Abstract
We herein report the case of a 39-year-old Japanese female with eosinophilic pneumonia associated with natalizumab. The patient with bronchial asthma had multiple sclerosis and was treated using natalizumab. The patient was referred to our department because of a persistent cough. A chest computed tomography (CT) scan revealed bilateral patchy consolidation surrounded by ground-glass opacity. A bronchoalveolar lavage (BAL) was performed. Eosinophil levels in the BAL fluid were increased and the patient was consequently diagnosed as eosinophilic pneumonia associated with natalizumab. Therefore, natalizumab treatment was discontinued. Subsequent chest CT findings showed a remarkable improvement without any treatment.
Introduction
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system (CNS). MS is considered to involve genetic and environmental factors.Citation1 Recently, the number of MS patients in Japan has been increasing.Citation2 The clinical symptoms of MS include loss of vision, cognitive impairment, and motor and sensory disturbance. Moreover, relapse and remission of the disease frequently occur.Citation3 Therefore, preventing recurrence is important. In Japan, five types of disease-modifying drugs such as IFNβ-1b, IFNβ-1a, fingolimod, natalizumab, and glatiramer acetate are currently available. Revealing the unknown adverse effects of these drugs is important. Therefore, we report a case of eosinophilic pneumonia (EP) associated with natalizumab, as a third-line treatment for MS and review the overall respiratory adverse events associated with natalizumab in Introduction to avoid confusion. Natalizumab is a humanized immunoglobulin G4 monoclonal antibody, which binds to the α4 subunit of α4β1 and α4β7 integrins and blocks their binding to endothelial receptors, thereby inhibiting the recruitment of immune cells to inflammatory tissues. The common adverse effects of natalizumab are fatigue and allergic reactions. Moreover, there is progressive multifocal leukoencephalopathy as a serious adverse effect.Citation4,Citation5
Case Report
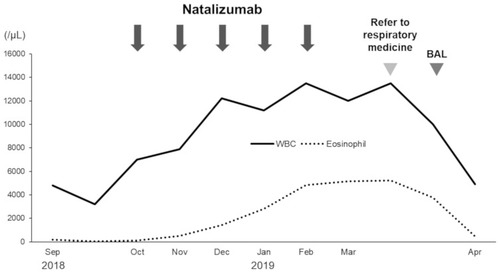
A 39-year-old Japanese female underwent treatment using natalizumab for relapsing-remitting multiple sclerosis (RRMS) in the division of neurology in our hospital. The patient was referred to division of respiratory medicine with a 3-month history of cough.
The patient was diagnosed with MS 9 years ago. She was treated in the neurology division since diagnosis. An initial treatment of interferon beta (IFN-β)-1b was discontinued because she was diagnosed as EP by bronchoscopy and needed steroid treatment. A second-line treatment using fingolimod was initiated 7 years ago. Treatment using natalizumab was initiated 5 months before the first visit to our department, because of poor control of MS. The patient was a never smoker, drank alcoholic beverages occasionally, and had a history of bronchial asthma. She did not have a history of keeping pets or taking new drugs including Chinese herbal medicines or supplements. There was no exposure that could trigger asthma exacerbations. Her vital signs were within a healthy range and no abnormalities were found in a physical examination. Her symptoms such as fever, difficulty breathing, night sweats, wheezing and weight loss were not observed. The results of an arterial blood gas test were also within a healthy range. A chest X-ray revealed patchy consolidation in the bilateral upper and left lower lung field (). A chest computed tomography (CT) scan showed bilateral patchy consolidation surrounded by ground-glass opacity (). Furthermore, laboratory tests demonstrated increased levels of white blood cells (13,500/μL), eosinophils (5220/μL), serum IgE (600.9 IU/mL), and surfactant protein D (112 ng/mL). No elevation in tumor markers (carcinoembryonic antigen (1.0 ng/mL), soluble cytokeratin-19 fragments (0.5 ng/mL), neuron-specific enolase (15.7 ng/mL)), serum β-D-glucan, anti-neutrophil cytoplasmic antibody (ANCA), and sialylated carbohydrate antigen KL-6 (130 U/mL) was noted (). The drug-induced lymphocyte stimulation tests were not performed in this case. The results of a pulmonary functional test were normal including carbon monoxide diffusing capacity, and fractional exhaled nitric oxide was 23 ppb. The normal results of the arterial blood gas test and pulmonary functional test might suggest inflammation of the lung was relatively mild. Bronchoalveolar lavage (BAL) from the right upper lobe was performed without transbronchial lung biopsy. No bacteria, fungi, and acid-fast bacillus were detected in the BAL fluid culture. Eosinophilia was revealed in the cell fraction of the BAL fluid (61% macrophages, 35% eosinophils, 4% lymphocytes) (). As a result, chest CT showed EP pattern. Moreover, other underlying diseases causing interstitial lung disease (ILD), such as cancer, fungal or bacterial infection, and ANCA-associated vasculitis, were excluded. Furthermore, there were no obvious abnormalities in the chest X-rays before natalizumab administration and no increase in peripheral blood eosinophils before natalizumab treatment. Therefore, natalizumab treatment was discontinued due to the possibility of EP associated with it. Peripheral blood eosinophilia and pulmonary infiltrates improved after the 6-week cessation of natalizumab treatment ( and ). Therefore, the patient was diagnosed as EP probably associated with natalizumab. Her symptoms of multiple sclerosis remained stable for a while after the cessation of natalizumab treatment. However, methylprednisolone pulse therapy was started because multiple sclerosis was exacerbated on MRI after EP subsided. After three cycles of methylprednisolone pulse therapy, fingolimod was started.
