Abstract
Objective
To explore the factors associated with improvement of lower urinary tract symptoms (LUTS) after transurethral plasmakinetic enucleation of the prostate (PKEP) and transurethral resection of the prostate (TURP) in patients with a small-volume prostate and bladder outlet obstruction (BOO).
Methods
The clinicopathologic data of 257 patients with BOO and a small-volume prostate from January 2013 to January 2018 were retrospectively collected preoperatively, 3 months postoperatively, and 12 months postoperatively. Patients were divided into postoperative success and failure groups based on the IPSS, IPSS-v, and IPSS-s. The relationship between each parameter and the improvement of postoperative LUTS was analyzed. Subgroup analysis was performed to compare the differences between the TURP and PKEP groups.
Results
Among patients followed up for 3 months postoperatively, multivariate analysis demonstrated that IPP, PUA, and post-PCB were significant predictors of postoperative IPSS improvement; TZI, IPP, and PUA were significant predictors of postoperative IPSS-v improvement; post-PCB and the surgical procedure were significant predictors of IPSS-s improvement; and IPP and PUA were significant predictors of postoperative Qmax improvement. Among patients followed up for 12 months postoperatively, multivariate analysis revealed that IPP, PUA, and post-PCB were significant predictors of postoperative IPSS improvement; PUA was a significant predictor of postoperative IPSS-v improvement; post-PCB was a significant predictor of IPSS-s improvement; and IPP and PUA were significant predictors of postoperative Qmax improvement. The post-PCB was significantly lower in the PKEP than the TURP group and the prostatic calculi removal rate was significantly higher in the PKEP than the TURP group.
Conclusion
Patients with a greater preoperative IPP and PUA and smaller post-PCB showed greater improvement of postoperative LUTS. PKEP might help to remove calculi from between the transitional and peripheral zones of prostate. Compared with conventional TURP, PKEP may improve the early postoperative storage symptoms of LUTS in patients with a small-volume prostate and BOO.
Introduction
Benign prostatic hyperplasia is the most common disease that causes voiding dysfunction in middle-aged and elderly men.Citation1 Transurethral resection of the prostate (TURP) is recognized as the gold standard treatment of benign prostatic hyperplasia. However, conservative medication and surgical treatments do not satisfactorily improve symptoms in patients with a small-volume prostate (<30 mL) and bladder outlet obstruction (BOO).Citation2–Citation4 In patients with a small-volume prostate, the prostate volume has been shown to be weakly correlated with lower urinary tract symptoms (LUTS); however, the morphological features of the prostate seem to be associated with LUTS.Citation5,Citation6 Therefore, we hypothesized that the morphological features of small-volume prostates can provide individualized treatment options for patients and further improve the efficacy of drugs and surgical treatments. This study was performed to investigate the predictors of surgical outcomes in patients with a small-volume prostate and BOO as well as improved postoperative LUTS in patients with BOO.
Materials And Methods
Ethics
This study was approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University and all patients provided written informed consent.
Patients
In total, 324 patients with BOO and a prostate volume of <30 mL as measured by transrectal ultrasonography were analyzed.Citation3,Citation7 All patients had LUTS, and conservative treatments (doxazosin 4mg/tamsulosin 0.2 mg once daily ± finasteride 5 mg once daily for 12 weeks) had been ineffective.Citation8–Citation10 All patients underwent TURP or transurethral plasmakinetic enucleation of the prostate (PKEP).Citation8 LUTS was evaluated using the International Prostate Symptom Score (IPSS). The following clinical data were collected 3 months preoperatively, 3 months postoperatively, and 12 months postoperatively: age, body mass index, surgical procedure, total prostate volume (TPV), transition zone volume (TZV), transition zone index (TZI, defined as TPV/TZV) (), intravesical prostatic protrusion (IPP), preoperative and postoperative prostatic calculi burden (pre-PCB and post-PCB),Citation11 maximum urinary flow rate (Qmax), preoperative residual urine, prostatic urethral angle (PUA), IPSS, IPSS voiding symptom score (IPSS-v), and IPSS storage symptom score (IPSS-s).Citation11,Citation12 The patients were divided into a successful operation group and failed operation group based on the IPSS, IPSS-v, and IPSS-s, and the correlation between each of the above parameters and the improvement of postoperative LUTS was analyzed. Moreover, the patients were divided into a TURP group and PKEP group according to the surgical procedure performed, and the difference in each of the above parameters between the two groups was compared. The inclusion criteria of this study should be all included the following:Citation8,Citation13–Citation15 ①prostate volume of ≤30 mL as measured by transrectal ultrasonography;Citation3,Citation7 ②Qmax of ≤15 mL or acute urinary retention or preoperative residual urine volume of ≥100 mL or presence of LUTS caused by benign prostatic obstruction;Citation16–Citation19 ③IPSS of >19 points; ④Preoperative cystoscopy to examine benign prostatic obstruction (BPO) and bladder trabeculation. ⑤An interest and ability to participate in this study. The exclusion criteria were as follows :Citation13,Citation20 ①postoperative pathology report for prostate cancer; ②history of diabetes; ③history of prostate tumor or bladder tumor; ④history of bladder calculi, neurogenic bladder, chronic prostatitis, urethral calculi, chronic cystitis, urethral stricture, or hypospadias; ⑤history of prostate biopsy; ⑥history of lower urinary tract surgery or pelvic radiation therapy; ⑦uncured postoperative urinary tract infection; ⑧history of medication affecting LUTS during follow-up; ⑨postoperative bladder neck stenosis. Initially, a total of 257 patients (including 111 TURP patients, 146 PKEP patients) were enrolled in this study when followed up to 3 months after surgery. However, there were increasing number of patients (including 14 TURP patients, 21 PKEP patients) receiving behavioral/physical/drug therapy due to dissatisfied postoperative outcome, who had to be excluded from this study. The rate of loss to follow-up (including 11 TURP patients, 9 PKEP patients) also gradually elevated. Finally, there were only 203 patients (including 86 TURP patients, 117 PKEP patients) at postoperative 12 months.
