Abstract
Hyperuricemia is usually associated with hypertension, diabetes mellitus, metabolic syndrome and chronic kidney disease. Accumulating data from epidemiological studies indicate an association of increased uric acid (UA) with cardiovascular diseases. Possible pathogenic mechanisms include enhancement of oxidative stress and systemic inflammation caused by hyperuricemia. Arterial stiffness may be one of the possible pathways between hyperuricemia and cardiovascular disease, but a clear relationship between increased UA and vascular alterations has not been confirmed. The review summarizes the epidemiological studies investigating the relationship between UA and arterial stiffness and highlights the results of interventional studies evaluating arterial stiffness parameters in patients treated with UA-lowering drugs.
Introduction
Uric acid (UA) is the final product of dietary and endogenous purine metabolism. While hyperuricemia has been classically associated with gouty arthritis, asymptomatic hyperuricemia is frequently found in metabolic syndrome, diabetes mellitus, chronic kidney disease or hypertension.Citation1–Citation3
Epidemiological studies have shown that hyperuricemia is a predictor of cardiovascular morbidity and mortality.Citation4,Citation5 Besides the association of increased UA levels with metabolic disturbances known to augment cardiovascular risk, a direct involvement of UA in cardiovascular disease has also been suggested.Citation6,Citation7
Arterial stiffness is the consequence of decreased arterial distensibility caused mainly by arterial structural alterations consisting of elastin degradation and an increase in stiff collagen. In clinical practice, arterial stiffness can be measured non-invasively using various parameters that investigate regional, local or systemic arterial stiffness. The most widely used parameter to evaluate regional arterial stiffness is pulse wave velocity (PWV), which increases with progressive stiffening of the arterial wall. PWV can be measured as the ratio between the distance traveled by the wave and the travel time, at various sites of the arterial tree. Carotid-femoral (aortic) pulse wave velocity (cfPWV) evaluates aortic stiffness, while carotid-radial pulse wave velocity (crPWV), brachial-ankle pulse wave velocity (baPWV) and brachial-radial pulse wave velocity (brPWV) measure both central and peripheral arterial stiffness. Aortic PWV, which proved to be an independent predictor of all-cause and cardiovascular mortality in various populations with different cardiovascular risk factors, is considered “the gold standard” measurement of great artery stiffness.Citation8 Age and hypertension are the most important determinants of arterial stiffness. Other cardiovascular risk factors, including diabetes, metabolic syndrome, obesity and chronic kidney disease, have also been associated with an increase in arterial stiffness.Citation9
Local arterial stiffness can be measured at a single site, at the level of a central artery (i.e. carotid artery) or more distally (i.e. femoral artery), using echo-tracking systems.Citation10 The cardio-ankle vascular index (CAVI), a parameter which, in contrast to PWV, is not influenced by blood pressure values, evaluates both the aorta and the peripheral arteries of the lower limbs.Citation11 Systemic arterial compliance can also be determined, but the techniques for its non-invasive measurement are complex and have poor precision. Moreover, the predictive value of systemic arterial stiffness for cardiovascular events has not yet been proved. When arteries are stiff, the pulse wave travels faster to peripheral sites and returns to the heart from reflection points, during ventricular systole, augmenting aortic systolic pressure. The increase in central systolic pressure is measured by the augmentation index (Aix), which is calculated as the difference between the second and first systolic peaks of the central pressure wave, divided by the central pulse pressure and expressed as a percentage. Aix depends on wave reflection and is not only influenced by arterial stiffness, but also by peripheral vascular tone.Citation10
Whether a cause-effect relationship exists between hyperuricemia and arterial stiffness or both are determined by the same etiological factors is not known.
Review Literature Sources and Aim
A PubMed/Medline, Web of Science, Embase and Goggle scholar data bases search was performed using the keywords “uric acid” combined with “cardiovascular disease”, “arterial stiffness”, “arterial stiffness and uric acid lowering drugs”, between 1996 and 2019. We have also made hand searches of the references of retrieved literature. Only articles written in English were included.
The aim of this narrative review is to present the pathogenic hypothesis that may explain the association between UA and arterial stiffness, as well as the results of epidemiological and clinical studies evaluating the possible role of UA in the development of arterial stiffness. Finally, the effects of the therapeutic intervention using UA-lowering drugs on arterial stiffness parameters are discussed.
UA Metabolism
The two purines, adenine and guanine, which are the main constituents of nucleotides, are finally converted to UA in a sequence of chemical reactions catalyzed by specific enzymes. Adenosine monophosphate (AMP) is initially converted to inosine, following two different enzymatic pathways; in one of them, AMP is transformed by deaminase into inosine monophosphate (IMP) and then by nucleotidase dephosphorylation into inosine, while, according to the other pathway, a phosphate group is removed by nucleotidase to form adenosine, which is subsequently, transformed by deaminase into inosine. Inosine is then converted to hypoxanthine by purine nucleoside phosphorylase (PNP). Hypoxanthine is oxidized to xanthine by xanthine oxidase (XO) and then xanthine is converted to UA by the same enzyme, XO. Guanine monophosphate (GMP) is metabolized to guanosine by nucleotidase and then guanosine is transformed into guanine by PNP. Subsequently, guanine deaminase converts guanine to xanthine, which is then oxidized to UA.Citation1,Citation12
UA is produced mainly by the liver, gut, muscles, kidneys and the vascular wall. Normal blood UA values are 1.5 to 6.0 mg/dl in women and 2.5 to 7 mg/dl in men.Citation1 Blood concentration is maintained due to complex regulatory mechanisms which control UA production and excretion. Increased circulating UA is the consequence of high intake, increased UA production, decreased renal elimination or a combination of these mechanisms. Diets rich in animal proteins (meat, seafood) are the principal exogenous source of purines. High intake of fructose is another dietary source of UA. Fructose is converted by fructokinase to fructose 1 phosphate, which reduces cellular phosphate and circulating ATP levels. The decrease in intracellular phosphate concentrations activates AMP deaminase, which catalyzes the transformation of AMP into inosine monophosphate and increases UA. Humans cannot oxidize UA because of the lack of uricase, which is present in other mammals and transforms UA into more soluble compounds, subsequently excreted by the kidneys. The largest part of circulating UA is represented by the soluble urate salt. When UA concentration exceeds 6.8 mg/dl, it crystallizes as monosodium urate.Citation1,Citation12
UA is eliminated by the kidneys (two thirds) and by the gastrointestinal tract (one third). In the kidney, UA is filtered by the glomeruli and then reabsorbed and secreted by the proximal and distal contorted tubes. Finally, the kidneys eliminate 10% of the filtered UA. Impaired renal function is an important cause of hyperuricemia.Citation1
Pathogenic Mechanisms Involved in UA-Related Arterial Stiffness
It has been shown that in physiological concentrations, UA is a potent antioxidant capable of scavenging reactive oxygen species and peroxynitrite.Citation13,Citation14 In experimental renal insufficiency, hyperuricemia was associated with improved endothelial function.Citation15 In humans, intravenous administration of UA improves endothelial function, reduces exercise-induced oxidative stress,Citation16 and increases antioxidant capacity.Citation17 A favorable effect of UA administration has also been reported in type 1 diabetes mellitus patients.Citation18 Moreover, low levels of UA, caused by decreased URAT1 (blood vessels and kidney proximal cells transporter) associated with SLC22A12 loss-of-function mutation, induce endothelial dysfunction.Citation19
The mechanisms involved in UA-related vascular stiffness are complex and incompletely elucidated.
