Abstract
Purpose
Treatment options appear lacking for patients with epileptic seizure-induced shoulder dislocations who are not candidates for shoulder and focus resection surgeries. To reduce shoulder joint dislocations caused by epileptic seizures and simultaneously reduce the frequency and intensity of seizures, we performed corpus callosotomy for two patients with medically intractable epilepsy that induced repetitive shoulder joint dislocations.
Materials and methods
A 21-year-old man (Patient 1) with bilateral temporal lobe epilepsy [Focal onset impaired awareness seizure (FIAS), 1/month; focal to bilateral tonic-clonic seizure (BTCS), 1/2–3 months], autism and intellectual disorder and a 34-year-old man (Patient 2) with left multi-lobar epilepsy (BTCS, 3–4/month; status epilepticus, 1/2–3 months), autism and intellectual disorder had suffered from repetitive seizure-induced shoulder dislocations (1/2–3 months for Patient 1; 3–4/month for Patient 2). Due to frequent seizures and uncooperativeness, they were not candidates for shoulder joint dislocation surgery. They were also not candidates for focus resection surgery due to multiple foci and uncooperativeness for invasive monitoring. We performed corpus callosotomy for both patients.
Results
Postoperatively, frequencies of both shoulder dislocations (2 in 5 years of follow-up for Patient 1; 1 in 5 months of follow-up for Patient 2) and epileptic seizures were drastically reduced.
Conclusions
For patients who are not candidates for focus resection and shoulder joint surgeries but who suffer from frequent shoulder joint dislocations, corpus callosotomy could be a treatment of last resort.
Introduction
Epileptic seizure has been reported to cause shoulder joint dislocation.Citation1 When a convulsing patient in a lateral decubitus position places their body weight on a fixed arm, the excessive force applied to the shoulder can result in dislocation.Citation2 This may lead to significant bone loss from the glenoid and humeral head, creating a situation in which repetitive shoulder dislocations may occur.Citation3 Generally, procedures such as Bankart repair or the Bristow procedure are considered for the treatment of shoulder joint dislocation.Citation4,Citation5 However, surgical strategies for shoulder joint dislocation require post-operative rehabilitation. In addition, these regimens require prevention of dislocation for a certain period of time.Citation6,Citation7 Patients with medically refractory epilepsy and intellectual disability and/or autism are predicted to be uncooperative in terms of postoperative treatment. Moreover, intellectual disorder is one of the risk factors for surgical site infection.Citation8 Surgical intervention for shoulder joint dislocation thus might not be a treatment option for such patients. Although many reports have described shoulder joint dislocation caused by epileptic seizures, little has been discussed regarding solutions.Citation2,Citation9 As repetitive shoulder joint dislocations are considered to be underestimated,Citation2,Citation3,Citation10 patients with epilepsy may frequently experience shoulder joint dislocations with severe pain and visit emergency rooms on every occasion simply for manual repositioning. However, longer-term treatment options appear lacking for patients with epileptic seizure-induced shoulder joint dislocations who are not candidates for shoulder and focus resection surgeries.
Patients with generalized seizure could be candidates for corpus callosotomy (CC).Citation11 This procedure reduces generalized tonic-clonic seizures and might turn them into focal onset seizures.Citation12
We encountered two patients with repetitive shoulder joint dislocation due to medically refractory bilateral tonic-clonic seizures (BTCS) who were not considered candidates for shoulder surgery due to possible postoperative uncooperativeness and frequent BTCS. We hypothesized that CC might reduce the frequency and intensity of BTCS along with the frequency of shoulder joint dislocations.