Surgical Procedures
TURP was performed at our center using bipolar technology as previously described.Citation21,Citation22
Plasmakinetic enucleation of the prostate (PKEP) was pioneered and promoted by Professor ChunXiao Liu and there were clear surgical images in his articles.Citation23 However, this procedure was still in the stage of “Exploration” according to The IDEAL (Idea, Development, Exploration, Assessment, Long term study) recommendations.Citation24 The technical details, indications, operator learning curves, and quality control are discussed widely until now.
The procedure at our center was performed using bipolar technology as previously described.Citation15,Citation25 All procedures were performed by a single urologist (Dr. Wei) at our center using 27 Fr continuous flow resectoscopy (Karl Storz, Tuttlingen, Germany) and the PlasmaKinetic SuperPulse System (Gyrus Medical, Cardiff, United Kingdom) with 100–120 W cutting power and 80 W coagulating power. Physiologic saline was used for irrigation. All patients were placed in the lithotomy position. The bladder neck, verumontanum, and ureteral orifices were observed first. The incision was started close to the verumontanum at the 5-o’clock and 7-o’clock positions and deep to the level of the surgical capsule. A cleavage plane between the detached lobe and the surgical capsule was created by inserting the tip of the resectoscope into the circular groove. During this time, if there were prostate stones between the adenoma and the surgical capsule, it will be exposed and removed. The entire adenoma was then spun-off 360° from the surgical capsule, remaining attached only to the bladder neck in the 6-o’clock position. Besides, the denuded blood vessels and hemorrhagic spots on the capsule surface were identified and coagulated. The devascularized adenoma was then resected into prostatic chips using the cutting loop. Finally, all fragments were extracted using an Ellik evacuator, and a 20 Fr 3-way Foley catheter was placed and connected to straight drainage until ≥7 daysCitation8 ().
Evaluation Methods
The parameters related to morphological features of the prostate in all patients who underwent transrectal ultrasonography were collected by a urologist in our center (ProSound Alpha 5 SV; Hitachi Aloka Medical, Ltd., Tokyo, Japan) from January 2013 to January 2018. TPV and TZV were calculated as follows: up–down diameter × left–right diameter × front–rear diameter × π/6. TZI was calculated as follows: TZI = TZV/TPV.Citation6 IPP was defined as the vertical distance in the sagittal plane from the base of the prostate to the tip of the prostate protruding into the bladder.Citation9,Citation19 The prostatic urethra was defined as the urethra from the base to the tip of the prostate, including the angle at the seminal colliculus. PUA was defined as the acute angle between a line from the base of the prostate to the seminal colliculus and another line from the tip of the prostate to the seminal colliculus.Citation26,Citation27 PCB was defined as the sum of the transverse diameters of all visible calculi within the prostate as measured by transrectal ultrasonography.Citation11 According to Homma et al,Citation28 a successful operation had occurred when the therapeutic efficacy was classified as good or excellent.Citation12
Statistical Methods
Statistical analysis was performed using SPSS 22.0 software (IBM Corp., Armonk, NY, USA). Numerical data were analyzed using the chi-square test or Fisher’s test. Nonparametric data were analyzed using the Mann–Whitney U-test. Logistic regression analysis was used to determine the risk factors associated with the improvement of postoperative LUTS. Receiver operating characteristic (ROC) curves were used to evaluate the diagnostic accuracy of each variable for improvement of postoperative LUTS. A P value of <0.05 was considered statistically significant. In this retrospective study using logistic regression analysis, the sample size was determined by empirical criterion, that is, the sample size should be 10 to 15 times the number of independent variables. We included 12 factors in univariate analysis; therefore, at least 180 cases should be included.Citation29 In addition, according to EPV (events per variable, that is, the number of events per independent variable) criteria used widely currently, EPV should be at least 10 as recommended.Citation30 Hence, the sample size of the 257 patients we included met the requirements.