Figure 1 Mechanisms involved in uric acid induced arterial stiffness. XO, xanthine oxidase; ROS, reactive oxygen species; URATv1, voltage- driven urate efflux transporter 1; NLRP3,nod-like receptor family protein 3; VSMCs, vascular smooth muscle cells.
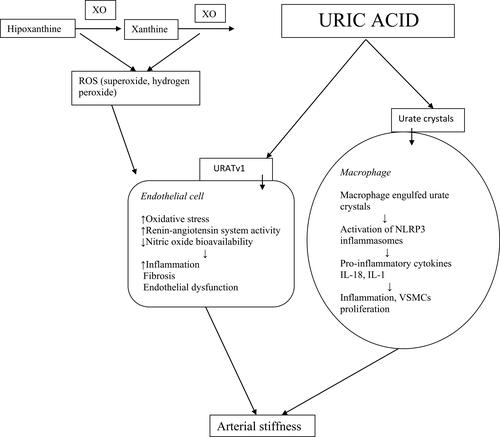
In particular environmental conditions, UA may induce oxidative stress, endothelial dysfunction and stimulate vascular inflammation and fibrosis. In the presence of native low-density lipoproteins, UA showed to have antioxidant effects but, after the oxidation of low-density lipoproteins, it induces pro-oxidant effects.Citation20
Important data regarding the involvement of UA in oxidative stress come from experimental studies. It has been proved that hyperuricemia stimulates nicotinamide adenine dinucleotide phosphate oxidase activity and, subsequently, oxygen species synthesis.Citation21 UA-induced oxidative stress may activate the Notch 1 pathway, which is involved in the UA inflammatory process. Administration of epigallocatechin-3-gallate, a flavanol derived from green tea extracts, with anti-inflammatory and antioxidant effects, decreased UA-induced inflammation and oxidative stress.Citation22
Hyperuricemia decreases nitric oxide production in endothelial cellsCitation23,Citation24 or, reacting with nitric oxide, reduces its activity.Citation25,Citation26 Renin-angiotensin system activation by increased UA may also impair endothelial nitric oxide production.Citation27 The decrease in nitric oxide bioavailability promotes endothelial dysfunction, increases vascular tone and may contribute to arterial stiffness.Citation28 The renin-angiotensin-aldosterone system is also responsible for an increased stiffness of the cytoskeleton in endothelial cellsCitation29 and extracellular matrix fibrosis.Citation30 Oxidative stress may mediate angiotensin 2 activation of TGF-ß1 which, subsequently, stimulates vascular fibrosis. TGF-ß1 induces proteoglycan, fibronectin and collagen synthesis which increases vascular wall stiffness.Citation31,Citation32 UA-induced oxidative stress may also stimulate the production of endothelin-1,Citation33 a potent vasoconstrictor known to increase arterial stiffness.Citation34
In cultured vascular smooth muscle cells (VSMCs), UA may induce monocyte chemoattractant protein-1Citation35 and cyclooxygenase 2,Citation36 promoting proliferation of VSMCs and inflammatory responses. The increase in platelet-derived growth factor A-chain expression, caused by hyperuricemia, may also stimulate VSMCs proliferation.Citation37
UA induces the transcription nuclear factor-ҡBCitation35 which has proinflammatory and proatherogenic effects in the vascular wall.Citation38 UA also stimulates C-reactive proteinCitation39 and production of proinflammatory cytokines, including TNF-α.Citation40 Moreover, C-reactive protein may increase the production of cellular adhesion molecules, promote cellular apoptosis and induce endothelial dysfunction and arterial stiffness.Citation41 A recent study has shown that UA can induce endothelial glycocalyx shedding which may contribute to increased oxidative stress and reduced nitric oxide bioavailability.Citation42
Besides its involvement in inflammation and oxidative stress, UA may also alter the natural turnover of non-cross-linked soluble elastin, thus reducing arterial wall distensibility.Citation43
Not only soluble UA but also the crystallized form of UA may cause vascular damage. UA crystals are recognized by toll-like receptor and induce inflammasome activation. Inflammasomes are protein structures that may play a role in the onset and development of several diseases, including gout, nonalcoholic fatty liver disease and vascular disease.Citation44
Two main mechanisms explaining the relationship between hyperuricemia and arterial stiffness have been proposed: one independent of urate crystal and the other, urate crystal dependent.Citation45 In the former mechanism, soluble UA may pass into the vascular wall via urate transporter GLUT 9 (glucose transporter) or URATv1 (voltage-driven urate efflux transporter 1) and initiate intracellular inflammation and oxidative stress.Citation46 In the latter mechanism, urate crystals, engulfed by vascular macrophages, activate nod-like receptor family protein 3 (NLRP3) inflammasome. Subsequently, inflammasome cleaves pro-IL-1ß into active IL-1ß, which induces inflammation and collagen formation.Citation47 Because UA starts to crystallize within the human body when serum UA level exceeds its solubility limit (6.8 mg/dl), this mechanism may be considered in patients with elevated UA.Citation45
Important data suggest that XO, the enzyme that catalyzes the final two reactions of purine catabolism leading to UA formation, may contribute to vascular damage, independently of UA.
