Abstract
Lung cancer is the leading cause of mortality worldwide. Non-small cell lung cancer (NSCLC) is a particularly aggressive cancer, the optimum management of which is still being determined. In the metastatic disease, the standard therapy is a platinum-based combination chemotherapy; however, in spite of available treatment options for patients who progress beyond first-line therapy, prognosis remains poor. Angiogenesis is a tightly regulated process which comprises a complex, complementary, and overlapping network. Inhibition of tumor-related angiogenesis has become an attractive target for anticancer therapy. Antiangiogenic strategy includes: monoclonal antibodies against vascular endothelial growth factor (VEGF) and VEGF receptor (VEGFR), small molecule inhibitors of VEGF tyrosine kinase activity, VEGF Trap, and a new class named “vascular disrupting agents,” tested in ongoing clinical trials which will further define their role in the management of NSCLC. BIBF 1120 is an investigational orally administered receptor tyrosine kinase inhibitor that has shown antiangiogenic and antineoplastic activity, inhibiting VEGFR, platelet-derived growth factor receptor, and fibroblast growth factor receptor tyrosine kinases, preventing tumor growth and interfering with the angiogenesis-signaling cascade and overcoming drug resistances.
Background
Non-small cell lung cancer (NSCLC) accounts for 85%–90% of all lung cancersCitation1 with a median survival time of 7.0–8.3 months, and 1-year survival rates of 29%–37% who progress beyond first-line therapy, with an overall 5-year survival rate of only 15% in the metastatic disease.Citation2
However, advances in the understanding of the biology of cancer have led to molecular targeted therapies. Tyrosine kinase inhibitors (TKIs) are the largest class of therapeutic agents in clinical use and under development that target angiogenesis.
Angiogenesis and neovascularization are critical for the growth, progression, and metastasis of solid tumors, including NSCLCs,Citation3–Citation5 so angiogenic pathways have become an important biologic target to inhibit tumor growth. Several pathways for vascularity and tumor neoangiogenesis have been identified, including vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and platelet-derived growth factor (PDGF) pathways.Citation5,Citation6
The VEGF pathway is critical to tumor angiogenesis and has become an important therapeutic target. The VEGF family consists of five glycoproteins (VEGF-A through VEGF-D and placental growth factor), which act by binding to their cognate tyrosine kinase receptor (VEGF receptor [VEGFR]).
Given their central role in angiogenesis, monoclonal antibodies against VEGF and TKIs directed towards VEGFRs have been developed.Citation6,Citation7
The FGF family of ligands comprises a number of growth factors with a broad spectrum of activity, including angiogenic activity.Citation8,Citation9 One such ligand, FGF-2, has been detected in high levels in patients with highly vascularized tumors, and its expression has been correlated with cancer progression and metastatic disease.Citation9 The PDGF pathway has also demonstrated angiogenic activity by way of recruiting pericytes and vascular smooth muscle cells, which are critical to the maturation of newly developing vasculature.Citation10 Studies suggest that FGF and PDGF may act synergistically to promote angiogenesis by reciprocally enhancing their activities on endothelial cells, pericytes, and vascular smooth muscle cells.Citation9,Citation10
BIBF 1120 (Vargatef™, Boehringer Ingelheim, Ingelheim, Germany) is an indolinone derivative potently blocking VEGFRs, PDGF receptors (PDGFRs) and FGF receptor (FGFR) kinase activity in enzymatic assays (half-maximal inhibitory concentration [IC50], 20–100 nmol/L). It also inhibits mitogen-activated protein kinase (MAPK) and Akt signaling pathways in endothelial cells, pericytes, and smooth muscle cells, resulting in inhibition of cell proliferation (half-maximal effective concentration [EC50], 10–80 nmol/L) and apoptosis. In all tumor models tested, BIBF 1120 is highly active at well tolerated doses (25–100 mg/kg daily, orally).
Antiangiogenic TKIs in NSCLC
Sorafenib
Sorafenib is an oral TKI with multiple targets, including VEGFR, PDGFR, RAF, c-KIT, rearranged during transfection (RET), and fms-like tyrosine kinase (FLT)-3.Citation11,Citation12 It has been approved by the United States Food and Drug Administration (FDA) as a single agent in the treatment of advanced renal cell carcinoma (RCC) and hepatocellular carcinoma,Citation13 and in preclinical models it also shows dose-dependent antitumor activity in NSCLC, either when administered alone or in combination with other chemotherapy agents such as vinorelbine and cisplatin and with targeted agents such as gefitinib.Citation14
Based on Phase I trials that included patients with NSCLC, the recommended dose of sorafenib for Phase II studies is 400 mg twice daily, given orally.Citation15
As a single agent in two Phase II studies, sorafenib shows an advantage either in progression-free survival (PFS) and in overall survival (OS) with respect to placebo; rash/hand-foot reactions, fatigue, hypertension, and diarrhea were the most common grade 3/4 toxicities.Citation16,Citation17
After a Phase I/II trial where sorafenib combined with carboplatin and paclitaxel showed a median PFS of 34 weeks with a good toxicity profile,Citation18 two Phase III trials were conducted to confirm the efficacy and feasibility of the combination treatment: the ESCAPE trial, with carboplatin paclitaxel; and the NEXUS trial, with gemcitabine/cisplatin.
Unfortunately neither of the trials met their primary endpoints: the ESCAPE trial was terminated early due the futility at interim analysis without any advantage in OS or disease control rate (DCR) and there was reported a higher rate of grade 5 drug-related adverse events (AEs) with sorafenib in patients with squamous cell histology;Citation19 and in the NEXUS (NCT00449033) trial, conducted exclusively in patients with nonsquamous NSCLC, sorafenib plus chemotherapy didn’t improve OS.Citation20
Another interesting combination studied was with agents that target the EGF, assuming that the possibility to overcome resistance to epidermal growth factor receptor (EGFR) TKIs in patients whose tumors express K-Ras mutations, or that simultaneous inhibition of both pathways (VEGF and EGF) may prove to be additive or synergistic.Citation21,Citation22
In a Phase II trial, sorafenib combined with erlotinib as first-line therapy met the primary endpoint with a rate of nonprogression at 6 weeks of 74%: 12 (24%) partial response (PR) and 25 (50%) stabilization of disease (SD). Patients with wild-type EGFR had a higher objective response rate (ORR) (19%) than previously reported for single-agent erlotinib/sorafenib.Citation23
Another Phase II trial, which randomized patients into one of four treatments (erlotinib, sorafenib, vandetanib, and erlotinib plus bexarotene), based on biomarker status, showed in the sorafenib arm a DCR of 58% (57 in 98 patients) but a higher DCR (61%, 11/18) in K-RAS-mutated patients, in contrast to 31% (4/13) in K-RAS-mutated patients treated with erlotinib regimen.Citation24
The authors of this paper, as monotherapy, have a large ongoing multicenter, Phase III, third/fourth-line placebo-controlled trial of sorafenib in patients with predominantly nonsquamous NSCLC (MISSION trial) planned to determine whether sorafenib plus best supportive care is an effective treatment for lung cancer compared with best supportive care alone. The estimated enrolment is 850 patients, and the primary endpoint of this study is OS.
