115
- Mulder BA, van Veldhuisen DJ, Crijns HJGM, et al. Effect of nebivolol on outcome in elderly patients with heart failure and atrial fibrillation: insights from SENIORS. Eur J Heart Fail. 2012;14:1171–1178. doi:10.1093/eurjhf/hfs100
- van Veldhuisen DJ, Cohen-Solal A, Böhm M, et al. Beta-blockade with nebivolol in elderly heart failure patients with impaired and preserved left ventricular ejection fraction. J Am Coll Cardiol. 2009;53:2150–2158. doi:10.1016/j.jacc.2009.02.046
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18(11):1609–1678. doi:10.1093/europace/euw295
- Rattka M, Pott A, Kühberger A, et al. Restoration of sinus rhythm by pulmonary vein isolation improves heart failure with preserved ejection fraction in atrial fibrillation patients. Europace. 2020:euaa101. doi:10.1093/europace/euaa101.
- Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321(13):1261–1274. doi:10.1001/jama.2019.0693
- Obokata M, Reddy YNV, Melenovsky V, et al. Myocardial injury and cardiac reserve in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2018;72(1):29–40. doi:10.1016/j.jacc.2018.04.039
- Hwang SJ, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63(25Pt A):2817–2827. doi:10.1016/j.jacc.2014.03.034
- Ahmed A, Rich MW, Fleg JL, et al. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation. 2006;114(5):397–403. doi:10.1161/CIRCULATIONAHA.106.628347
- Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi:10.1056/NEJMoa1313731
- Morris JH, Chen L. Exercise training and heart failure: a review of the literature. Card Fail Rev. 2019;5:57–61. doi:10.15420/cfr.2018.31.1
- Pandey A, Parashar A, Kumbhani D, et al. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail. 2015;8:33–40. doi:10.1161/CIRCHEARTFAILURE.114.001615
- Solomon SD, JJV M, Anand IS, et al. Angiotensin–neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–1620. doi:10.1056/NEJMoa1908655
- Nassif ME, Kosiborod M. Effects of sodium glucose cotransporter type 2 inhibitors on heart failure. Diabetes Obes Metab. 2019;Suppl 2(S2):19–23. doi:10.1111/dom.13678
- Anker SD, Butler J, Filippatos GS, et al. Evaluation of the effects of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: rationale for and design of the EMPEROR-preserved trial. Eur J Heart Fail. 2019;21:1279–1287. doi:10.1002/ejhf.1596
- Wintrich J, Kindermann I, Ukena C, et al. Therapeutic approaches in heart failure with preserved ejection fraction: past, present, and future [published online ahead of print, 2020 Mar 31]. Clin Res Cardiol. 2020. doi:10.1007/s00392-020-01633-w
- Maggioni AP, Dahlström U, Filippatos G, et al. EURObservational research programme: regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot). Eur J Heart Fail. 2013;15(7):808–817. doi:10.1093/eurjhf/hft050
- Sandhu AT, Heidenreich PA. Heart failure management with ambulatory pulmonary artery pressure monitoring. Trends Cardiovasc Med. 2018;28(3):212–219. doi:10.1016/j.tcm.2017.09.002
- Abraham WT, Perl L. Implantable hemodynamic monitoring for heart failure patients. J Am Coll Cardiol. 2017;70(3):389–398. doi:10.1016/j.jacc.2017.05.052
- Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377(9766):658–666. doi:10.1016/S0140-6736(11)60101-3
- Abraham WT, Stevenson LW, Bourge RC, et al. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the CHAMPION randomised trial. Lancet. 2016;387(10017):453–461. doi:10.1016/S0140-6736(15)00723-0
- Adamson PB, Abraham WT, Bourge RC, et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. 2014;7(6):935–944. doi:10.1161/CIRCHEARTFAILURE.113.001229
- Lindenfeld J, Abraham WT, Maisel A, et al. Hemodynamic-GUIDEd management of Heart Failure (GUIDE-HF). Am Heart J. 2019;214:18–27. doi:10.1016/j.ahj.2019.04.014
- Hasenfuß G, Hayward C, Burkhoff D, et al. A transcatheter intracardiac shunt device for heart failure with preserved ejection fraction (REDUCE LAP-HF): a multicentre, open-label, single-arm, Phase 1 trial. Lancet. 2016;387(10025):1298–1304. doi:10.1016/S0140-6736(16)00704-2
- Feldman T, Komtebedde J, Burkhoff D, et al. Transcatheter interatrial shunt device for the treatment of heart failure: rationale and design of the randomized trial to REDUCE Elevated Left Atrial Pressure in Heart Failure (REDUCE LAP-HF I). Circ Heart Fail. 2016;9(7):e003025. doi:10.1161/CIRCHEARTFAILURE.116.003025
- Shah SJ, Feldman T, Ricciardi MJ, et al. One-year safety and clinical outcomes of a transcatheter interatrial shunt device for the treatment of heart failure with preserved ejection fraction in the Reduce Elevated Left Atrial Pressure in Patients With Heart Failure (REDUCE LAP-HF I) trial: a randomized clinical trial. JAMA Cardiol. 2018;3(10):968–977. doi:10.1001/jamacardio.2018.2936