During the catalytic process, XO reduces oxygen resulting in reactive oxygen species (hydrogen peroxide and superoxide anion). In physiological conditions, XO-derived reactive oxygen species may have favorable biologic effects, including the modulation of systemic redox balance and the defense reaction against bacterial infections. However, increased superoxide production by XO induces oxidative stress, systemic inflammation and, subsequently, endothelial dysfunction and vascular damage.Citation1,Citation14 The mechanism of UA induced arterial stiffness is schematically represented in .
XO is expressed in endothelial cells.Citation48 Experimental studies have shown that XO is involved in the progression of the atherosclerotic process, while XO inhibitors may reduce proatherogenic mediators and vascular lesions. In the aortic cells of the experimental atherosclerosis model of ApoE-/- mice, the authors reported an increased level of endothelial XO, which was associated with the development of endothelial dysfunction. The administration of febuxostat, a potent inhibitor of XO, reduces atherosclerotic lesions.Citation49
The activity of XO has also been studied in experimental models of arterial stiffness. In female rodents fed a Western-type diet rich in fat and fructose, XO inhibition with allopurinol improved Western diet-induced vascular stiffness. Favorable vascular effects were accompanied by decreased oxidative stress and aortic fibrosis.Citation50 Male mice fed a Western diet developed hyperuricemia, oxidative stress and proteinuria. Allopurinol administration for 16 weeks significantly reduced plasma UA, vascular XO activity, oxidative stress, arterial stiffness and proteinuria.Citation51
UA and Arterial Stiffness in Community-Based Studies
The relationship between UA and arterial stiffness has been evaluated in cross-sectional and prospective large population studies. Some of these studies investigated a possible gender influence on this relationship. Separate analyses of central and peripheral arterial stiffness parameters in relation to UA have also been made.
Cross-Sectional Studies
The relationship between UA and arterial stiffness parameters has been studied in various large populations and different ethnic groups. Gender differences in the relationship between UA and arterial stiffness have also been investigated.
In the Framingham Heart Study, which included 4140 participants with a low burden of risk factors, UA levels were significantly associated with arterial stiffness measured as cfPWV and crPWV. The correlation persisted after adjustment for conventional risk factors including age, sex, hypertension, body mass index, fasting plasma glucose, insulin levels, animal protein intake and renal function. However, the authors mentioned that the strength of the association diminished after these adjustments, suggesting that UA may not be a significant clinical contributor to arterial stiffness in healthy adults. The results of this study suggested the hypothesis that UA alone may not be sufficient to cause arterial damage, and that its role might be more important in patients with increased cardiovascular risk.Citation52
In the Korean Multi-Rural Communities Cohort study, the authors reported a positive linear correlation between serum UA levels and baPWV in both males and females.Citation53 In a study which included a population from the Western region of Beijing, with a homogenous lifestyle, subjects with higher UA had a 1.593 times risk of higher cfPWV and a tendency to higher CAVI. However, serum UA did not correlate with carotid intima-media thickness. Because cfPWV is a measure of arterial function, while CAVI is a measure of arterial function and structure and carotid intima-media thickness is a measure of arterial structure, the authors speculated that their results suggest a predominant effect of UA on arterial function and not on arterial structure. The associations did not differ between genders.Citation54 A large Japanese study, which included 27,360 healthy subjects, found significant positive correlations between UA and CAVI in both genders, even though UA values associated with increased CAVI were lower in females than in males.Citation55
Some other studies found a stronger association between UA and arterial stiffness in women than in men, or a significant association only in women but not in men. In a retrospective cross-sectional study that included 66,917 middle aged Korean subjects with a mean age of 39.4 years and low cardiovascular risk, serum UA quintiles were linearly correlated with baPWV in women, while in men, a J-shaped correlation was found. The associations were more consistent and stronger in women than in men. The authors suggested that high UA may have unfavorable vascular effects even in low-risk populations, but they underlined the possible influence of the ethnic lifestyle on their results.Citation56 A previous Korean study conducted in asymptomatic healthy subjects with a mean age of 50.6±8.9 years who underwent a health examination failed to find any association between UA and arterial stiffness in women and men younger than 55 years. High-normal or greater UA levels were associated with an increased risk of arterial stiffness, measured as baPWV, in women older than 55 years, but not in men. In this study, possible associated conditions, including a history of hypertension, diabetes mellitus, coronary artery disease, peripheral artery disease, chronic kidney disease and gout, as well as medications such as allopurinol, febuxostat, colchicine, probenecid, and benzbromarone represented exclusion criteria. Subjects with a coronary artery calcium score greater than 100 were also excluded. The authors concluded that healthy older women are exposed to hyperuricemia and have an increased risk of arterial stiffness.Citation57
In a community-based study of a Southwestern Chinese population, the authors reported a positive association between increased UA and arterial stiffness measured as CAVI in females, while in males the results were not statistically significant.Citation58 In a study on 5116 Chinese inhabitants of Beijing, UA was independently correlated with cfPWV only in women and not in men. The authors reported a higher number of current smokers and hypertensive patients among men who were much younger, with much lower serum total cholesterol, low-density lipoprotein cholesterol and triglycerides compared to women, and suggested that heterogeneity of cardiovascular risk factors between men and women might have contributed to the discrepancies regarding their results.Citation59 In a Japanese study carried out in 982 individuals who underwent health screening, a significant direct correlation between UA and baPWV was found only in women.Citation60 Similarly, in an apparently healthy Taiwanese population, high UA was correlated with baPWV only in women, independently of classical cardiovascular risk factors.Citation61
The mechanism that determines gender differences in the association between UA and arterial stiffness is not known. Young women seem to be protected by estrogens which favor renal UA elimination, whereas older women may have an increased risk of hyperuricemia and arterial stiffness.Citation57
It has been hypothesized that women with hyperuricemia may have a relatively increased risk of hyperinsulinemia and hyperglycemia compared to men, which may expose them to vascular alterations associated with hyperuricemia. This increased hyperinsulinemia and hyperglycemia in women was proposed as a possible explanation for the stronger association of serum UA with coronary artery disease in women than in men.Citation62 It has been suggested that women may have an increased vascular hypersensitivity to UA damage because cardiovascular events and worse prognosis linked to UA are more frequent in women. In the LIFE (Losartan Intervention For Endpoint reduction in hypertension) study, the association between serum UA levels and cardiovascular outcomes was significant only in women after adjustment for the Framingham risk score.Citation63 In another study, serum UA was independently associated with silent brain infarcts only in women.Citation64 Additionally, a possible increased risk of coronary heart disease related to hyperuricemia, in women and not in men has been suggested.Citation65