Case series
Case 1
Patient 1 was a 21-year-old, right-handed man with bilateral temporal lobe epilepsy, autism and intellectual disorder (Tanaka-Binet intelligence scale: 7 years 1 month old). He exhibited focal onset impaired awareness seizures (FIAS) and focal to BTCSs at 8 years old, both of which proved medically intractable (FIAS, 1/month; BTCS, 1/2–3 months). Electroencephalography (EEG) showed epileptiform discharges over bilateral temporal regions. Brain magnetic resonance imaging (MRI) showed no abnormalities except for relative atrophy for his age. He was currently on lacosamide (LCM) and levetiracetam (LEV). He had previously been administered topiramate, carbamazepine and lamotrigine. He had undergone vagus nerve stimulation therapy that had not prevented BTCS and had suffered frequent shoulder joint dislocations.
Case 2
Patient 2 was a 34-year-old, right-handed man with left multi-lobar epilepsy, autism and intellectual disorder (Tanaka-Binet intelligence scale, 5 years 10 months old) who exhibited BTCS at 29 years old. At first, since seizures were brief and appeared on an annual basis, he had not been on any medications. However, as seizures exacerbated in intensity and frequency, LEV was started. As seizures finally reached daily frequency, LCM was added. However, seizures were medically intractable, and he also had started exhibiting status epilepticus (SE). EEG for Patient 2 showed independent epileptiform discharges over bilateral frontal, temporal and parietal lobes, with a left predominance.
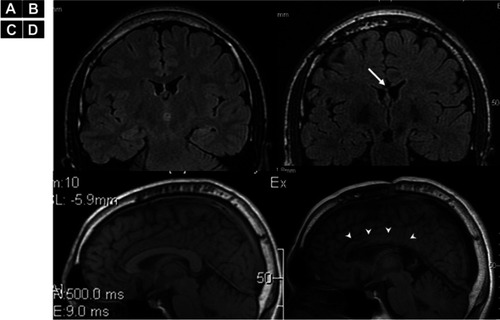
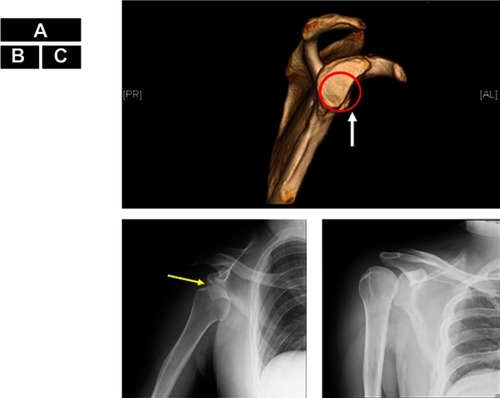
As seizures in both patients caused right shoulder dislocation every time, they had visited the emergency room for shoulder repositioning, sometimes requiring general anesthesia (). Their orthopedic surgeons did not regard either patient as a candidate for shoulder surgery due to the relatively high frequency of seizures and likely uncooperativeness after shoulder surgery. Discussions regarding epilepsy surgery were held in our case conferences, where the patients were considered unsuitable for focus resection surgery due to the multiple foci and predicted uncooperativeness with invasive monitoring. To reduce BTCS in both patients and SE in Patient 2, we performed total CC for Patient 1 () and anterior callosotomy for Patient 2, as these procedures were considered to have the possibility of preventing shoulder dislocation. The reason for anterior callosotomy in Patient 2 was simply the preference of patient’s family, as they were concerned about the risks of disconnection syndrome.Citation13 The etiology of epilepsy was unclear in both patients. However, as both were patients with autism spectrum disorder and epilepsy, shared underlying neurobiological mechanisms might have been involved,Citation14 such as genetic issuesCitation15 or mammalian target of the rapamycin pathway.Citation16
Figure 1 Shoulder dislocation in Patient 2. A normal glenoid cavity conforms to the red circle. The anterior edge of the glenoid cavity is straightened (white arrow) indicating bone defect, at the 2:00–6:00 position (A). The humeral head (yellow arrow) is displaced downward (B). The humeral head has been repositioned and returned to its original position (C).