- Obokata M, Reddy Yogesh NV, Shah SJ, et al. Effects of interatrial shunt on pulmonary vascular function in heartfailure with preserved ejection fraction. J Am Coll Cardiol. 2019;74(21):2539–2550. doi:10.1016/j.jacc.2019.08.1062
- Kaye DM, Petrie MC, McKenzie S, et al. Impact of an interatrial shunt device on survival and heart failure hospitalization in patients with preserved ejection fraction. ESC Heart Fail. 2019;6(1):62–69. doi:10.1002/ehf2.12350
- Kaye DM, Nanayakkara S. Interatrial shunt device for heart failure with preserved ejection fraction. Front Cardiovasc Med. 2019;6:143. doi:10.3389/fcvm.2019.00143
- Mahfoud F, Lüscher TF, Andersson B, et al. Expert consensus document from the European Society of Cardiology on catheter-based renal denervation. Eur Heart J. 2013;34(28):2149–2157. doi:10.1093/eurheartj/eht154
- Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373(9671):1275–1281. doi:10.1016/S0140-6736(09)60566-3
- Donazzan L, Mahfoud F, Ewen S, et al. Effects of catheter-based renal denervation on cardiac sympathetic activity and innervation in patients with resistant hypertension. Clin Res Cardiol. 2016;105(4):364–371. doi:10.1007/s00392-015-0930-4
- Brandt MC, Mahfoud F, Reda S, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol. 2012;59(10):901–909. doi:10.1016/j.jacc.2011.11.034
- Mahfoud F, Urban D, Teller D, et al. Effect of renal denervation on left ventricular mass and function in patients with resistant hypertension: data from a multi-centre cardiovascular magnetic resonance imaging trial. Eur Heart J. 2014;35(33):2224–31b. doi:10.1093/eurheartj/ehu093
- Schirmer SH, Sayed MM, Reil JC, et al. Atrial remodeling following catheter-based renal denervation occurs in a blood pressure- and heart rate-independent manner. JACC Cardiovasc Interv. 2015;8(7):972–980. doi:10.1016/j.jcin.2015.02.014
- Mahfoud F, Böhm M, Azizi M, et al. Proceedings from the European clinical consensus conference for renal denervation: considerations on future clinical trial design. Eur Heart J. 2015;36(33):2219–2227. doi:10.1093/eurheartj/ehv192
- Patel HC, Rosen SD, Hayward C, et al. Renal denervation in heart failure with preserved ejection fraction (RDT-PEF): a randomized controlled trial. Eur J Heart Fail. 2016;18(6):703–712. doi:10.1002/ejhf.502
- Bhatt DL, Kandzari DE, O’Neill WW, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393–1401. doi:10.1056/NEJMoa1402670
- Kandzari DE, Böhm M, Mahfoud F, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391(10137):2346–2355. doi:10.1016/S0140-6736(18)30951-6
- Böhm M, Kario K, Kandzari DE, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395(10234):1444–1451. doi:10.1016/S0140-6736(20)30554-7
- Weber MA, Mahfoud F, Schmieder RE, et al. Renal denervation for treating hypertension: current scientific and clinical evidence. JACC Cardiovasc Interv. 2019;12(12):1095–1105. doi:10.1016/j.jcin.2019.02.050
- Shah SJ, Katz DH, Selvaraj S, et al. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131(3):269–279. doi:10.1161/CIRCULATIONAHA.114.010637
- Obokata M, Reddy YNV, Borlaug BA. The role of echocardiography in heart failure with preserved ejection fraction: what do we want from imaging? Heart Fail Clin. 2019;15(2):241–256. doi:10.1016/j.hfc.2018.12.004
- Harada T, Obokata M. Obesity-related heart failure with preserved ejection fraction: pathophysiology, diagnosis, and potential therapies. Heart Fail Clin. 2020;16(3):357–368. doi:10.1016/j.hfc.2020.02.004
- Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136(1):6–19. doi:10.1161/CIRCULATIONAHA.116.026807
- Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53(13):1119–1126. doi:10.1016/j.jacc.2008.11.051
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67–119. doi:10.1093/eurheartj/ehv317
- Vachiéry JL, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53:1801897. doi:10.1183/13993003.01897-2018
- Melenovsky V, Hwang SJ, Lin G, et al. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3452–3462. doi:10.1093/eurheartj/ehu193
- Gorter TM, van Veldhuisen DJ, Bauersachs J, et al. Right heart dysfunction and failure in heart failure with preserved ejection fraction: mechanisms and management. Position statement on behalf of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20(1):16–37. doi:10.1002/ejhf.1029
- Hoeper MM, Lam CSP, Vachiery JL, et al. Pulmonary hypertension in heart failure with preserved ejection fraction: a plea for proper phenotyping and further research. Eur Heart J. 2017;38(38):2869–2873. doi:10.1093/eurheartj/ehw597
- Borlaug BA, Obokata M. Is it time to recognize a new phenotype? Heart failure with preserved ejection fraction with pulmonary vascular disease. Eur Heart J. 2017;38(38):2874–2878. doi:10.1093/eurheartj/ehx184
- Gorter TM, Obokata M, Reddy YNV, Melenovsky V, Borlaug BA. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J. 2018;39(30):2825–2835. doi:10.1093/eurheartj/ehy331