Other studies, however, found a direct correlation between UA levels and arterial stiffness only in men. In a Chinese study which included employees of a factory and their families, UA was moderately associated with cfPWV in the entire population and in men. The authors speculated that the lack of association in women might have been determined by the small number of women with increased UA levels.Citation66 In healthy Korean subjects with normal UA levels, high-normal UA levels were associated with arterial stiffness only in men after adjustment for confounders. Serum UA levels above 5.2 mg/dl predicted increased baPWV in healthy Korean men.Citation67 Among 3588 healthy Brazilian subjects, serum UA was linearly associated with cfPWV in men, but not in women.Citation68
The relationship between UA and arterial stiffness at different central or peripheral sites was evaluated in several studies that aimed to identify a possible preferential site for UA alteration of arterial distensibility. Three large Chinese studies found a positive correlation between UA and central artery stiffness.Citation58,Citation59,Citation69
The Cardiometabolic Risk in Chinese Study, which comprised a large sample of Chinese adults, reported that serum UA levels were significantly associated with central arterial stiffness measured as cfPWV, but not with peripheral arterial stiffness, evaluated as crPWV and carotid-dorsalis pedis artery PWV, independently of conventional risk factors, including sex, body mass index, lipids and glucose metabolism. The authors also reported that the relationship between UA and cfPWV was stronger in patients with normal blood pressure than in those with high blood pressure, suggesting a more important role of UA in arterial stiffness before the development of hypertension. They also found that high heart rate associated with increased UA may have synergistic effects on central arterial stiffness.Citation69
In a study performed in a community from the Western region of Beijing, cfPWV, crPWV, CAVI and carotid intima-media thickness were evaluated in relation to UA. People with higher UA had a significantly increased risk of high cfPWV and a tendency to increased risk of higher CAVI. There were no correlations between UA values and crPWV or carotid intima-media thickness.Citation58
In the community-based investigation of 5116 Chinese inhabitants of Haidian or Daxing District in Beijing, the impact of UA on regional PWV revealed that UA levels were not correlated with peripheral arterial stiffness parameters, crPWV and carotid-ankle PWV either in men or in women. The authors reported an independent correlation between UA and cfPWV, which was found only in women and not in men. Concomitantly, wave reflection was evaluated by the measurement of Aix75. The analysis of the correlation revealed a negative linear relationship between UA and Aix75 in the entire population, which disappeared after correction for classical risk factors.Citation59
Some other studies documented a correlation of UA only with peripheral markers of arterial stiffness. In a Korean community-based study, peripheral arterial stiffness measured as femoral-dorsalis pedis artery PWV and not aortic PWV was independently correlated with UA. This relationship was observed in women and only in non-smoking men with normal blood pressure.Citation70
However, several studies reported no association between UA and arterial stiffness.
In a study that selected 248 men and 371 women enrolled in the Brisighella Heart Study population survey, who were not taking antihypertensive, antidiabetic, lipid-lowering and UA-lowering drugs, cfPWV and carotid intima-media thickness were measured together with multiple metabolic parameters. The authors reported that patients in the third and fourth quartiles of UA had an increased prevalence of hypertension and metabolic syndrome. Increased UA was independently correlated with carotid intima-media thickness, but not with cfPWV. The linear correlation between UA and cfPWV disappeared after adjustment for age.Citation71 In a community-dwelling Chinese population study which included 623 men and 917 women, serum UA was correlated with cfPWV in univariate analysis but the correlation disappeared in multivariate analysis.Citation72 Arterial stiffness evaluated as CAVI in 1243 asymptomatic Chinese subjects was not independently correlated with UA either in men or in women.Citation73
In another study conducted in 1276 Korean adults without atherosclerotic cardiovascular disease, diabetes, renal disease or systemic disease, treatment affecting serum UA levels or medications for hypertension or dyslipidemia, serum UA was not associated with arterial stiffness evaluated as baPWV and heart femoral PWV.Citation74
The results of cross-sectional studies evaluating the relationship between UA and arterial stiffness in the general population are conflicting. Several explications may be speculated. The populations included were of different ethnicities and had various cardiovascular risk factors, which might have influenced vascular parameters and serum UA values. Moreover, the adjustment did not consider in all studies the protein intake or the administration of diuretics. The different parameters and methods used to measure arterial stiffness might also have contributed to the discrepancies between studies. Moreover, the effects of UA on vascular structure and function, along the arterial tree, are not clear and remain to be established by further research.
Prospective Studies
Several studies prospectively evaluated the relationship between UA and arterial stiffness. A subgroup of subjects from the Baltimore Longitudinal Study of Aging, which included community-dwelling elderly individuals, had repeated measurements of UA and cfPWV at baseline and over a median follow-up period of 6 years. The study found a significant independent relationship between increased serum UA and increased cfPWV over time in men but not in women. This gender difference was attributed to the fact that men had higher values of UA than women and longtime exposure to increased UA might have contributed to vascular alterations. The exclusion of subjects with high UA levels determined the loss of the significant association between UA and cfPWV. The study also showed a greater increase in PWV with aging in male subjects with higher serum UA values independently of age, blood pressure, renal function and metabolic parameters.Citation75 In another study, which evaluated 407 normotensive subjects (in 2002 at baseline and in 2011 or 2012 at the end of the study), serum UA predicted increased baPWV after adjustment for classical risk factors, including age, sex, body mass, systolic blood pressure and lipid parameters. Serum UA was also a predictor of decreased renal function.Citation76
The relationship between serum UA and peripheral arterial stiffness measured as crPWV was investigated in a prospective study of a population in the Pingguoyuan area (China). Higher baseline UA (corresponding to the third and fourth quartiles) was an independent predictor for increased crPWV after a period of 4.8 years of follow-up. The separate evaluation of patients with or without metabolic syndrome found that UA levels did not correlate with peripheral arterial stiffness in the metabolic syndrome group, while in non-metabolic syndrome patients, an independent relationship between UA and crPWV was reported, after adjustment for classical risk factors, including age, gender, diabetes mellitus, blood pressure and parameters of lipid metabolism. The results of this study indicated an important role of high-normal UA levels in predicting peripheral arterial stiffness, particularly in healthy subjects.Citation77
UA and Arterial Stiffness in Various Conditions
Hyperuricemia, Hypertension and Arterial Stiffness
Hyperuricemia is frequently seen in patients with hypertension,Citation78–Citation80 but a causal relationship between them has not yet been established. However, several experimental and clinical studies suggest a possible pathogenetic role for UA in the development of hypertension.