Figure 1 A chest X-ray revealed patchy consolidation in the bilateral upper and left lower lung field (white arrow).
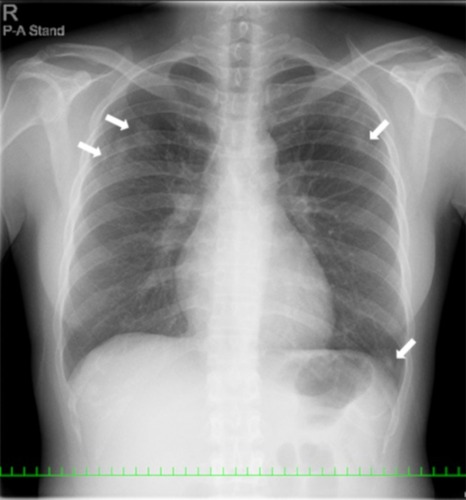
Figure 2 CT scans showed bilateral patchy consolidation surrounded by ground-glass opacity (white arrow).
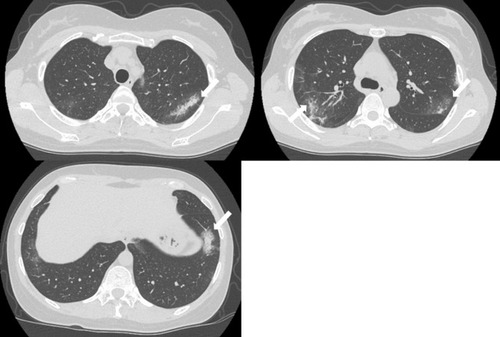
Figure 3 BAL fluid showed increased numbers of eosinophils with Papanicolaou staining (A, ×400). Under high magnification, the eosinophils were stained light green with nuclear localization (B, ×800).
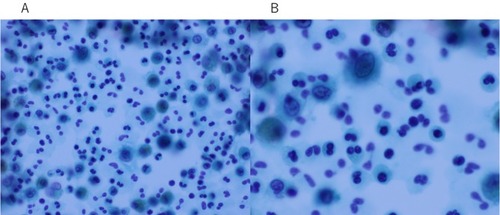
Figure 4 A chest X-ray and chest CT images showed an improvement in lung infiltrates after the discontinuation of natalizumab.