Sunitinib
Sunitinib is another multitargeted oral TKI; it targets VEGFR- 2, PDGFR, FGFR, FLT-3, RET, and c-KIT,Citation25,Citation26 approved by the FDA as a single agent for the treatment of patients who have advanced RCC and for patients with imatinib-resistant or -intolerant gastrointestinal stromal tumors.Citation27
In NSCLC, sunitinib has been studied as a single agent in two dosing schedules: continuous and intermittent. A Phase II study examined the role of continuous daily dosing (CDD) sunitinib 37.5 mg in 47 previously treated patients with advanced NSCLC, with 2.1% PR, 23.4% SD, while the median PFS and OS were 11.9 weeks and 37.1 weeks, respectively.Citation26 A second Phase II study evaluated a different dosing schedule: sunitinib 50 mg/d for 4 weeks followed by a 2-week break. PRs were observed in seven patients (ORR, 11.1%; 95% confidence interval [CI], 4.6%–21.6%); SD for ≥8 weeks was observed in 18 patients (28.6%). The median PFS was 12.0 weeks (95% CI, 10.0–16.1 weeks) with a median OS of 23.4 weeks (95% CI, 17.0–28.3).Citation27 Both studies reported fatigue, hypertension, dyspnea, and bleeding.
Data with sunitinib in combination with chemotherapy are limited. In a Phase I dose-escalation study, sunitinib in combination with cispatin/gemcitabine as first-line advanced NSCLC therapy, the maximum tolerated dose (MTD) was identified as sunitinib 37.5 mg (schedule 2/1), gemcitabine 1250 mg/m2, and cisplatin 80 mg/m2, showing an antitumor activity with a manageable toxicity profile,Citation28 as well as in another Phase I in combination with docetaxel (75 mg/m2), in several advanced solid tumors including NSCLC.Citation29
As in the case of sorafenib, sunitinib has been evaluated in combination with EGFR inhibitor, erlotinib, in a double-blind Phase II study, in patients with platinum refractory NSCLC: patients are randomized to erlotinib alone versus the combination of erlotinib plus sunitinib in the second-line treatment.
Diarrhea and fatigue are the most frequent grade 3/4 toxicities, with no erlotinib interaction on sunitinib: two patients had a durable PR and two patients reported SD for 16 weeks. The Phase II portion of this trial is ongoing.Citation30
A Phase III randomized, multicenter clinical trial (SUN1087) will evaluate the combination of erlotinib at standard dose plus sunitinib at CDD versus erlotinib plus placebo given in 4-week cycles in 956 advanced NSCLC, which have received one or two chemotherapy regimens including platinum-based therapy.Citation31
Vandetanib
Vandetanib (ZD6474) is a novel, orally available, adenosine-5-triphosphate (ATP)-mimetic small molecule targeting VEGFR-2, EGFR, and RET tyrosine kinase,Citation32 and blocks multiple intracellular signaling pathways involved in tumor growth, progression, and angiogenesis.Citation33
In a Phase I trial, single-agent vandetanib was well tolerated in a variety of solid tumors at 300 mg daily,Citation31 so in a randomized double-blind Phase II trial it was compared with gefitinib at standard dose, as a first-line treatment, with a crossover design: OS was similar between these two arms but the crossover should be a confounder. The most common AEs relating to vandetanib were diarrhea, rash, and asymptomatic QTc (QT interval corrected for heart rate) prolongation.Citation34
In other Phase II trials, vandetanib (100 or 300 mg daily) was evaluated in combination with docetaxel (75 mg/m2 3-weekly) as second-line treatment for advanced or metastatic NSCLC patients,Citation37 or with carboplatin plus paclitaxelCitation38 in first line. Both studies demonstrated a prolonged PFS, but without any advantage in OS.Citation35,Citation36
The ZODIAC and the ZEAL, two randomized, double-blind Phase III trials, compared vandetanib 100 mg plus docetaxel or pemetrexed to docetaxel or pemetrexed alone, respectively: while the first combination showed an improvement in median PFS with a positive trend, but not statistically significant, in OS,Citation37 the combination with pemetrexed didn’t meet the first endpoint, the PFS.Citation38
In a Phase III trial (Zactima Efficiacy when Studied versus Tarceva [ZEST]), vandetanib versus erlotinib was evaluated in 1240 pretreated patients with advanced NSCLC; vandetanib did not prolong PFS or increase ORR, but there was a higher incidence of diarrhea and hypertension in the vandetanib group, whereas skin rash was more common in the erlotinib arm.Citation39
In the ZEPHYR (Zactima Efficacy trial for NSCLC Patients with History of EGFR-TKI and chemo-Resistance) trial, a Phase III, randomized, double-blind, multicenter study that evaluated the efficacy of vandetanib plus best supportive care (BSC) versus BSC alone in patients with advanced NSCLC after failure of prior therapy with an EGFR inhibitor. The primary endpoint of a superior OS for patients receiving vandetanib was not met. However, significant advantages favoring vandetanib were observed for the PFS (hazard ratio 0.63; 95.2% CI 0.54–0.74; P < 0.0001), response rate (RR) (2.6% versus 0.7%; P = 0.028), and DCR at 8 weeks (30% versus 16%; P < 0.0001).Citation40
Cediranib
Cediranib (AZD2171) targets VEGFR, c-KIT, and PDGFR signaling.Citation41,Citation42 Two Phase I studies have evaluated cediranib (30 or 45 mg) in combination respectively with carboplatin area under the curve (AUC) 6 and paclitaxel 200 mg/m2 or with cisplatin 80 mg/m2 and gemcitabine 1250 mg/m2, with no dose-limiting toxicities during the first cycle with both doses. There was a good DCR, and the recommended Phase II/III dose of cediranib was 30 mg/d, with fatigue, nausea, diarrhea, anorexia, and hypertension the most common toxicities.Citation43,Citation44
After the failure in the BR.24 trial, where cediranib 30 mg/d combined with carboplatin/paclitaxel or placebo improved RR but not median PFS, and with a high toxicity profile,Citation45 in the BR.29 trial (NCT00795340) cediranib was evaluated at a lower dosage (20 mg/d) combined with the same chemotherapeutic regimen versus chemotherapy plus placebo as first-line treatment in advanced NSCLC.