Results
In total, 257 patients (including 111 TURP patients and 146 PKEP patients) were included in this study. The Qmax, IPSS efficacy grade, and success rate are shown in . The flow diagram of this study is shown in .
Table 1 Criteria For Determining The Efficacy Of Individual Domains (IPSS, IPSS-S, IPSS-V And Function) And Proportion Of Patients For Each Efficacy Grade
The results of the univariate analysis are shown in . Among patients followed up for 3 months postoperatively, IPP, PUA, pre-PCB, post-PCB, and surgical procedure were significantly different between the two groups based on the IPSS (P < 0.05); TZV, TZI, IPP, and PUA were significantly different between the two groups based on the IPSS-v (P < 0.05); IPP, pre-PCB, post-PCB, and surgical procedure were significantly different between the two groups based on the IPSS-s (P < 0.05); and IPP, PUA and post-PCB surgical procedure were significantly different between the two groups based on Qmax (P < 0.05). Among patients followed up for 12 months postoperatively, PSA, IPP, PUA, pre-PCB, post-PCB, and surgical procedure were significantly different between the two groups based on the IPSS (P < 0.05); PUA was significantly different between the two groups based on the IPSS-v (P < 0.05); pre-PCB, post-PCB, surgical procedure, and Pre-PVR were significantly different between the two groups based on the IPSS-s (P < 0.05); and IPP, PUA, and post-PCB were significantly different between the two groups based on Qmax (P < 0.05).
Table 2 Univariate Analysis Of Risk Factors For Postoperative Outcomes In Patients With Small-Volume Prostate
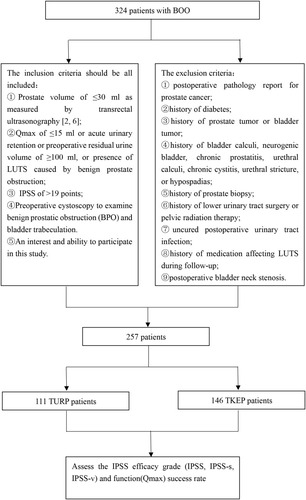
The results of the multivariate analysis are shown in . Among patients followed up for 3 months postoperatively, IPP, PUA, and post-PCB were the predictors of postoperative IPSS improvement (IPP cut-off, 6.5 mm; PUA cut-off, 38.5°; post-PCB cut-off, 5.6 mm) (). TZI, IPP, and PUA were the predictors of postoperative IPSS-v improvement (TZI cut-off point, 0.48; IPP cut-off point, 8.3 mm; PUA cut-off point, 39.5°) (). Post-PCB and the surgical procedure were the predictors of IPSS-s improvement (post-PCB cut-off, 5.1 mm) (). Finally, IPP and PUA were the predictors of postoperative Qmax improvement (IPP cut-off, 8.3 mm; PUA cut-off, 38.5°) (). Among patients followed up for 12 months postoperatively, IPP, PUA, and post-PCB were the predictors of postoperative IPSS improvement (IPP cut-off, 8.9 mm; PUA cut-off, 38.0°; post-PCB cut-off, 5.6 mm) (). PUA was the predictors of postoperative IPSS-v improvement (PUA cut-off point, 39.0°) (). Post-PCB was the predictors of IPSS-s improvement (post-PCB cut-off, 5.1 mm) (). Finally, IPP and PUA were the predictors of postoperative Qmax improvement (IPP cut-off, 8.9 mm; PUA cut-off, 38.0°) ().
Figure 2 ROC curve of improved postoperative IPSS (A), IPSS-v (B), IPSS-s (C) and Qmax (D) in patients followed up for 3 months postoperatively.
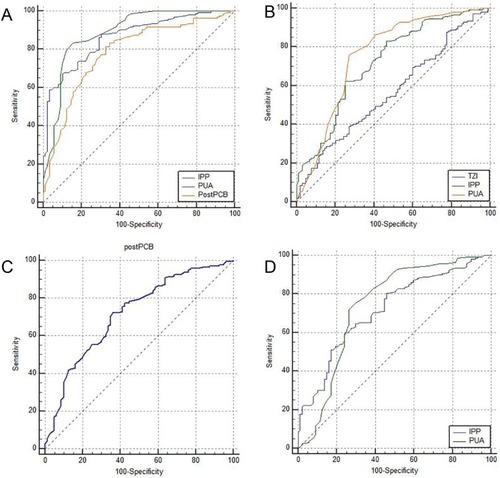
Figure 3 ROC curve of improved postoperative IPSS (A), IPSS-v (B), IPSS-s (C) and Qmax (D) in patients followed up for 12 months postoperatively.