Results
After the CC, FIAS remained in both patients, but BTCS became rare and SE disappeared in Patient 2, even though we did not change any medications post-operatively. Right after the callosotomy, intra-operative electrocorticography (ECoG) strip electrodes in bilateral frontal cortices showed reductions in epileptiform discharges for both patients. Generalized discharges were only seen in Patient 2, and were drastically reduced and lateralized to the left hemisphere (). Clinically, seizure frequency and intensity were reduced, but seizure semiology itself did not change after the callosotomy. As of the time of writing, Patient 1 has experienced only three BTCS events with two shoulder joint dislocations within the 5 years after CC, whereas previously he had experienced shoulder dislocations once every 2–3 months. Patient 2 has experienced no BTCS for the 5 months since CC, although one spontaneous shoulder joint dislocation was seen within the same period; previously he had experienced shoulder dislocations 3–4 times/month (). Before CC, even though both patients had used shoulder-immobilizing braces, BTCSs had induced shoulder dislocation. After CC, immobilization therapy using shoulder immobilizer braces allowed each patient to maintain their shoulder in the correct position due to the reduced occurrence of BTCSs. Both were also able to perform range of motion and muscle-strengthening exercises, which required shoulder joint immobilization for at least several weeks. No neurological abnormalities were seen except for transient alien’s hand in Patient 1 and transient mutism in both patients.
Figure 3 Electro Corticography (ECoG) of Pre- and post-corpus callosotomy for Patient 2. (A) Pre-corpus callosotomy ECoG monitoring from bilateral frontal lobe cortices shows generalized poly-spikes. (B) Post-corpus callosotomy ECoG shows reduced generalized poly-spikes lateralized to the left hemisphere. The epileptiform discharges on the right side are eleminated (arrow). As scalp EEG showed epileptiform discharges predominantly in the left hemisphere, we used an 8-contact strip on the left hemisphere and a 6-contact strip on the right hemisphere.
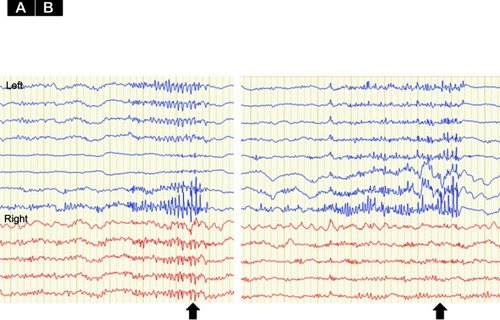
Table 1 Clinical information and data between pre- and post-callosotomy
Discussion
As both patients obtained reductions in seizure frequency and intensity, the occurrence of shoulder joint dislocation was reduced. Patients with medically intractable epilepsy currently benefit from epilepsy focus resection surgery. However, if severe intellectual disorders are present, both epilepsy focus resection surgery and shoulder joint surgery may become much more difficult. These patients thus need to attend the emergency room for shoulder repositioning, which could reduce quality of life for both patients and caregivers. Shoulder joint dislocation caused by epileptic seizures is said to be underestimated.Citation2,Citation3,Citation10 Many patients suffer from shoulder joint dislocation due to frequent seizures without any treatments being suggested. For patients who are unsuitable for shoulder and focus resection surgeries and who suffer from frequent shoulder dislocation, CC could offer a good treatment of last resort.
Conclusion
For patients who are not candidates for focus resection or shoulder-repositioning surgeries and who are suffering from frequent shoulder dislocations, corpus callosotomy could represent a good last-resort treatment.
Ethics approval
The parents of the patients who were not considered capable of providing legal consent, provided written informed consent for publication of these case details. The ethics committee at Seirei Hamamatsu General Hospital approved this study.
Author contributions
Neurosurgical operation: AF, NI. Acquisition of data: TO, KS and AF. Analysis and interpretation of data: AF, TO, and HE. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgement
No funding was received for this case series. We are grateful to Dr. Masayuki Abe from the Department of Orthopedic Surgery for advice regarding shoulder dislocation and manuscript preparation.