Currently, two Phase II studies are accruing patients: cediranib combined with pemetrexed or in combination with carboplatin plus paclitaxel. Preliminary results haven’t shown any significant improvement in PFS, OS, or RR with the addition of cediranib as first-line therapy in previously untreated patients with NSCLC.Citation46,Citation47
Axitinib
Axitinib (AG-013736) is an orally bio-available TKI that targets VEGFR, PDGFR, and colony-stimulating factor-1 receptor,Citation48 inhibiting the pro-angiogenic VEGF-1, -2, and -3 and PDGFRs inhibiting angiogenesis, vascular permeability, and blood flow in a wide range of tumor types.Citation49
In a Phase I trial (N = 47), axitinib combined with carboplatin plus paclitaxel in patients previously untreated, or cisplatin plus gemcitabine in patients who received prior treatment for metastatic disease, the determined MTD was axitinib 5 mg twice a day (bid). Most common toxicities were fatigue, hypertension, headache, and diarrhea,Citation50 with strong evidence of clinical activity.Citation51
An open-label, multicenter Phase II study evaluated the efficacy and safety of axitinib in advanced NSCLC patients previously treated with chemotherapy and/or radiotherapy. The majority of patients (75%) had adenocarcinoma, with a good DCR and an OS similar in patients receiving axitinib as a single agent in first-line therapy, with a good toxicity profile.Citation52
Pazopanib
Pazopanib is a potent and selective multitargeted receptor TKI of VEGFR-1, VEGFR-2, VEGFR-3, PDGFR-α and PDGFR-β, and c-KIT that blocks tumor growth and inhibits angiogenesis. Pazopanib is currently being studied in a number of different tumor types, and clinical trials are ongoing in RCC, breast cancer, ovarian cancer, soft tissue sarcoma, NSCLC, cervical cancer, and other solid tumors.Citation53
In a Phase I trial, patients with advanced-stage refractory solid tumors including NSCLC were enrolled into sequential dose-escalating cohorts of axitinib (50 mg three times weekly to 2000 mg once daily and 300–400 mg twice daily). A monotherapy dose of 800 mg once daily was selected for Phase II studies.Citation54 The most frequent drug-related AEs were hypertension, diarrhea, hair depigmentation, and nausea, the majority of which were of grade 1/2.
Interestingly, early Phase II data for stage IA to IIA NSCLC have been reported in the neo-adjuvant setting for this agent,Citation55 at 800 mg/d for 2–6 weeks before surgery. Among 35 patients enrolled, three PRs were observed. Significant toxicities included pneumonia, rash, urinary tract infection, blood potassium elevation, lymphopenia, dyspnea, and transaminase elevation (all grade 3).Citation56 Based on these promising data, further studies with pazopanib in multiple stages of NSCLC are planned.
Motesanib
Motesanib (AMG 706) is a small oral, multikinase inhibitor, molecule antagonist of VEGFR-1, -2, and -3, PDGFR, KIT, and RET. Preclinical studies demonstrated inhibition of VEGF-induced angiogenesis and inhibition of tumor growth in vivo.Citation57
In a Phase Ib study, motesanib was combined with carboplatin plus paclitaxel showing the same RR as the same regimen plus panitumumab (17%) in advanced NSCLC. In another arm of this study motesanib was combined with panitumumab showing no benefit in terms of RR. Common motesanib-related AEs observed were fatigue (60% of patients), diarrhea (53%), hypertension (38%), anorexia (27%), and nausea (22%).Citation58
On this basis a phase II trial was organized where 181 patients were randomly assigned to three treatment arms: paclitaxel/carboplatin for 6 cycles maximum plus motesanib, continuously or intermittent orally, versus the same chemotherapic regimen plus bevacizumab: motesanib continuously assumed plus carboplatin/paclitaxel had ORR median PFS and OS similar to carboplatin/paclitaxel plus bevacizumab. The most common all-grade toxicities included cholecystitis, hemorrhagic events, deep vein thrombosis, and pulmonary embolism (3%, 2%, 3%).Citation59
A Phase III, multicenter, randomized, placebo-controlled, double-blind trial (MONET-1) is ongoing to evaluate the addition of motesanib to paclitaxel and carboplatin compared with the same chemotherapy regimen plus placebo in advanced NSCLC patients. Since November 2008, subjects were excluded from the enrolment because of a higher rate of early mortality rate and incidence of hemoptysis in the motesanib group compared with the placebo group in the squamous population.Citation60
BIBF 1120
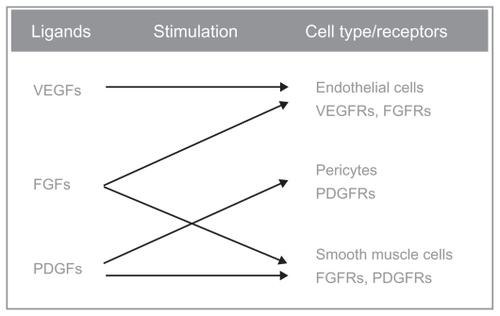
BIBF 1120, an orally administered indolinone derivative, is a novel, potent, triple angiokinase inhibitor, which simultaneously acts on three key receptor families involved in angiogenesis: VEGFR, FGFR, and PDGFR-α and -β. BIBF 1120 competitively binds to the ATP-binding site of receptor tyrosine kinases and inhibits downstream intracellular signaling. Biochemical assays demonstrate that BIBF 1120 inhibits a narrow range of kinases at pharmacologically relevant concentrations: VEGFR types 1, 2, and 3, PDGFR-α and PDGFR-β, FGFR types 1, 2, and 3, FLT-3, and members of the Src familyCitation61 (). Inhibition of these receptors found on endothelial cells, tumor cells, and pericytes allows BIBF 1120 to potentially prevent both tumor growth and dissemination, and also provides a possible solution to intrinsic and acquired resistance observed with other single or dual angiogenesis inhibitors.Citation63
Figure 1 Triple mechanism of action of BIBF-1120: it inhibits all three VEGFR subtypes, PDGFR-α and PDGFR-β and FGFR types 1, 2, and 3. Other targets of this drug are the FLT-3 (inhibition of acute myelogenous leukemia cell proliferation), and members of the Src-family (Src, Lyn, and Lck).
Moreover, BIBF 1120 has a sustained duration of cellular action blocking, with 50 nmol/L BIBF 1120; the in-vitro auto-phosphorylation of VEGFR-2 for around 32 hoursCitation62 indicates the potential for a long-lasting antiangiogenic effect.