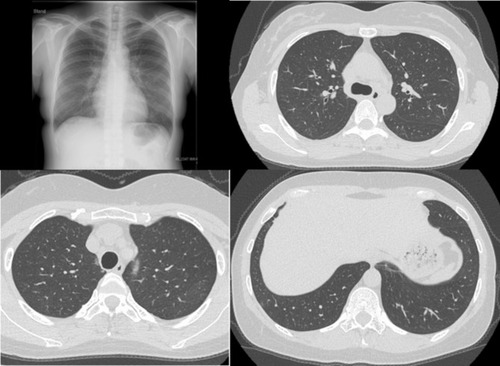
Table 1 Laboratory Findings On The Initial Visit
Discussion
Recently, MS phenotypes have been classified into five distinct subtypes: RRMS, clinically isolated syndrome, radiologically isolated syndrome, primary-progressive MS, and secondary progressive MS. The majority of patients with MS follow the course of RRMS.Citation6 Early treatment is effective in the relapsing-remitting phase, and it has been reported that IFN-β improves prognosis compared with placebo.Citation7 However, when IFN-β cannot be used because of side effects or if it is poorly effective, a second-line treatment should be initiated. Natalizumab and fingolimod are reported to be effective as a drug after second-line treatments.Citation8,Citation9 Natalizumab is a humanized immunoglobulin G4 monoclonal antibody, which binds to the α4 subunit of α4β1 and α4β7 integrins and blocks their binding to endothelial receptors. As a result, natalizumab can inhibit the influx of lymphocytes to the CNS and attenuate inflammation. It has been reported that the number of circulating lymphocytes, monocytes, eosinophils, and basophils increase during natalizumab treatment.Citation4,Citation5 A few cases of hypereosinophilia (≥1500/μL) have been reported as an adverse event of natalizumab treatment.Citation10 On the other hand, it has also been reported that natalizumab could induce adverse events such as nontuberculous mycobacteria, mycobacterium tuberculosis and paracoccidioidomycosis, sarcoidosis and Crohn’s disease.Citation11–Citation15 However, there has been only one case of suspected EP.Citation16 In that case, the proportion of eosinophils in BAL was 12%, and it did not meet the criteria of EP. Hence, this was the first case with concurrent hypereosinophilia and EP as adverse event of natalizumab. The summary of cases with respiratory adverse events associated with natalizumab is shown in . However, the biologic agents are reported to have something to do with EP.Citation17,Citation18
Table 2 Summary Of Cases With Respiratory Adverse Events Associated With Natalizumab
A diagnosis of drug-induced ILD requires correct identification of the drug, singularity of the drug, temporal eligibility, characteristic clinical symptoms, imaging results, BAL results and pathologic patterns of the reaction to the specific drug and the exclusion of other causes for the ILD.Citation19 A diagnosis of EP requires both characteristic clinical imaging features and a demonstration of alveolar eosinophilia consisting of at least 25% of the eosinophils in the BAL.Citation20 In the current case, a chest CT scan showed lung infiltrates consistent with an EP pattern, and eosinophils in the BAL were increased. Furthermore, the patient had no other causes for the ILD such as cancer, fungal or bacterial infection, and ANCA-associated vasculitis. It is noteworthy that hypereosinophilia and lung infiltrates were improved only by the discontinuation of natalizumab, which seemed to be the causative agent of the EP. Therefore, the patient was diagnosed with EP associated with natalizumab. Although the details of the pathophysiology of EP were unknown, it was thought to be that there was an involvement of T helper 2 (Th2) cell-mediated allergic reaction. Asthma is a chronic inflammatory airway disease associated with Th2 cell cytokines, such as interleukin-4 (IL-4), IL-5, and IL-13, which promote airway eosinophilia.Citation21 Moreover, Marchand E et al reported that the prevalence of asthma in patients diagnosed with idiopathic chronic EP was 64%.Citation22 It should be noted that she had a past history of IFN-β treatment-induced eosinophilia, although there is little structural similarity between IFN-β and natalizumab. Drug hypersensitivity in the past appears to have an increased tendency to develop sensitivity to new drugs.Citation23 Therefore, it is possible that the patient could be susceptible to an allergic reaction to natalizumab. As for possible molecular-biological mechanism of natalizumab-induced EP, it binds to α4β1 and α4β7 integrins and could induce endothelial injury. As a result, IL-33 and vascular endothelial growth factor (VEGF) are released from injured endothelial cells, recruit eosinophil to the lung, and activate macrophage and dendritic cells, leading to T-helper cell type 2 (Th2) immune responses. T-lymphocytes further promote recruitment and activation of eosinophil in the lung via production of IL-5. Indeed, Abbas M et al reported 3 cases of hypereosinophilia in patients with multiple sclerosis treated with natalizumab and they show a Th2 cell-driven condition, suggesting that natalizumab could activate Th2 lymphocytes in genetically predisposed individuals.Citation10
Conclusion
In conclusion, clinicians should consider the possibility of EP after the initiation of natalizumab treatment. It is important to highlight new adverse events when they are encountered.
Ethics
The Ethics Committee at Kobe University/Kobe University Graduate School of Medicine confirms that ethics approval for case report or case series are waived.
Consent For Publication
Written informed consent for publication of the case details included accompanying images was obtained from the patient.
Disclosure
All of the authors report that they have no conflicts of interest to disclose in this work.