In rodents, hyperuricemia caused by uricase inhibitor oxonic acid determined increased blood pressure, while treatment with the XO inhibitor allopurinol or the uricosuric substance benziodarone prevented the development of hypertension.Citation81
In humans, a number of observational studies reported an independent association between UA and arterial stiffness and/or wave reflection parameters in hypertensive patients.Citation82–Citation85 In 1225 newly diagnosed, never-treated hypertensive patients, increased UA was independently correlated with cfPWV in both genders.Citation84 In contrast, in 222 untreated hypertensive patients with gout, increased cfPWV was associated with mild hyperuricemia in univariate analysis, but the association weakened after correction for classical risk factors and lost statistical significance after further adjustment for albuminuria. The authors emphasized the fact that they adjusted their results for albuminuria, a parameter that was not taken into consideration in other studies.Citation86 However, it was shown that increased values of serum UA accompany microalbuminuria.Citation87
Tsioufis et alCitation88 studied 450 consecutive subjects with never-treated, newly diagnosed (within the last 2 years) stage I–II essential hypertension and found that UA was independently associated with C-reactive protein and adiponectin levels, but the association with cfPWV disappeared after adjustment for confounders. The authors speculate that the close correlation of UA with C-reactive protein and adiponectin may suggest that systemic inflammation and hypoadiponectinemia which accompany hyperuricemia may mediate UA-associated vascular damage. Another study conducted in newly diagnosed hypertensive patients from the HARVEST study, with a mean age of 31±8 years, found that increased UA values were associated with low pulse pressure amplification, but not with aortic PWV or Aix.Citation89
In a study investigating carotid structural and functional parameters in treated hypertensive patients, UA was independently correlated with the internal carotid resistive index (a measure of carotid stiffness and microangiopathy) in women, but not in men.Citation90 All the other parameters, including intima-media thickness, Young’s elastic modulus, arterial compliance and the stiffness index, were not associated with UA after adjustment for confounders. The internal carotid resistive index is an early sign of vascular damage which precedes intima-media thickening,Citation91 and it has been speculated that hyperuricemia may be associated with an increased risk of intracranial microvascular damage.Citation90
In another study, which comprised 366 treated hypertensive subjects with various vascular risk factors, including diabetes mellitus and metabolic syndrome, UA was independently correlated with cfPWV only in women, after adjustment for classical risk factors. Moreover, UA was positively correlated with the ambulatory arterial stiffness index in women, but not in men.Citation92
In prospective studies, increased UA predicted the development of hypertension, after adjusting for traditional cardiovascular risk factors.Citation93–Citation98 Asymptomatic hyperuricemia in patients without comorbidities was an independent predictor for the development of hypertension.Citation99 Hyperuricemia was also a predictor for the development of hypertension in patients with prehypertension, after adjusting for classical risk factors.Citation100 Tomiyama et alCitation101 also found a longitudinal association between hyperuricemia and hypertension in 3274 Japanese men without hypertension followed up over a period of 8 years. UA, baPWV, C-reactive protein, estimated glomerular filtration rate and blood pressure were measured annually. After adjustment for classical risk factors, baPWV and C-reactive protein showed a longitudinal correlation with the new onset of hypertension. This study suggested that arterial stiffness and inflammation may be the link between hyperuricemia and the development of hypertension. The association was present only in men and not in women. The lack of correlation in women was explained by the small number of women included in the study and by the high threshold used to define hyperuricemia, similar for both genders – 7.0 mg/dl. In subjects with treated hypertension, a longitudinal relationship between increased UA and reduced flow-mediated vasodilatation was also found.Citation102 The influence on endothelial function may further explain UA increase in wave reflection and arterial stiffness.
UA and Arterial Stiffness in Diabetes Mellitus and Metabolic Syndrome
It has been shown that UA is related to insulin resistanceCitation44 and represents an independent predictor for the development of type 2 diabetes mellitus.Citation103,Citation104 Moreover, an independent predictive value of elevated UA for vascular complications and mortality of diabetic patients has been reported.Citation105 On the other hand, pathophysiological mechanisms induced by obesity and metabolic syndrome may cause hyperuricemia. Metabolic syndrome associated hyperinsulinemia increases urate reabsorption in the renal proximal tubule, augmenting serum UA.Citation106 Adipose tissue dysfunction in obese subjects may be associated with increased XO activity and UA synthesisCitation107 leading to oxidative stress and chronic low-grade inflammation. Arterial stiffness has been associated with diabetes mellitus and metabolic syndrome. Endothelial dysfunction, increased formation of advanced glycation end-products causing cross-linking with stiff collagen formation, low-grade inflammation and activation of the renin-angiotensin-aldosterone system are some important mechanisms that may contribute to arterial stiffness development in diabetes.Citation108
The relationship between UA and arterial stiffness has been investigated in several cross-sectional studies in diabetic and metabolic syndrome subjects, leading to conflicting results.
A study, which cross-sectionally investigated the association between serum UA levels and cfPWV and crPWV in male patients with newly diagnosed type 2 diabetes mellitus, found that UA was an independent predictor of both central (cfPWV) and peripheral arterial stiffness (crPWV).Citation109 In elderly Chinese (1020 subjects older than 60 years) with metabolic syndrome, UA correlated with baPWV after adjustment for gender, age, blood pressure, body mass index, serum creatinine, high-density lipoprotein, and insulin resistance.Citation110
Negative results were reported in a cross-sectional study including 614 adults from the Maastricht study with a mean age of 58.7±8.5 years, of whom 23.2% with diabetes mellitus, which evaluated the relationship of UA with cfPWV, carotid and femoral artery compliance, and carotid artery Young’s elastic modulus. UA was not independently associated with aortic, carotid or femoral arterial stiffness after adjustment for classical cardiovascular risk factors either in non-diabetic or in diabetic patients.Citation111
Large prospective studies including subjects with impaired glucose metabolism are needed to clarify the role of UA in the development of arterial stiffness in this high cardiovascular risk population.