Clinical studies demonstrate maximum BIBF 1120 plasma concentrations occurred mainly 1–4 hours after oral administration.Citation63 No deviation from dose proportionality in the pharmacokinetics of BIBF 1120 has been observed. BIBF 1120 showed a high apparent volume of distribution during the terminal phase, both after single dose and at steady state, which might indicate a high tissue distribution of the drug. In addition, there was no decrease in exposure over time during continuous daily treatment with BIBF 1120.
From clinical investigation, the cleavage of [14C]BIBF 1120 by esterase-catalyzed hydrolysis is the prevalent metabolic reaction; cytochrome P450 (CYP450)-dependent metabolism was found to be minor. The terminal half-life of BIBF 1120 was determined to be 19 hours. BIBF 1120 is mainly excreted via the liver ().
Table 1 Kinase inhibition: comparing BIBF 1120 with other small-molecule VEGFR inhibitors
Pharmacodynamic and pharmacokinetic profile
BIBF 1120 binds to the ATP-binding site in the cleft between the NH2 and the COOH terminal lobes of the kinase domain. It inhibits targeted kinases including all three VEGFR subtypes (IC50, 13–34 nmol/L), PDGFR-α and PDGFR-β (IC50, 59 and 65 nmol/L), and FGFR types 1, 2, and 3 (IC50, 69, 37, and 108 nmol/L, respectively); as well as in corresponding human and rodent kinases. In addition, BIBF 1120 inhibits FLT-3 (inhibition of acute myelogenous leukemia cell proliferation), and members of the Src-family (Src, Lyn, and Lck).
By contrast, receptor tyrosine kinases, such as EGFR and HER2, InsR, IGF-IR, or the cell cycle kinases CDK1, CDK2, and CDK4 were not inhibited at concentrations below 1000 nmol/L.
After oral administration, maximum BIBF 1120 plasma concentrations occurred mainly 1–4 hours after administration. Citation61 BIBF 1120 showed a high apparent volume of distribution during the terminal phase, both after single dose and at steady state, which might indicate a high tissue distribution of the drug, without any decrease in exposure over time during continuous daily treatment with BIBF 1120. From clinical investigation, the cleavage of [14C]BIBF 1120 by esterase-catalyzed hydrolysis is the prevalent metabolic reaction; CYP450-dependent metabolism was found to be minor. The terminal half-life of BIBF 1120 was determined to be 19 hours. BIBF 1120 is mainly excreted via the liver.
In the Hilberg experiment, the inhibition of cell proliferation and apoptosis of endothelial cells derived from umbilical veins (HUVEC) and skin micro-vessels (HSMEC) was obtained with BIBF 1120 (EC50, <10 nmol/L) and was preceded by inhibition of MAPK and Akt phosphorylation. Inhibition of basic-FGF-stimulated HUVEC proliferation required higher drug concentrations (EC50, 290 nmol/L), although activation of both MAPK and Akt was at least partially suppressed at concentrations down to 100 nmol/L. On the pericytes, BIBF 1120 inhibited proliferation of PDGF-BB-stimulated bovine retinal pericytes with an EC50 of 79 nmol/L. In cultures of human vascular smooth muscle cells, BIBF 1120 blocks the activation of MAPK or of Akt at concentrations down to 100 nmol/L.
Clinical efficacy
Due to its unique triple-targeting profile, the potential of BIBF 1120 to prevent both tumor growth and dissemination while also avoiding problems such as redundancy or resistance in advanced solid tumors was investigated.
Phase I
Based on several Phase I, BIBF 1120 monotherapy, dose-escalation trials, the MTD of BIBF 1120 was defined as 250 mg bid in Caucasian patients and 200 mg bid in Japanese patientsCitation64,Citation65 divided into two daily administrations more tolerable and without additional toxicity. The most frequent AEs were nausea, diarrhea, vomiting, abdominal pain, and fatigue of a mild-to-moderate intensity. Occasionally grade 3 or 4 reversible liver enzyme elevations (alanine amino-transferase and aspartate-amino-transferase) were observed, without any drug-related bleeding events.
Furthermore, Phase I dose-escalation studies investigating BIBF 1120 in combination with standard chemotherapy regimens have also been conducted. One study investigated the MTD of continuous oral treatment with BIBF 1120 in combination with standard-dose pemetrexed (500 mg/m2) in patients with recurrent NSCLC who had been treated with one prior platinum-based chemotherapy regimen,Citation66 while a second study investigated the safety, tolerability, and MTD of BIBF 1120 in combination with carboplatin (AUC 6) and paclitaxel (200 mg/m2) in previously untreated patients with advanced-stage (IIIB/IV) NSCLC.Citation67
The recommended dose of BIBF 1120 was maintained to be 200 mg bid even when combined with standard regimens for NSCLC; they obtained a good safety profile, with high tolerability and an optimal pharmacokinetic effect of BIBF 1120 when used in combined with standard chemotherapy. The AE profiles observed were comparable to those in the BIBF 1120 monotherapy trials, except for toxicities commonly related to the chemotherapy agent.
Promising efficacy effect was shown in both studies, but especially in the pemetrexed one: they enrolled 31 patients, and 26 received the treatment. Of the 26 patients treated, 21 completed the initial 21-day treatment cycle and were eligible to continue in the second one and beyond. Thirteen patients (50%) had stable disease as the best overall response, while eight patients showed progressive disease as best response; three patients had missed follow-up radiology data due to early treatment termination. Nine patients completed four cycles of combination therapy, and seven patients went on to receive BIBF 1120 monotherapy. One patient with a complete response obtained after 44 days after initiating treatment completed the study and has remained on 100 mg bid BIBF 1120 monotherapy for more than 3 years.
The most common reasons for study discontinuation were disease progression (57.7%) and dose-limiting toxicities (19.2%). Median PFS for all 26 treated patients was approximately 5.4 months.
Furthermore, dynamic contrast enhanced magnetic resonance imaging results demonstrated an antiangiogenic effect of BIBF 1120 in a substantial number of patients.
Phase II
The key Phase II evidence for BIBF 1120 in NSCLC has been obtained from a double-blind, two-arm, randomized monotherapy studyCitation68,Citation69 in patients with relapsed, advanced NSCLC of any histology. The primary endpoints were PFS and ORR.
Secondary endpoints included characterization of the safety and pharmacokinetic profiles of BIBF 1120, as well as OS.
A total of 73 patients were randomized with an Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–2 with locally advanced or metastatic relapsed NSCLC after failure of first- or second-line chemotherapy to continuous twice-daily treatment with 150 (37 patients) or 250 mg (36 patients) BIBF 1120 until disease progression ().
Figure 2 Demographic distribution of population from a Phase II randomized double-blind study with BIBF 1120 as monotherapy in advanced non-small cell lung cancer.