References
- Sawcer S, Franklin RJ, Ban M. Multiple sclerosis genetics. Lancet Neurol. 2014;13:700–709. doi:10.1016/S1474-4422(14)70041-924852507
- Osoegawa M, Kira J, Fukazawa T, et al. Temporal changes and geographical differences in multiple sclerosis phenotypes in Japanese: nationwide survey results over 30 years. Mult Scler. 2009;15:159–173. doi:10.1177/135245850809837218987106
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi:10.1016/S0140-6736(08)61620-718970977
- Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi:10.1056/NEJMoa04439716510744
- Ransohoff RM. Natalizumab for multiple sclerosis. N Engl J Med. 2007;356:2622–2629. doi:10.1056/NEJMct07146217582072
- Katz Sand I. Classification, diagnosis, and differential diagnosis of multiple sclerosis. Curr Opin Neurol. 2015;28:193–205. doi:10.1097/WCO.000000000000020625887774
- Goodin DS, Reder AT, Ebers GC, et al. Survival in MS: a randomized cohort study 21 years after the start of the pivotal IFNβ-1b trial. Neurology. 2012;78:1315–1322. doi:10.1212/WNL.0b013e3182535cf622496198
- Spelman T, Kalincik T, Zhang A, et al. Comparative efficacy of switching to natalizumab in active multiple sclerosis. Ann Clin Transl Neurol. 2015;2:373–387. doi:10.1002/acn3.18025909083
- Bergvall N, Makin C, Lahoz R, et al. Relapse rates in patients with multiple sclerosis switching from interferon to fingolimod or glatiramer acetate: a US claims database study. PLoS One. 2014;9:e88472. doi:10.1371/journal.pone.008847224516663
- Abbas M, Lalive PH, Chofflon M, Simon HU, Chizzolini C, Ribi C. Hypereosinophilia in patients with multiple sclerosis treated with natalizumab. Neurology. 2011;77:1561–1564. doi:10.1212/WNL.0b013e318233b39121975205
- de Masson A, Maillart E, Veziris N, Meyssonnier V, Papeix C, Caumes E. Cavitary pulmonary disease in a patient treated with natalizumab. Presse Med. 2014;43:1009–1012. doi:10.1016/j.lpm.2013.12.01224742610
- Hradilek P, Zeman D, Tudik I, Zapletalova O, Ulmann V. Asymptomatic lung disease caused by Mycobacterium kansasii as an opportunistic infection in a patient treated with natalizumab for relapsing-remitting multiple sclerosis. Mult Scler. 2014;20:639–640. doi:10.1177/135245851350157223959714
- Dahdaleh D, Altmann DM, Malik O, Nicholas RS. Breathlessness, night sweats, and weight loss on natalizumab. Lancet. 2012;380:726–727. doi:10.1016/S0140-6736(12)61401-922920745
- Almeida KJ, Barreto-Soares RV, Campos-Sousa RN, Campos-Sousa MG, Bor-Seng-Shu E. Pulmonary paracoccidioidomycosis associated with the use of natalizumab in multiple sclerosis. Mult Scler. 2018;24:1002–1004. doi:10.1177/135245851876309129649930
- Parisinos CA, Lees CW, Wallace WA, Satsangi J. Sarcoidosis complicating treatment with natalizumab for Crohn’s disease. Thorax. 2011;66:1109–1110. doi:10.1136/thx.2010.15576221233484
- Curto E, Munteis-Olivas E, Balcells E, Domínguez-Álvarez MM. Pulmonary eosinophilia associated to treatment with natalizumab. Ann Thorac Med. 2016;11:224–226. doi:10.4103/1817-1737.18576227512514
- Bartal C, Sagy I, Barski L. Drug-induced eosinophilic pneumonia: A review of 196 case reports. Medicine (Baltimore). 2018;97:e9688. doi:10.1097/MD.000000000000968829369189
- Jin F, Wang ST. Chronic eosinophilic pneumonia after trastuzumab and radiation therapy for breast cancer: a case report. Medicine (Baltimore). 2019;98:e14017. doi:10.1097/MD.000000000001401730608451
- Camus P, Fanton A, Bonniaud P, Camus C, Foucher P. Interstitial lung disease induced by drugs and radiation. Respiration. 2004;71:301–326. doi:10.1159/00007963315316202
- Cottin V. Eosinophilic lung diseases. Clin Chest Med. 2016;37:535–556. doi:10.1016/j.ccm.2016.04.01527514599
- Lambrecht BN, Hammad H, Fahy JV. The cytokines of asthma. Immunity. 2019;50:975–991. doi:10.1016/j.immuni.2019.03.01830995510
- Marchand E, Etienne-Mastroianni B, Chanez P, Lauque D, Leclerc P, Cordier JF. Idiopathic chronic eosinophilic pneumonia and asthma: how do they influence each other? Eur Respir J. 2003;22:8–13. doi:10.1183/09031936.03.0008560312882444
- Leslie C, Paul A. Patterson’s Allergic Diseases. 7th ed Wolters Kluwer Health;2009: 239–314.