UA and Arterial Stiffness in Chronic Kidney Disease (CKD)
The progression of CKD is accompanied by a gradual increase in arterial stiffness.Citation112 The possible mechanisms involved in the development of arterial stiffness in CKD are complex and incompletely elucidated. Alteration of the extracellular matrix caused by increased matrix metalloproteinase production, degradation of elastic fibers, accumulation of advanced glycation end products, increased oxidative stress and systemic inflammation, activation of the renin-angiotensin-aldosterone system and vascular calcification are some important processes involved in arterial stiffening in CKD.Citation113 Once developed, arterial stiffness increases systolic blood pressure and decreases diastolic blood pressure, leading to increased pulse pressure. This increased pulsatility is transmitted to distal arteries, including glomerular microcirculation, and may worsen kidney function.Citation114
Hyperuricemia is a common finding in CKD, which persists in dialysis patientsCitation115 and also, in a great proportion of patients after renal transplantation.Citation116
It has been shown that in patients with CKD, hyperuricemia is independently associated with an increased risk of cardiovascular and all-cause mortality.Citation117 Hyperuricemia-induced vascular damage may be one possible cause of this increased mortality in CKD patients. However, the results of studies investigating the relationship between UA and arterial stiffness in CKD patients are conflicting. In a cross-sectional study that included 399 patients with hypertensive CKD, serum UA was associated with cfPWV only in women, but the significance disappeared in adjusted analysis. Nevertheless, UA was independently associated with Aix in the entire population and also in men.Citation118 These results suggest a predominant influence of increased UA on wave reflection, which may be explained by UA alteration of endothelial function. It has been shown that endothelial dysfunction and microvascular alterations may modify Aix without influencing aortic PWV.Citation10
A study in 645 subjects on peritoneal dialysis found a significant direct association between baPWV and UA in young male patients. The results were not significant in women, and the authors explained this gender difference by the lower UA levels in women. In a subgroup of male patients with low levels of UA, the significance of the association was lost.Citation119
After renal transplantation, no significant correlation between serum UA and PWV, Aix, small and large artery elasticity and systemic vascular resistance was found. The authors explained this lack of association by the multiple non-traditional factors that might have influenced this relationship after renal transplantation, including various donor-related aspects, duration of dialysis prior to transplantation, graft function, and immunosuppressive therapy. In the subgroup that received UA-lowering treatment, there was no significant amelioration of arterial parameters.Citation120
Although CKD patients have increased UA levels and arterial stiffness, the results of studies evaluating the relationship between UA and vascular parameters are not conclusive. The various cardiovascular risk factors associated in CKD patients may impact in different ways both vascular and kidney structure and function and may influence the relation between UA and arterial stiffness.
UA and Arterial Stiffness in Patients with Manifest Atherosclerosis
In a study conducted in patients with stable angina pectoris or acute coronary syndromes, the relationship of arterial stiffness and arterial wave reflection with serum UA, total bilirubin and inflammatory status, evaluated as neutrophil to lymphocyte (N/L) ratio, was investigated cross-sectionally. The results indicated that increased arterial stiffness and arterial wave reflection were associated with a higher N/L ratio, higher serum UA levels, and lower serum total bilirubin levels both in stable and unstable coronary artery disease. The authors suggested that increased UA together with a higher N/L ratio and low bilirubin levels may represent an increased risk of arterial stiffness in coronary syndromes.Citation121 In stroke survivors, UA was independently associated with increased carotid PWV.Citation122 Future studies must test if treating hyperuricemia in clinically manifest atherosclerosis may favorably impact arterial stiffness and patients’ prognosis.
UA and Arterial Stiffness in Postmenopausal Women
Two Korean studies conducted in postmenopausal women found a direct correlation between UA and arterial stiffness.Citation123,Citation124 One of these studies found that UA was an important determinant of arterial stiffness after adjustment for risk factors in 841 postmenopausal women aged 50 years or older.Citation123 In the other study, which included 293 postmenopausal women with an abnormal range of serum UA, a J-shaped relationship between UA and arterial stiffness was reported. Patients in the first quartile of serum UA had a more increased risk for an elevated baPWV than subjects in the second quartile. The highest risk for increased arterial stiffness was in the fourth quartile. The authors speculated that reduced UA may have increased systemic oxidant activity and favored arterial stiffness, while patients in the second quartile of UA may have benefited from the antioxidant properties of UA, with a reduced risk of arterial stiffness. The greatest values of baPWV in the fourth quartile may have been the result of increased oxidative stress induced by high levels of UA. The correlations persisted after adjustment for age, body mass index, smoking status, alcohol intake, regular exercise, history of hypertension or diabetes, total cholesterol level, resting heart rate, and neutrophil-lymphocyte ratio. However, the authors mentioned that they did not take into consideration diuretic or allopurinol treatments which might have influenced UA levels.Citation124
Interventional Studies
The effect of UA-lowering drugs on arterial stiffness and wave reflection parameters was tested in interventional studies. UA-lowering medication acts by three main mechanisms: inhibition of UA synthesis (XO inhibitors), increase in UA renal elimination (uricosuric medication) and reduction in UA by oxidation to allantoin (uricase).Citation125
XO Inhibitor Drugs
The most important UA-lowering agents are XO inhibitors, which are classified as purinic (i.e. allopurinol) or non-purinic (i.e. febuxostat). Accumulating data indicate that XO inhibitors reduce the risk of major cardiovascular complicationsCitation126–Citation130 and mortality, particularly in high-risk patients.Citation130 Allopurinol is a common indication for hyperuricemia treatment in patients with gout.