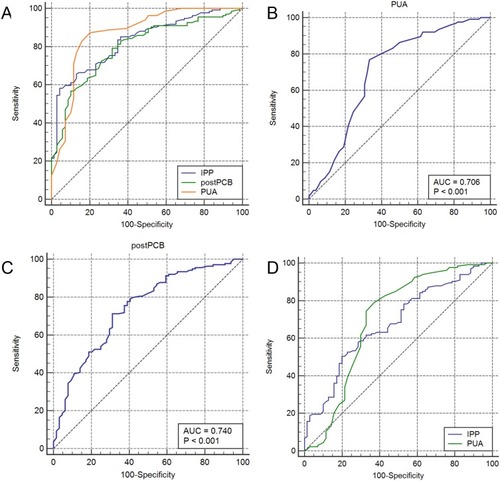
Figure 4 Zonal classification of the prostate and prostatic calculi.
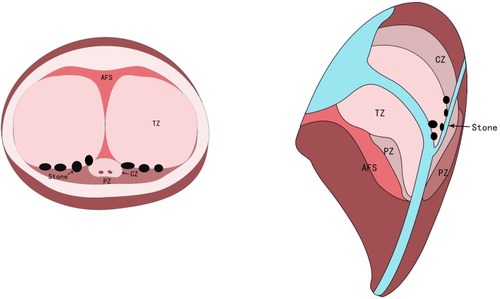
Figure 5 Prostate calculi between the adenoma and the surgical capsule were removed using electrocution during PKEP. (A) Prostatic calculi at 5-o’clock position (B) Prostatic calculi at 7-o’clock position. a: prostatic calculi; b: surgical capsule; c: electric cut loops; d: 5-o’clock position of hyperplasia gland lobe in prostate apex; e: 7-o’clock position of hyperplasia gland lobe in prostate apex.
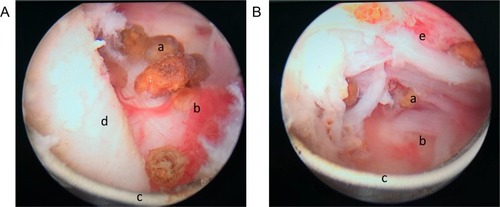
Table 3 Multivariate Analysis Of Risk Factors For Successful Treatment In Patients With Small-Volume Prostate
The ROC curve analysis results are shown in . Among patients followed up for 3 months postoperatively, the area under the curve (AUC) of IPP, PUA, and post-PCB was 0.865, 0.888, and 0.785, respectively, for postoperative IPSS improvement; the AUC of TZI, IPP, and PUA were 0.567, 0.721, and 0.758, respectively, for postoperative IPSS-v improvement; the AUC of post-PCB was 0.717 for postoperative IPSS-s improvement; the AUC of IPP and PUA was 0.717 and 0.737, respectively, for postoperative Qmax improvement. Among patients followed up for 12 months postoperatively, the area under the curve (AUC) of IPP, PUA, and post-PCB were 0.830, 0.870, and 0.798, respectively, for postoperative IPSS improvement; the AUC of PUA was 0.706 for postoperative IPSS-v improvement; the AUC of post-PCB was 0.740 for postoperative IPSS-s improvement; the AUC of IPP and PUA was 0.676 and 0.698, respectively, for postoperative Qmax improvement.
Table 4 Evaluation The Utility Of Different Variables For Predicting Treatment Success Using Receiver Operating Curve (ROC) Analysis
The postoperative PCB was significantly lower in the PKEP than the TURP group, and the removal rate of the prostate calculi was significantly higher in the PKEP than the TURP group (P < 0.05) ().
Table 5 Comparison Of Clinical Parameters Between The Two Surgical Procedures
Discussion
Previous studies have shown that the prostate volume is weakly correlated with LUTSCitation2,Citation6,Citation20 in patients with a small-volume prostate and BOO. In the present study, neither TPV nor TZV was a predictor of postoperative Qmax, IPSS, IPSS-s, or IPSS-v improvement in patients with a small-volume prostate and BOO. Therefore, other parameters reflecting the morphological features of the prostate, such as IPP, TZI, PUA, and PUB, were included in our study. These parameters are highly correlated with LUTS in patients with a small prostate.Citation6,Citation20,Citation26 The aim of this study was to investigate the role of these quantitative morphological features in postoperative LUTS improvement in patients with small-volume prostates.