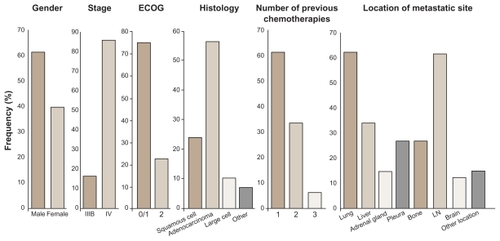
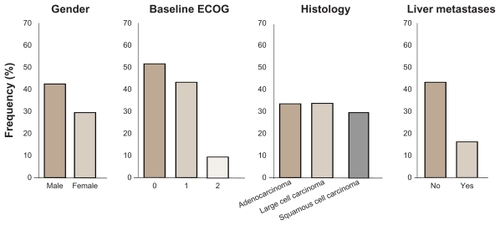
The median PFS of all patients (n = 73) was 6.9 weeks, and the median OS was 21.9 weeks with no significant difference between the two groups; the DCR was 59%. One PR was observed in the 250 mg bid arm, with a 74% reduction in tumor size for up to 9 months; 20 patients showed tumor shrinkage as best response, and the stable disease rate was 48%. Stratifying patients for PS, an ECOG PS 0–1 (n = 57) had a median PFS of 2.9 months, with a median OS of 9.5 months. Three patients maintained clinical benefit for more than 1 year, and four patients achieved a maximum decrease of at least 25% in tumor size ().
Figure 3 Demographic distribution by overall clinical response status from Phase II randomized double-blind study with BIBF 1120 as monotherapy in advanced non-small cell lung cancer.
With respect to physical functioning and global health status, 67.8% and 82.1% of all patients remained stable or showed an improvement within the first 42 days as measured by the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ)-C30. More than 50% of patients reported stable or improved cough, dyspnea, and pain on day 42 (87.5%, 58.9%, and 57.1% for each symptom, respectively) as measured by the EORTC QLQ-LC13. Twenty-two percent of patients discontinued before day 42 (19% and 26% for the 150 and 250 mg BIBF 1120 bid dose cohorts, respectively).
The majority of AEs reported were mild to moderate in nature and predominantly related to the gastrointestinal tract (nausea, diarrhea, and vomiting). Severe drug-related bleeding and hypertension were not observed, and there were no major differences in toxicity with regards to histology.
The BIBF 1120 tolerability was comparable between the two doses, with the exception of a higher frequency of liver enzyme elevations in the higher dose group ().
Table 2 Most frequent adverse events linked to different doses of BIBF 1120 as monotherapy from Phase II randomized doubleblind study in advanced non-small cell lung cancer
Steady state was reached by day 15 for both groups. The BIBF 1120 pre-dose plasma concentrations on days 15, 29, and 43 were stable during all this period for both doses, with no deviation from dose proportionality. Moderate-to-high inter-patient variability of BIBF 1120 pre-dose plasma concentrations was observed. In both arms, BIBF 1120 plasma concentrations increased within the first 3 hours after the first drug administration. There was only slight accumulation of BIBF 1120 plasma concentrations from day 1 to day 43 for both dose groups.
These Phase II data confirmed the promising single-agent activity of BIBF 1120 in patients suffering from recurrent NSCLC, warranting further development of BIBF 1120 in the Phase III setting.
Phase III development program
The BIBF 1120 Phase III clinical development program is currently underway, with patients being recruited into two pivotal studies, LUME-Lung 1 and 2. The LUME-Lung study program is investigating the potential benefit of adding BIBF 1120 to standard chemotherapy in patients with advanced NSCLC in the second-line setting. Based on the overall safety profile from Phase I and II investigations, BIBF 1120 200 mg bid is the recommended Phase III dose for a combination of BIBF 1120 with pemetrexed and docetaxel. Besides the primary endpoint of PFS, both trials are statistically powered to give adequate information on OS ().
Figure 4 Phase III trials ongoing with BIBF 1120 in combination with chemotherapy: actually the LUME-Lung 1 trial stopped to recruit new patients, while the LUME-Lung 2 is continuing recruitment.
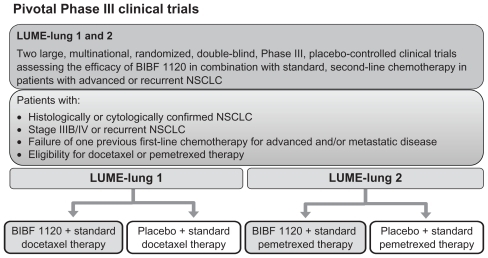
LUME-Lung 1 is a multicenter, randomized (1:1), double- blind study to investigate the efficacy and safety of BIBF 1120 200 mg bid plus standard docetaxel therapy compared with placebo plus standard docetaxel therapy in patients with stage IIIB/IV or recurrent NSCLC (all histologies) after relapse or failure of first-line chemotherapy.Citation70 Patients continue in 21-day treatment cycles upon completion of a minimum of four courses of combination therapy, or as long as they tolerate therapy or do not develop progression and do not meet one of the withdrawal criteria; patients who are ineligible for further combination therapy can continue to receive BIBF 1120/placebo monotherapy until disease progression or withdrawal criteria are met. The primary endpoint of the study is the PFS, while secondary endpoints are: OS, tumor response according to modified Response Evaluation Criteria In Solid Tumors (RECIST) criteria, clinical improvement, AEs (according to Common Terminology Criteria for Adverse Events [CTCAE] version 3.0), changes in safety laboratory parameters, and quality of life.
LUME-Lung 2 is a multicenter, randomized, double-blind study to investigate the efficacy and safety of BIBF 1120 200 mg bid plus standard pemetrexed therapy compared with placebo plus standard pemetrexed therapy in patients with stage IIIB/IV or recurrent nonsquamous NSCLC after relapse or failure of first-line chemotherapy.Citation71 The trial will be carried out by investigators who specialize in the treatment of NSCLC. Written informed consent will be obtained prior to randomization.
A total of 1300 patients will be enrolled, with each arm containing 650 patients. Patients will be assigned to receive either: pemetrexed (500 mg/m2 intravenously on day 1) and BIBF 1120 (200 mg twice daily) on days 2–21 of each 21-day cycle, or pemetrexed on day 1 plus placebo twice daily on days 2–21 of each 21-day cycle.