Disclosure
The authors report no conflicts of interest in this work.
References
- Buhler M, Gerber C. Shoulder instability related to epileptic seizures. J Shoulder Elbow Surg. 2002;11(4):339–344. doi:10.1067/mse.2002.12452412195251
- DeToledo JC, Lowe MR. Seizures, lateral decubitus, aspiration, and shoulder dislocation: time to change the guidelines? Neurology. 2001;56(3):290–291. doi:10.1212/wnl.56.3.29011235662
- Thangarajah T, Lambert S. The management of recurrent shoulder instability in patients with epilepsy: a 15-year experience. J Shoulder Elbow Surg. 2015;24(11):1723–1727. doi:10.1016/j.jse.2015.04.00826119633
- Donohue MA, Mauntel TC, Dickens JF. Recurrent shoulder instability after primary Bankart repair. Sports Med Arthrosc. 2017;25(3):123–130. doi:10.1097/JSA.000000000000015928777214
- Kawasaki T, Hasegawa Y, Kaketa T, et al. Midterm clinical results in rugby players treated with the Bristow procedure. Am J Sports Med. 2018;46(3):656–662. doi:10.1177/036354651774056729172635
- Givon U, Oran A, Blankstein A, Pritsch M. Use of botulinum neurotoxin injections to treat recurrent shoulder dislocations in a patient with severe epilepsy: a case report. J Bone Joint Surg Am. 2011;93(19):e112(111–113). doi:10.2106/JBJS.J.00419
- Fedorka CJ, Mulcahey MK. Recurrent anterior shoulder instability: a review of the Latarjet procedure and its postoperative rehabilitation. Phys Sportsmed. 2015;43(1):73–79. doi:10.1080/00913847.2015.100554325598036
- Toth R, Szanto P, Prodan Z, et al. Down syndrome and postoperative complications after paediatric cardiac surgery: a propensity-matched analysis. Interact Cardiovasc Thorac Surg. 2013;17(4):691–697. doi:10.1093/icvts/ivt26723832837
- Gosens T, Poels PJ, Rondhuis JJ. Posterior dislocation fractures of the shoulder in seizure disorders–two case reports and a review of literature. Seizure. 2000;9(6):446–448. doi:10.1053/seiz.2000.041810986005
- Schulz TJ, Jacobs B, Patterson RL Jr. Unrecognized dislocations of the shoulder. J Trauma. 1969;9(12):1009–1023.5358646
- Graham D, Tisdall MM, Gill D. Corpus callosotomy outcomes in pediatric patients: a systematic review. Epilepsia. 2016;57(7):1053–1068. doi:10.1111/epi.1340827237542
- Ono T, Baba H, Toda K, Ono K. Hemispheric asymmetry of callosal neuronal participation in bilaterally synchronous epileptiform discharges. Seizure. 2009;18(1):7–13. doi:10.1016/j.seizure.2008.05.00518565768
- Jea A, Vachhrajani S, Widjaja E, et al. Corpus callosotomy in children and the disconnection syndromes: a review. Childs Nerv Syst. 2008;24(6):685–692. doi:10.1007/s00381-008-0626-418373102
- Keller R, Basta R, Salerno L, Elia M. Autism, epilepsy, and synaptopathies: a not rare association. Neurol Sci. 2017;38(8):1353–1361. doi:10.1007/s10072-017-2974-x28455770
- Stafstrom CE, Benke TA. Autism and epilepsy: exploring the relationship using experimental models. Epilepsy Curr. 2015;15(4):206–210. doi:10.5698/1535-7511-15.4.20626316869
- Russo E, Follesa P, Citraro R, et al. The mTOR signaling pathway and neuronal stem/progenitor cell proliferation in the hippocampus are altered during the development of absence epilepsy in a genetic animal model. Neurol Sci. 2014;35(11):1793–1799. doi:10.1007/s10072-014-1842-124889758