The effects of allopurinol on parameters of arterial stiffness and wave reflection have been investigated in randomized control trials, which included subjects with various cardiovascular risk factors. A meta-analysis of the randomized controlled studies published before the end of 2015 found a favorable effect of allopurinol in improving Aix. No significant beneficial effects were reported for aortic PWV.Citation131
However, in a recent randomized controlled study, which included patients with mild-moderate chronic heart failure (New York Heart Association functional class I–III), short-term (3 month) low-dose allopurinol (300 mg daily) induced a significant reduction of UA levels (38% of the baseline UA level), without amelioration of cfPWV, aortic Aix and baPWV. The authors supposed that the important baseline alteration of vascular parameters may explain the negative results of their study.Citation132
The precise mechanisms involved in the vascular effects of allopurinol are not known, but the decrease in oxidative stress,Citation133–Citation135 systemic inflammation,Citation136 advanced glycation end productsCitation137 or reduction of blood pressure valuesCitation136,Citation138 under allopurinol treatment may all contribute to improving arterial stiffness. Moreover, it has been shown that the amelioration of endothelial function is mainly related to the reduction of oxidative stress and not to the degree of UA lowering. The predominant effect of allopurinol on Aix compared to aortic PWV has been explained by the fact that the decrease in oxidative stress which improves endothelial function predominantly influences wave reflection and Aix.Citation131 The short duration of allopurinol treatment, which might have been insufficient to influence great artery structure and aortic PWV, may be another explanation.
Febuxostat, a novel non-purinic XO inhibitor, has a very strong hypouricemic effect which is linked to its ability to block both isoforms of XO, whereas allopurinol inhibits only the reduced form.Citation139 Experimentally, febuxostat showed to have an important effect on endothelial UA because it reduces UA sequestrated along the arterial endothelium, with direct consequences on vascular oxidative stress.Citation140
In humans, febuxostat has greater antioxidant, renoprotective and anti-atherogenic effects than allopurinol.Citation141,Citation142 In a study that compared cfPWV in patients with chronic tophaceous gout treated with allopurinol or febuxostat for one year, the results indicated a significant increase in cfPWV in patients treated with allopurinol compared to those treated with febuxostat, who presented no changes in cfPWV.Citation143 In contrast to these studies showing superior protective vascular effects of febuxostat compared to allopurinol, a meta-analysis that evaluated the effects of UA-lowering medication on cardiovascular outcomes found no statistical differences between febuxostat and allopurinol in the incidence of cardiovascular events. Moreover, the authors reported a trend for less favorable cardiovascular outcomes with febuxostat.Citation144
Preliminary results of CARES (Cardiovascular Safety of Febuxostat and Allopurinol in Participants With Gout and Cardiovascular Comorbidities) trial, which investigated over 6000 patients with gout and cardiovascular diseases and compared cardiovascular outcomes associated with febuxostat to those associated with allopurinol, indicate that febuxostat was not inferior to allopurinol regarding the rates of cardiovascular events, but all-cause and cardiovascular mortality was higher with febuxostat than with allopurinol.Citation145 The most recent meta-analysis of randomized clinical trials, which investigated the safety of febuxostat compared to allopurinol or placebo, found an increased risk of cardiovascular death associated with febuxostat treatment, while the risk of cardiovascular events was not increased by febuxostat. The authors recommend caution when deciding to give febuxostat to high cardiovascular risk patients.Citation146
Uricosuric Medication
Among medications known to induce renal uric acid elimination, sodium-glucose transport protein 2 (SGLT-2) inhibitors were the most studied drugs in relation to arterial stiffness.
SGLT-2 inhibitors are a new class of hypoglycemic medication which reduces plasma glucose by inhibiting its renal reabsorption. Concomitantly, SGLT-2 reduces UA due to an uricosuric effect. SGLT-2 inhibitors have been shown to reduce cardiovascular disease, total mortality and heart failure hospitalizations. The mechanisms involved in these favorable cardiovascular effects are partly unknown, but blood pressure lowering, increased natriuresis, weight loss and decrease in arterial stiffness are discussed.Citation147
Few studies have investigated the effects of SGLT-2 inhibitors on arterial stiffness and UA. In a study conducted in 20 type 2 diabetic patients with hypertension, the association of canagliflozin with metformin and amlodipin for 6 months significantly lowered blood pressure, UA and cfPWW as compared to baseline parameters. In addition to blood pressure reduction, a decrease in UA was mentioned as one of the possible mechanisms involved in vascular protection by canagliflozin.Citation148 In another study of 19 patients with type 2 diabetes mellitus, anagliptin, a dipeptidyl peptidase-4 inhibitor, was replaced by tofogliflozin, a SGLT-2 inhibitor. After 6 months of treatment, CAVI was significantly reduced, concomitantly with markers of visceral obesity, advanced glycation end products and UA.Citation149 Empagliflozin, a potent and highly selective SGLT-2 inhibitor,Citation150 was shown to reduce blood pressure and improve measures of arterial stiffness in diabetic patients.Citation151,Citation152
However, the protective cardiovascular effect of SGLT-2 inhibitors has been shadowed by the results from post-marketing studies which indicate significant adverse effects, such as an increased risk of diabetic ketoacidosis and leg amputations.Citation153
Drugs That Increase UA Removal
Urate oxidase is used as a drug for the treatment of acute hyperuricemia in cancer patients receiving chemotherapy and in refractory gout patients.
In a small study conducted in ten patients with diabetes and ten healthy controls, infusion of intravenous urate oxidase reduced UA levels, but did not influence endothelial function and Aix, suggesting that UA reduction may not have direct vascular effects in diabetic patients.Citation154
Conclusions
The association of UA with arterial stiffness is documented in studies developed in the general population and also, in various conditions including menopause, hypertension, diabetes mellitus or chronic kidney disease. The increased oxidative stress and systemic inflammation associated with elevated levels of UA may be one of the possible mechanisms explaining this association. It is not clear whether UA is only a marker of increased arterial stiffness or an active mediator in the development of vascular alteration. Moreover, it has been speculated that oxidative stress induced by XO in the process of UA synthesis, but not UA itself, is the main cause of vascular damage, because UA in normal concentrations is a potent antioxidant and a possible vasculoprotector. However, it is supposed that hyperuricemia plays a role in the development of arterial hypertension and that UA-induced hypertension is mediated by arterial stiffness.Citation45 It is not clear whether there is a gender specific relationship between UA and arterial stiffness. Furthermore, the effect of UA along the arterial tree has not yet been established.
Lowering UA may improve arterial stiffness, but this effect is more evident with XO inhibitors. It is supposed that the favorable effects of XO inhibitors are particularly related to their ability to reduce oxidative stress and are less correlated with the amount of UA lowering. Future large interventional studies are needed to clarify if UA-lowering drugs are efficient in reducing vascular alterations and, subsequently, cardiovascular risk. This would add evidence to support the indication for hyperuricemia treatment in order to prevent cardiovascular events.