IPP is a risk factor for the failure of conservative treatment in patients with benign prostatic hyperplasia and LUTS.Citation9 The IPP-affected part of the prostate can act as a spherical valve, resulting in aggravation of mechanical obstruction at the bladder outlet and further aggravation of mechanical obstruction caused by the prostate volume. Patients with a high IPP exhibit a lower Qmax, more serious storage symptoms, and a higher incidence of acute urinary retention.Citation31 Additionally, patients with a higher preoperative IPP attain better surgical outcomes.Citation19 However, some studies have indicated that only PUA and TZI were independent risk factors for LUTS in patients with a small-volume prostate (<30 mL).Citation6,Citation32 Kang et alCitation6 indicated that IPP, TZV, and TZI were not risk factors for Qmax and LUTS in patients with small-volume prostates. In the present study, although TZI was a risk factor for postoperative IPSS-v reduction, further ROC curve analysis revealed that TZI had lower diagnostic efficiency (<0.7) that was significantly poorer than that of the other morphological parameters of the prostate (such as IPP and PUA). Kuei et alCitation9 reported that patients with higher IPP tended to have larger prostates. However, the higher IPP was not associated with the IPSS. PV, TZV, and TZI play important roles in influencing the IPSS and Qmax, largely accounting for prolongation and compression of the prostatic urethra by the enlarged prostate. As a result, the morphological features of the prostate, such as IPP and PUA, contribute little to the improvement of LUTS. However, the effects of these morphological parameters on LUTS increased in patients with a small-volume prostate and BOO. In previous studies of patients with a small-volume prostate, LUTS appeared and Qmax was reduced to ≤10 mL/s when the PUA was ≥43.5°C.Citation6,Citation27,Citation33 The reduction of the Qmax may be attributed to the decrease in the kinetic energy of the urine flow.Citation6 In the present study of patients with a small-volume prostate, patients with a higher IPP and preoperative PUA had a higher postoperative Qmax and lower postoperative IPSS and IPSS-v. However, TZV and TZI were not associated with the improvement of the postoperative IPSS and Qmax.
Acute or chronic prostatitis may be attributed to the formation of prostatic calculi.Citation34,Citation35 The ducts and acinus of the prostate may swell due to stimulation by the prostatic calculi, which leads to changes in the structure of the normal glandular epithelial cells, aggravates the inflammation of the prostate, and increases the volume of the prostate calculi. Moreover, the presence of large and rough prostatic calculi has been shown to be an independent risk factor for severe LUTS.Citation14,Citation36 Enlarged prostatic calculi can further block the prostatic duct, which aggravates the inflammation of the prostateCitation37 and induces storage symptoms by stimulating contraction of the smooth muscle in the prostate stroma and bladder neck.Citation14 The presence of large prostatic calculi is also an important risk factor for BOO.Citation38 Therefore, a large volume or \high PCB may be associated with more severe LUTS.Citation4,Citation11,Citation13,Citation14,Citation20,Citation36,Citation39 However, some studies have indicated that prostatic calculi are not associated with LUTS.Citation40,Citation41 Yang et alCitation14 evaluated mild calcification (one or multiple small foci without a coarse shadow) and moderate/marked calcification (three or more hyperechoic foci, largest diameter of ≥3 mm with a coarse shadow) in their study of LUTS and reported that moderate/marked calcification may lead to moderate/severe LUTS. Park and ChooCitation11 found that a decrease in the Qmax, LUTS, and the severity of prostatitis were associated with the size of prostatic calculi rather than the number or distribution of prostatic calculi.Citation13,Citation14,Citation19 This discrepancy may due to the ineffective and inconsistent definitions of the size, number, and distribution of prostatic calculi.Citation4,Citation14,Citation36,Citation40 Thus, we used the definition of PCB as described by Park and ChooCitation11 to quantitatively identify the effect of the volume of the prostatic calculi on the improvement of postoperative LUTS in the present study. We demonstrated that patients with a large postoperative PCB had a poor improvement in their postoperative storage LUTS regardless of the degree of preoperative PCB. Thus, it is important to reduce the PCB and attenuate the effect of prostatic calculi in patients with LUTS and severe PCB. Relieving the inflammation and infection and controlling the formation and development of prostatic calculi caused by medication (nonsteroidal anti-inflammatory drugs or antibiotics) can relieve the LUTS to some extent.Citation11,Citation14,Citation34
Severe LUTS may be caused by prostatic calculi between the transition zone and the peripheral zone.Citation38,Citation39 The volume of the calculi at the tip of the prostate is larger than that at the base of the prostate. Anatomically, the calculi at the tip of the prostate are more likely to be associated with LUTS.Citation11 Surgical removal of these prostatic calculi may relieve the burden of the prostate and improve the LUTS.Citation39,Citation42 However, a systematic controlled study is needed to further explore these issues.Citation11 In the present study, we found that postoperative PCB was significantly associated with postoperative improvement of the IPSS and IPSS-s. A higher postoperative calculus removal rate was correlated with greater improvement of postoperative LUTS. This is the first comparison of the effect of TURP and PKEP on the removal rate of prostatic calculi and the improvement of postoperative LUTS. This study revealed that PKEP has anatomical advantages in the removal of calculi between the peripheral zone and transition zone as well as calculi at the tip of the prostate. PKEP was associated with a higher removal rate of prostatic calculi than TURP.