The primary endpoint will be PFS. Secondary endpoints include objective tumor response (at baseline and every 6 weeks) assessed according to modified RECIST criteria and OS.73 Safety will be evaluated using CTCAE version 3.0. Quality of life will be measured with various self-assessment questionnaires, including the EuroQoL EQ-5D, EORTC QLQ-C30, and EORTC QLQ-LC13. Patients will be treated until unacceptable toxicity or disease progression occurs or until another withdrawal criterion is met. Patients who discontinue combination therapy due to toxicity with either pemetrexed or BIBF 1120 and have not developed disease progression may continue with either blinded BIBF 1120 monotherapy, pemetrexed monotherapy, or placebo if they received at least 4 cycles of combination therapy and have not fulfilled any of the withdrawal criteria. If less than 4 cycles of combination therapy were received, patients may continue pemetrexed monotherapy. These patients will continue on treatment until disease progression or one of the other withdrawal criteria is met. These studies are currently ongoing.
Conclusion
Tumor angiogenesis remains a critical target for the treatment of patients with NSCLC. Multiple mechanisms are responsible for this aspect of tumor growth and, as a result, several approaches to treatment are necessary. A number of antiangiogenic TKIs currently under development may provide additional treatment options for patients in the future. Research continues to reveal novel pathways and targets. As oncologists strive to improve patients’ lives, toxicity remains an important consideration. Ongoing Phase III trials will bring into focus the changing role of these agents for patients with NSCLC.
BIBF 1120 differs from other angiogenesis inhibitors not only in its distinctive VEGFR-, PDGFR-, and FGFR-targeting profile, but also with regard to its sustained cellular duration of action and its pharmacokinetic profile.
Assuming its convenient oral application and good tolerability, no severe bleeding, skin reactions, hypertension, or hematological side effects are observed in patients suffering from all histologies.
In Phase II trials, the predominant dose-limiting toxic effects were reversible liver enzyme elevations, mostly in patients receiving BIBF 1120 doses above the MTD, and the most frequent AEs requiring dose adjustment or discontinuation were elevated liver enzymes, which were fully reversible and responded rapidly within 2 weeks of treatment discontinuation or dose reduction.
In patients who experienced nausea or vomiting, no dose reductions were necessary, without any differences between male and female patients. These kinds of side effects were treated with common antiemetic agents like metoclopramide, dimenhydrinate, or 5-HT3 receptor antagonist.
In all Phase II trials and in the Phase III ongoing trial, the squamous cell histology is not an exclusion criteria linked to the BIBF 1120: in the LUME-Lung 2 study, the nonsquamous histology is required by the selected chemotherapeutic agent, pemetrexed.
This is particularly important for patients with squamous cell NSCLC who are ineligible for treatment with bevacizumab and who develop more grade 3 or 4 AEs than the adenocarcinoma patients in trials with other oral antiangiogenic agents.
In this context, the potential use of BIBF 1120 in patients with squamous histology will be investigated as part of the pivotal Phase III LUME-Lung 1 study, which will therefore provide more substantial data regarding the efficacy and safety of BIBF 1120 in this key patient population.
Due to its unique and multiple targeting profile, BIBF 1120 has the potential to effectively prevent both tumor growth and dissemination, while also avoiding problems such as redundancy or resistance across the complex signaling networks. Clinical trials have demonstrated that, due to the non-CYP450-mediated metabolism of BIBF 1120, drug–drug interactions are not expected and, to date, no comedications have been excluded from BIBF 1120 trials.
This is of potential benefit both in considering combination with other cancer therapeutics and in taking into account medication being taken for comorbidities, which is a key issue for the majority of late-stage NSCLC patients. BIBF 1120 showed comparable efficacy data to other angiogenesis inhibitors in similar patient populations.
The adequate selection of patients based on clinical factors and ECOG score should be considered the first and most important step to identify the most appropriate kind of patient population.
In line with Phase I and II trials, BIBF 1120 represents a new and very interesting alternative in the NSCLC treatment as a monotherapy or in combination with chemotherapeutic agents. Phase III trials are actually ongoing and they will clarify whether the combination with chemotherapeutic agents will be a feasible choice, and if the use of BIBF 1120 will become part of a multimodality strategy with other targeted agents.
Disclosure
The authors report no conflicts of interest in this work.
References
- American Cancer SocietyDetailed guide: lung cancer – non-small cellWhat is non-small cell lung cancer?American Cancer Society2010 Available from: http://www.cancer.org/Cancer/LungCancer-Non-SmallCell/DetailedGuide/non-small-cell-lung-cancer-what-is-non-small-cell-lung-cancerAccessed April 21, 2011
- National Comprehensive Cancer Network (NCCN)Clinical practice guidelines in OncologyTMNon-small cell lung cancer. V.2.2010National Comprehensive Cancer Network2011 Available from: http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdfAccessed September 8, 2011
- CarmelietPJainRKAngiogenesis in cancer and other diseasesNature200040724925711001068
- FolkmanJKlagsbrunMAngiogenic factorsScience19872354424472432664
- KeedyVLSandlerABInhibition of angiogenesis in the treatment of non-small cell lung cancerCancer Sci2007981825183017892508
- FerraraNKerbelRSAngiogenesis as a therapeutic targetNature200543896797416355214
- LohelaMBryMTammelaTAlitaloKVEGFs and receptors involved in angiogenesis versus lymphangiogenesisCurr Opin Cell Biol20092115416519230644
- PrestaMDell’EraPMitolaSMoroniERoncaRRusnatiMFibroblast growth factor/fibroblast growth factor receptor system in angiogenesisCytokine Growth Factor Rev20051615917815863032
- CaoYCaoRHedlundEMRegulation of tumor angiogenesis and metastasis by FGF and PDGF signaling pathwaysJ Mol Med20088678578918392794
- MurakamiMSimonsMFibroblast growth factor regulation of neovascularizationCurr Opin Hematol20081521522018391788