This study had some limitations. First, this was a retrospective study. Only the patients who were experiencing LUTS and needed surgical intervention were selected in this study. We did not include all patients with small-volume prostate and LUTS.Citation40 Second, as one of the common causes of obstruction in patients with small prostates, bladder neck stenosis could affect the improvement of postoperative LUTS. In order to avoid the bias, we excluded patients with postoperative bladder neck stenosis. However, the catheter was indwelled for at least 7 days after the operation to prevent bladder neck stenosis. Third, the cut-off point of the PUA in this study was different from that reported by Kang et alCitation5. The potential reason for this difference is that the PUA was measured in the stationary state under transrectal ultrasonography in our study; however, the PUA may change during the process of urination, especially in patients with good urethral compliance. Furthermore, PUA may also be altered by the crimp position and compression of the ultrasound probe in patients undergoing transrectal ultrasonography. Therefore, further studies should be performed to address this issue.Citation6 Besides, we believe that magnetic resonance imaging (MRI) can be used to accurately measure PUA in natural position (standing position) in future studies. Fourth, only some of the patients in this study underwent urodynamics studies. Because of the limited number of patients in this study, further investigation cannot be performed. We can only attempt to exclude those patients with LUTS caused by bladder dysfunction, nervous system diseases, or other psychological factors based on a detailed medical history, physical examination, and preoperative cystoscopy.Citation2 Fifth, this study only compared the effect of TURP and PKEP on removing prostate stones. Indeed, HoLEP could also remove stones between the apex, periapical and transitional areas of the prostate although the prostate gland is anatomically exfoliated. However, whether HoLEP can achieve sufficient clearance of prostate stones still needs further study.Citation12 Sixth, we merely compared postoperative functional outcomes between TURP and PKEP. There was no significant difference between these two groups in terms of complications according to the Clavien grading system. Short follow-up time might be responsible for this. Further research into complications between TURP and PKEP is needed. Seventh, with the extension of postoperative follow-up time, the number of patients lost to follow-up or receiving behavioral/physical/drug therapy due to dissatisfied postoperative outcome increased gradually, which resulted in research difficulties. Hence, we initially identified postoperative 12 months as the endpoint of follow-up to ensure the integrity of data and avoid bias. Further research with a larger sample size and multicenter is needed, especially in the aspect of long-term postoperative outcome.
Conclusion
This study was performed to provide clinical guidance in surgical treatment for BOO in patients with small-volume prostates and unsatisfactory medical treatment results. Patients with a greater preoperative IPP and PUA (IPP of ≥6.5 mm and PUA of ≥38.5°) and a smaller post-PCB (post-PCB of ≤5.6 mm) achieve greater improvement of postoperative LUTS. PKEP might help to remove the calculi from between the transitional and peripheral zones of the prostate. Compared with conventional TURP, PKEP may improve the early postoperative storage symptoms of LUTS in patients with small-volume prostates and BOO.
Ethics And Informed Consent Statements
This study was conducted in accordance with the Declaration of Helsinki. This study was approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University and all patients provided written informed consent.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Ke ZB, Cai H, Wu YP, et al. Identification of key genes and pathways in benign prostatic hyperplasia. J Cell Physiol. 2019;234(11):19942–19950. doi:10.1002/jcp.2859231187492
- Kang M, Kim M, Choo MS, Paick JS, Oh SJ. Urodynamic features and significant predictors of bladder outlet obstruction in patients with lower urinary tract symptoms/benign prostatic hyperplasia and small prostate volume. Urology. 2016;89:96–102. doi:10.1016/j.urology.2015.11.02726683755
- Hashimoto M, Shimizu N, Sugimoto K, et al. Efficacy of adding dutasteride to alpha-blocker therapy treated benign prostatic hyperplasia patients with small volume prostate (<30 mL). Low Urin Tract Symptoms. 2017;9(3):157–160. doi:10.1111/luts.1212726991643
- Cao JJ, Huang W, Wu HS, et al. Prostatic calculi: do they matter? Sex Med Rev. 2018;6(3):482–491. doi:10.1016/j.sxmr.2017.10.00329157875
- Guneyli S, Ward E, Thomas S, et al. Magnetic resonance imaging of benign prostatic hyperplasia. Diagn Interv Radiol. 2016;22(3):215–219. doi:10.5152/dir27015442
- Kang DH, Lee JY, Hah YS, et al. Correlation of prostatic urethral angle with the severity of urinary symptom and peak flow rate in men with small prostate volume. PLoS One. 2014;9(8):e104395. doi:10.1371/journal.pone.010439525127394
- Singh K, Sinha RJ, Sokhal A, Singh V. Does prostate size predict the urodynamic characteristics and clinical outcomes in benign prostate hyperplasia? Urol Ann. 2017;9(3):223–229. doi:10.4103/0974-7796.21002928794586
- Xu N, Chen SH, Xue XY, et al. Older age and larger prostate volume are associated with stress urinary incontinence after plasmakinetic enucleation of the prostate. Biomed Res Int. 2017;2017:6923290. doi:10.1155/2017/692329028466017
- Kuei CH, Liao CH, Chiang BJ. Significant intravesical prostatic protrusion and prostatic calcification predict unfavorable outcomes of medical treatment for male lower urinary tract symptoms. Urol Sci. 2016;27(1):13–16. doi:10.1016/j.urols.2015.01.003
- Zhang J, Li X, Yang B, et al. Alpha-blockers with or without phosphodiesterase type 5 inhibitor for treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: a systematic review and meta-analysis. World J Urol. 2019;37(1):143–153. doi:10.1007/s00345-018-2370-z29948047
- Park B, Choo SH. The burden of prostatic calculi is more important than the presence. Asian J Androl. 2017;19(4):482–485. doi:10.4103/1008-682X.18119327184549
- Suh YS, Ko KJ, Kim TH, Sung HH, Lee KS. Efficacy of holmium laser transurethral incision of the prostate in symptomatic mild-to-moderate benign prostate enlargement based on preoperative characteristics. Low Urin Tract Symptoms. 2018;10(3):231–236. doi:10.1111/luts.1216828436120
- Han JH, Kwon JK, Lee JY, et al. Is periurethral calcification associated with urinary flow rate and symptom severity in men with lower urinary tract symptoms-benign prostatic hyperplasia? A retrospective review. Urology. 2015;85(5):1156–1161. doi:10.1016/j.urology.2015.01.03825818911
- Yang HJ, Huang KH, Wang CW, Chang HC, Yang TK. Prostate calcification worsen lower urinary tract symptoms in middle-aged men. Urology. 2013;81(6):1320–1324. doi:10.1016/j.urology.2013.02.02123561714
- Luo YH, Shen JH, Guan RY, Li H, Wang J. Plasmakinetic enucleation of the prostate vs plasmakinetic resection of the prostate for benign prostatic hyperplasia: comparison of outcomes according to prostate size in 310 patients. Urology. 2014;84(4):904–910. doi:10.1016/j.urology.2014.06.02525150180
- Braeckman J, Denis L. Management of BPH then 2000 and now 2016 - From BPH to BPO. Asian J Urol. 2017;4(3):138–147. doi:10.1016/j.ajur.2017.02.00229264222
- Cumpanas AA, Botoca M, Minciu R, Bucuras V. Intravesical prostatic protrusion can be a predicting factor for the treatment outcome in patients with lower urinary tract symptoms due to benign prostatic obstruction treated with tamsulosin. Urology. 2013;81(4):859–863. doi:10.1016/j.urology.2012.12.00723375910
- Gratzke C, Bachmann A, Descazeaud A, et al. EAU guidelines on the assessment of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2015;67(6):1099–1109. doi:10.1016/j.eururo.2014.12.03825613154
- Lee JW, Ryu JH, Yoo TK, et al. Relationship between intravesical prostatic protrusion and postoperative outcomes in patients with benign prostatic hyperplasia. Korean J Urol. 2012;53(7):478–482. doi:10.4111/kju.2012.53.7.47822866219
- Han JH, Lee JY, Kwon JK, Lee JS, Cho KS. Clinical significance of periurethral calcification according to the location in men with lower urinary tract symptoms and a small prostate volume. Int Neurourol J. 2017;21(3):220–228. doi:10.5213/inj.1732732.36628954459
- Samir M, Tawfick A, Mahmoud MA, et al. Two-year follow-up in bipolar transurethral enucleation and resection of the prostate in comparison with bipolar transurethral resection of the prostate in treatment of large prostates. Randomized controlled trial. Urology. 2019. doi:10.1016/j.urology.2019.07.029