- BlumenscheinGRJrGatzemeierUFossellaFPhase II, multicenter, uncontrolled trial of single-agent sorafenib in patients with relapsed or refractory, advanced non-small-cell lung cancerJ Clin Oncol2009274274428019652055
- WilhelmSMCarterCTangLBAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesisCancer Res2004647099710915466206
- NEXAVAR (sorafenib) tablets, oral [package, insert]Wayne, NJBayer Health-Care Pharmaceuticals, Inc2009
- CarterCChenCBrinkCSorafenib is efficacious and tolerated in combination with cytostatic agents in preclinical models of human non-small cell lung carcinomaCancer Chemother Pharmacol20075918319516724239
- MinamiHKawadaKEbiHPhase I and pharmacokinetic study of sorafenib, an oral multikinase inhibitor, in Japanese patients with advanced refractory solid tumorsCancer Sci2008991492149818477034
- BlumenscheinGRJrGatzemeierUFossellaFPhase II, multicenter, uncontrolled trial of single-agent sorafenib in patients with relapsed or refractory, advanced non-small-cell lung cancerJ Clin Oncol2009274274428019652055
- SchillerJHLeeJWHannaNHTraynorAMCarboneDPA randomized discontinuation Phase II study of sorafenib versus placebo in patients with non-small cell lung cancer who have failed at least two prior chemotherapy regimens: E2501J Clin Oncol (Meeting Abstracts)20082615 Supplabstr 8014
- SchillerJHFlahertyKTRedlingerMSorafenib combined with carboplatin/paclitaxel for advanced non-small cell lung cancer: a Phase I subset analysisJ Clin Oncol (Meeting Abstracts)20062418 Supplabstr 7194
- ScagliottiGNovelloSvonPJPhase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancerJ Clin Oncol2010281835184220212250
- Bayer HealthCare Pharmaceuticals Inc, Onyx Pharmaceuticals IncPhase 3 trial of Nexavar in first-line advanced non-small cell lung cancer does not meet primary endpoint of overall survival Press release2010614 Available from: http://www.onyx-pharm.com/view.cfm/685/Phase-3-Trial-of-Nexavar-in-First-Line-Advanced-Non-Small-Cell-Lung-Cancer-Does-Not-Meet-Primary-Endpoint-of-Overall-SurvivalAccessed August 9, 2011
- HerbstRSJohnsonDHMininbergEPhase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small cell lung cancerJ Clin Oncol2005232544255515753462
- TonraJRDeeviDSCorcoranESynergistic antitumor effects of combined epidermal growth factor receptor and vascular endothelial growth factor receptor-2 targeted therapyClin Cancer Res2006122197220716609035
- LindJSDingemansAMGroenHJA multicenter, Phase II study of erlotinib and sorafenib in chemotherapy-naive patients with advanced non-small cell lung cancerClin Cancer Res2010163078308720395213
- HerbstRSBlumenscheinGRJrKimESSorafenib treatment efficacy and KRAS biomarker status in the Biomarker-Integrated Approaches of Targeted Therapy for Lung Cancer Elimination (BATTLE) trialJ Clin Oncol (Meeting Abstracts)20102815 Suppl abstr 7609
- SUTENT® (sunitinib malate) capsules, oral [package insert]New York, NYPfizer Inc2010
- NovelloSScagliottiGVRosellRPhase II study of continuous daily sunitinib dosing in patients with previously treated advanced non-small cell lung cancerBr J Cancer20091011543154819826424
- SocinskiMANovelloSBrahmerJRMulticenter, Phase II trial of sunitinib in previously treated, advanced nonsmall-cell lung cancerJ Clin Oncol20082665065618235126
- ReckMFrickhofenNCedresSSunitinib in combination with gemcitabine plus cisplatin for advanced nonsmall cell lung cancer: a Phase I dose-escalation studyLung Cancer2010
- RobertFSandlerASchillerJHSunitinib in combination with docetaxel in patients with advanced solid tumors: a Phase I dose-escalation studyCancer Chemother Pharmacol201066466968020043166
- PfizerA study in patients with non-small cell lung cancer testing if erlotinib plus SU011248 (sunitinib) is better than erlotinib alone (SUN1058)ClinicalTrialsgov [website on the Internet]Bethseda, MDUS National Library of Medicine2011 [updated August 26, 2011]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00265317. NLM identifier: NCT00265317Accessed September 12, 2011
- PfizerA study in patients with non-small cell lung cancer to test if erlotinib plus SU011248 is better than erlotinib aloneClinicalTrials gov [website on the Internet]Bethseda, MDUS National Library of Medicine2011 [updated July 6, 2011]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00457392. NLM identifier: NCT00457392Accessed September 12, 2011
- HennequinLFStokesESThomasAPNovel 4-anilinoquinazolines with C-7 basic side chains: design and structure activity relationship of a series of potent, orally active, VEGF receptor tyrosine kinase inhibitorsJ Med Chem2002451300131211881999
- WedgeSROgilvieDJDukesMZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administrationCancer Res2002624645465512183421
- HoldenSNEckhardtSGBasserRClinical evaluation of ZD6474, an orally active inhibitor of VEGF and EGF receptor signaling, in patients with solid, malignant tumorsAnn Oncol2005161391139715905307
- NataleRBBodkinDGovindanRVandetanib versus gefitinib in patients with advanced non-small cell lung cancer: results from a two-part, double-blind, randomized Phase II trialJ Clin Oncol2009272523252919332730
- HeymachJVJohnsonBEPragerDRandomized, placebo-controlled Phase II study of vandetanib plus docetaxel in previously treated non small-cell lung cancerJ Clin Oncol2007254270427717878479
- HerbstRSSunYEberhardtWEVandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, Phase 3 trialLancet Oncol7201011761962620570559
- De BoerRArrietaOGottfriedMVandetanib plus pemetrexed versus pemetrexed as second-line therapy in patients with advanced non-small cell lung cancer (NSCLC): a randomized, double-blind Phase III trial (ZEAL)J Clin Oncol (Meeting Abstracts)20092715 Supplabstr 8010
- NataleRBThongprasertSGrecoFAVandetanib versus erlotinib in patients with advanced non-small cell lung cancer (NSCLC) after failure of at least one prior cytotoxic chemotherapy: a randomized, double-blind Phase III trial (ZEST)J Clin Oncol (Meeting Abstracts)20092715 Supplabstr 8009
- LeeJHirshVParkKVandetanib versus placebo in patients with advanced non-small cell lung cancer (NSCLC) after prior therapy with an EGFR tyrosine kinase inhibitor (TKI): a randomized, double-blind Phase III trial (ZEPHYR)J Clin Oncol201028abstr 7525
- HeckmanCAHolopainenTWirzeniusMThe tyrosine kinase inhibitor cediranib blocks ligand-induced vascular endothelial growth factor receptor-3 activity and lymphangiogenesisCancer Res2008684754476218559522
- WedgeSRKendrewJHennequinLFAZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancerCancer Res2005654389440015899831