- Wei Y, Xu N, Chen SH, et al. Bipolar transurethral enucleation and resection of the prostate versus bipolar resection of the prostate for prostates larger than 60gr: a retrospective study at a single academic tertiary care center. Int Braz J Urol. 2016;42(4):747–756. doi:10.1590/S1677-5538.IBJU.2015.022527564286
- Liu C, Zheng S, Li H, Xu K. Transurethral enucleation and resection of prostate in patients with benign prostatic hyperplasia by plasma kinetics. J Urol. 2010;184(6):2440–2445. doi:10.1016/j.juro.2010.08.03720952005
- Sedrakyan A, Campbell B, Merino JG, et al. IDEAL-D: a rational framework for evaluating and regulating the use of medical devices. Bmj. 2016;353:i2372. doi:10.1136/bmj.i237227283585
- Chen S, Zhu L, Cai J, et al. Plasmakinetic enucleation of the prostate compared with open prostatectomy for prostates larger than 100 grams: a randomized noninferiority controlled trial with long-term results at 6 years. Eur Urol. 2014;66(2):284–291. doi:10.1016/j.eururo.2014.01.01024502959
- Bang WJ, Kim HW, Lee JY, et al. Prostatic urethral angulation associated with urinary flow rate and urinary symptom scores in men with lower urinary tract symptoms. Urology. 2012;80(6):1333–1337. doi:10.1016/j.urology.2012.08.05823206777
- Cho KS, Kim JH, Kim DJ, et al. Relationship between prostatic urethral angle and urinary flow rate: its implication in benign prostatic hyperplasia pathogenesis. Urology. 2008;71(5):858–862. doi:10.1016/j.urology.2008.01.01918343484
- Homma Y, Kawabe K, Tsukamoto T, et al. Estimate criteria for efficacy of treatment in benign prostatic hyperplasia. Int J Urol. 1996;3(4):267–273. doi:10.1111/iju.1996.3.issue-48844281
- Demidenko E. Sample size determination for logistic regression revisited. Stat Med. 2007;26(18):3385–3397. doi:10.1002/sim.277117149799
- van Smeden M, Moons KG, de Groot JA, et al. Sample size for binary logistic prediction models: beyond events per variable criteria. Stat Methods Med Res. 2019;28(8):2455–2474. doi:10.1177/096228021878472629966490
- Lee LS, Sim HG, Lim KB, Wang D, Foo KT. Intravesical prostatic protrusion predicts clinical progression of benign prostatic enlargement in patients receiving medical treatment. Int J Urol. 2010;17(1):69–74. doi:10.1111/j.1442-2042.2009.02409.x19919641
- Choi J, Ikeguchi EF, Lee SW, et al. Is the higher prevalence of benign prostatic hyperplasia related to lower urinary tract symptoms in Korean men due to a high transition zone index? Eur Urol. 2002;42(1):7–11. doi:10.1016/S0302-2838(02)00222-112121722
- Cho KS, Kim J, Choi YD, Kim JH, Hong SJ. The overlooked cause of benign prostatic hyperplasia: prostatic urethral angulation. Med Hypotheses. 2008;70(3):532–535. doi:10.1016/j.mehy.2007.07.01217761390
- Dessombz A, Meria P, Bazin D, Daudon M. Prostatic stones: evidence of a specific chemistry related to infection and presence of bacterial imprints. PLoS One. 2012;7(12):e51691. doi:10.1371/journal.pone.005169123272143
- Gandaglia G, Briganti A, Gontero P, et al. The role of chronic prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia (BPH). BJU Int. 2013;112(4):432–441. doi:10.1111/bju.2013.112.issue-423650937
- Kim WB, Doo SW, Yang WJ, Song YS. Influence of prostatic calculi on lower urinary tract symptoms in middle-aged men. Urology. 2011;78(2):447–449. doi:10.1016/j.urology.2010.12.05621689847
- Ficarra V. Is chronic prostatic inflammation a new target in the medical therapy of lower urinary tract symptoms (LUTS) due to benign prostate hyperplasia (BPH)? BJU Int. 2013;112(4):421–422. doi:10.1111/bju.2013.112.issue-423879899
- Calleja R, Yassari R, Wilkinson EP, Webb R. Bladder outflow obstruction caused by prostatic calculi. Sci World J. 2004;4(Suppl 1):46–47. doi:10.1100/tsw.2004.45
- Hyun JS. Clinical significance of prostatic calculi: a review. World J Mens Health. 2018;36(1):15–21. doi:10.5534/wjmh.1701829076299
- Kim SH, Jung KI, Koh JS, et al. Lower urinary tract symptoms in benign prostatic hyperplasia patients: orchestrated by chronic prostatic inflammation and prostatic calculi? Urol Int. 2013;90(2):144–149. doi:10.1159/00034264323295780
- Park SW, Nam JK, Lee SD, Chung MK. Are prostatic calculi independent predictive factors of lower urinary tract symptoms? Asian J Androl. 2010;12(2):221–226. doi:10.1038/aja.2009.7519966831
- Goyal NK, Goel A, Sankhwar S. Transurethral holmium-YAG laser lithotripsy for large symptomatic prostatic calculi: initial experience. Urolithiasis. 2013;41(4):355–359. doi:10.1007/s00240-013-0571-x23715770