- LaurieSAGauthierIArnoldAPhase I and pharmacokinetic study of daily oral AZD2171, an inhibitor of vascular endothelial growth factor tyrosine kinases, in combination with carboplatin and paclitaxel in patients with advanced non-small-cell lung cancer: the National Cancer Institute of Canada clinical trials groupJ Clin Oncol2008261871187818398152
- GossGShepherdFALaurieSA Phase I and pharmacokinetic study of daily oral cediranib, an inhibitor of vascular endothelial growth factor tyrosine kinases, in combination with cisplatin and gemcitabine in patients with advanced non-small cell lung cancer: a study of the National Cancer Institute of Canada Clinical Trials GroupEur J Cancer20094578278819091548
- GossGDArnoldAShepherdFARandomized, double-blind trial of carboplatin and paclitaxel with either daily oral cediranib or placebo in advanced non-small-cell lung cancer: NCIC Clinical Trials Group BR24 studyJ Clin Oncol201028495519917841
- GadgeelSMWozniakAEdelmanMJCediranib, a VEGF receptor 1, 2, and 3 inhibitor, and pemetrexed in patients (pts) with recurrent non-small cell lung cancer (NSCLC)J Clin Oncol (Meeting Abstracts)20092715 Supple19007
- DyGKMandrekarSJNelsonGDA randomized Phase II study of gemcitabine (G) and carboplatin (C) with or without cediranib (AZD2171 [CED]) as first-line therapy in advanced non-small cell lung cancer (NSCLC)J Clin Oncol (Meeting Abstracts)20102815 Supplabstr 7603
- ChoueiriTKAxitinib, a novel anti-angiogenic drug with promising activity in various solid tumorsCurr Opin Investig Drugs20089658671
- KellyRJRixeOAxitinib (AG-013736)Recent Results Cancer Res2010184334420072829
- MartinLPKozloffMFKrzakowskiMAxtinib (AG-013736; AG) combined with chemotherapy in patients (pts) with advanced non-small cell lung cancer (NSCLC) and other solid tumorsJ Clin Oncol (Meeting Abstracts)20092715 Supplabstr 3559
- RugoHSHerbstRSLiuGPhase I trial of the oral antiangiogenesis agent AG-013736 in patients with advanced solid tumors: pharmacokinetic and clinical resultsJ Clin Oncol2005235474548316027439
- SchillerJHLarsonTOuSHEfficacy and safety of axitinib in patients with advanced non-small-cell lung cancer: results from a Phase II studyJ Clin Oncol2009273836384119597027
- SchutzFAChoueiriTKSternbergCNPazopanib: clinical development of a potent anti-angiogenic drugCrit Rev Oncol Hematol201177316317120456972
- HurwitzHIDowlatiASainiSPhase I trial of pazopanib in patients with advanced cancerClin Cancer Res2009154220422719509175
- AltorkiNGuarinoMLeePPreoperative treatment with pazopanib (GW786034) a multikinase angiogenesis inhibitor in early stage non-small cell lung cancer (NSCLC): a proof-of-concept Phase II studyJ Clin Oncol (Meeting Abstracts)20082615 Supplabstr 7557
- CarlomagnoFVitaglianoDGuidaTZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks, oncogenic RET kinaseCancer Res2002627284729012499271
- PolverinoACoxonAStarnesCAMG 706, an oral, multikinase inhibitor that selectively targets vascular endothelial growth factor, platelet-derived growth factor, and kit receptors, potently inhibits angiogenesis and induces regression in tumor xenograftsCancer Res2006668715872116951187
- BlumenscheinGRJrReckampKStephensonGJPhase 1b study of motesanib, an oral angiogenesis inhibitor, in combination with carboplatin/paclitaxel and/or panitumumab for the treatment of advanced non-small cell lung cancerClin Cancer Res20101627929020028752
- BlumenscheinGRKabbinavarFFMenonHRandomized, open-label Phase II study of motesanib or bevacizumab in combination with paclitaxel and carboplatin (P/C) for advanced nonsquamous non-small cell lung cancer (NSCLC)J Clin Oncol (Meeting Abstracts)20102815 Supplabstr 7528
- AmgenMONET1-MOtesanib NSCLC Efficacy and Tolerability StudyClinicalTrialsgov [website on the Internet]Bethseda, MDUS National Library of Medicine2007 [updated September 1, 2011]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00460317. NLM identifier: NCT00460317Accessed October 4, 2011
- HilbergFRothGJKrssakMBIBF1120: triple angiokinase inhibitor with sustained receptor blockade and good anti-tumor efficacyCancer Res200868124774478218559524
- HilbergFBrandstetterIRothGJIn vitro and in vivo efficacy of BIBF 1120, a small molecule triple angiokinase inhibitor, in combination with taxanes. Proceedings AACR-NCI-EORTCInt Conf Mol Targets Cancer Ther200559A19
- StopferPRothWMrossKBPharmacokinetic characterization of BIBF 1120, an orally active triple angiokinase inhibitor (VEGFR, PDGFR, FGFR) inadvanced cancer patientsEur J Cancer Suppl200641226
- LeeCPTaylorNJAttardGA Phase I study of BIBF 1120, an orally active triple angiokinase inhibitor (VEGFR, PDGFR, FGFR) given continuously to patients with advanced solid tumours, incorporating dynamic contrast enhanced magnetic resonance imaging (DCE-MRI)J Clin Oncol (Meeting Abstracts)20062418 Supplabstr 3015
- KanedaHOkamotoISatohTPhase I dose-escalation study of continuous oral treatment with the angiokinase inhibitor BIBF 1120 in patients with advanced solid tumours (and presentation)Eur J Cancer Suppl200972abstr 1243
- HannaNEllisPStopferPA Phase I study of continuous oral treatment with the triple angiokinase inhibitor BIBF 1120 together with pemetrexed in previously treated patients with non-small cell lung cancerJ Thoracic Oncol200728S717
- CamidgeDRConklingPStephensonJShapiroDA Phase I study of continuous oral treatment with the triple angiokinase inhibitor BIBF 1120 together with carboplatin and paclitaxel in patients with advanced non-small cell lung cancer (NSCLC)J Thoracic Oncol200728S730
- Von PawelJKaiserREschbachCEfficacy, safety and pharmacokinetic (PK) results of a Phase II study with the triple angiokinase inhibitor BIBF 1120 in patients suffering from advanced non small cell lung cancer (NSCLC)J Thoracic Oncol200834S61
- ReckMKaiserREschbachCA Phase II double-blind study to investigate efficacy and safety of two doses of the triple angiokinase inhibitor BIBF 1120 in patients with relapsed advanced non-small-cell lung cancerAnn Oncol20112261374138121212157
- ClinicalTrials.govLUME-Lung 1: BIBF 1120 plusdocetaxel as compared to placebo plus docetaxel in 2nd line non small cell lung cancer2008 Available from: http://clinicaltrials.gov/ct2/show/NCT00805194?term=BIBF+1120&rank=6Accessed April 15, 2009
- ClinicalTrials.govLUME-Lung 2: BIBF 1120 plus pemetrexed compared to placebo plus pemetrexed in 2nd line nonsquamous NSCLC2008 Available from: http://clinicaltrials.gov/ct2/show/NCT00806819?term=BIBF+1120&rank=4Accessed April 15, 2009
- EisenhauerEATherassePBogaertsJNew response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1)Eur J Cancer20094522824719097774