Disclosure
The authors report no other conflicts of interest in this work.
References
- Maiuolo J, Oppedisano F, Gratteri S, Muscoli C, Mollace V. Regulation of uric acid metabolism and excretion. Int J Cardiol. 2016;213:8–14. doi:10.1016/j.ijcard.2015.08.109
- Feig DI, Kang D-H, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359(17):1811–1821. doi:10.1056/NEJMra0800885
- Chaudhary K, Malhotra K, Sowers J, Aroor A. Uric acid key ingredient in the recipe for cardiorenal metabolic syndrome. Cardiorenal Med. 2013;3:208–220. doi:10.1159/000355405
- Wang JG, Staessen JA. Raised concentrations of serum creatinine and uric acid and the risks of mortality and cardiovascular disease. Cardiovasc Rev Rep. 2002;23:393–399.
- Wang JG, Staessen JA, Fagard RH, Birkenhager WH, Gong L, Liu L. Prognostic significance of serum creatinine and uric acid in older Chinese patients with isolated systolic hypertension. Hypertension. 2001;37:1069–1074. doi:10.1161/01.hyp.37.4.1069
- Niskanen LK, Laaksonen DE, Nyyssonen K, et al. Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men: a prospective cohort study. Arch Intern Med. 2004;164:1546–1551. doi:10.1001/archinte.164.14.1546
- Verdecchia P, Schillaci G, Reboldi G, et al. Relation between serum uric acid and risk of cardiovascular disease in essential hypertension. The PIUMA study. Hypertension. 2000;36:1072–1078. doi:10.1161/01.hyp.36.6.1072
- Vlachopoulos C, Azaouridis K, Stefanidis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi:10.1093/eurheartj/ehq024
- Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension. 2009;54:1328–1336. doi:10.1161/HYPERTENSIONAHA.109.137653
- Laurent S, Cockroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi:10.1093/eurheartj/ehl254
- Sun C-K. Cardio-ankle vascular index (CAVI) as an indicator of arterial stiffness. Integrated Blood Pressure Control. 2013;6:27–38. doi:10.2147/IBPC.S34423
- El Ridi R, Tallima H. Physiological functions and pathogenic potential of uric acid: a review. J Adv Res. 2017;8:487–493. doi:10.1016/j.jare.2017.03.003
- Backer BF. Towards the physiological function of uric acid. Review. Free Radic Biol Med. 1993;14(6):615–631. doi:10.1016/0891-5849(93)90143-i
- Glantzounis GK, Tsimoyannis EC, Kappas AM, Galaris DA. Uric acid and oxidative stress. Curr Pharm Des. 2005;11(32):4145–4151. doi:10.2174/138161205774913255
- Kurra V, Eraranta A, Jolma P, et al. Hyperuricemia, oxidative stress and carotid artery tone in experimental renal insufficiency. Am J Hypertens. 2009;22:964–970. doi:10.1038/ajh.2009.109
- Waring WS, Convery A, Mishra V, Shenkin A, Webb DJ, Maxwell SR. Uric acid reduces exercise-induced oxidative stress in healthy adults. Clin Sci (Lond). 2003;105:425–430. doi:10.1042/CS20030149
- Waring WS, Webb DJ, Maxwell SR. Systemic uric acid administration increases serum antioxidant capacity in healthy volunteers. J Cardiovasc Pharmacol. 2001;38:365–371. doi:10.1097/00005344-200109000-00005
- Waring WS, McNight JA, Webb DJ, Maxwell SR. Uric acid restores endothelial function in patients with type 1 diabetes and regular smoking. Diabetes. 2006;55:3127–3132. doi:10.2337/db06-0283
- Sugihara S, Hisatome I, Kuwabara M, et al. Depletion of uric acid due to SCL22A12 (URAT1) loss-of-function mutation causes endothelial dysfunction in hypouricemia. Circ J. 2015;79(5):1125–1132. doi:10.1253/circj.CJ-14-1267
- Patterson RA, Horsley ET, Leake DS. Prooxidant and antioxidant properties of human serum ultrafiltrates toward LDL: important role of uric acid. J Lipid Res. 2003;44:512–521. doi:10.1194/jlr.M200407-JLR200
- Sánchez-Lozada LG, Soto V, Tapia E, et al. Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am J Renal Physiol. 2008;295:F1134– F1141. doi:10.1152/ajprenal.00104.2008
- Xie H, Sun J, Chen Y, Zong M, Li S, Wang Y. EGCG attenuates uric acid-induced inflammatory and oxidative stress responses by medicating the NOTCH pathway. Oxid Med Cell Longev. 2015;2015:214836. doi:10.1155/2015/214836
- Choi Y-J, Yoon Y, Lee K-Y. Uric acid induces endothelial dysfunction by vascular insulin resistance associated with the impairment of nitric oxide synthesis. FASEB J. 2014;28(7):3197–3204. doi:10.1096/fj.13-247148
- Zharikov S, Krotova K, Hu H, et al. Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells. Am J Physiol Cell Physiol. 2008;295:C1183–C1190. doi:10.1152/ajpcell.00075.2008
- Jin M, Yang F, Yang I, et al. Uric acid, hyperuricemia and vascular diseases. Front Biosci. 2012;17:656–669. doi:10.2741/3950
- Suzuki T. Nitrosation of uric acid induced by nitric oxide under aerobic conditions. Nitric Oxide. 2007;16:266–273. doi:10.1016/j.niox.2006.10.008
- Khosla UM, Zharikov S, Finch JL, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–1742. doi:10.1111/j.1523-1755.2005.00273.x
- Corry DB, Eslami P, Yamamoto K, Nyby MD, Makino H, Tuck ML. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular rennin-angiotensin system. J Hypertens. 2008;26:269–275. doi:10.1097/HJH.0b013e3282f240bf
- Drüppel V, Kusche-Vihrog K, Grossmann C, et al. Long-term application of the aldosterone antagonist spironolactone prevents stiff endothelial cell syndrome. FASEB J. 2013;27:3652–3659. doi:10.1096/fj.13-228312
- Izzo JL Jr. Systolic hypertension, arterial stiffness, and vascular damage: role of the renin-angiotensin system. Blood Press Monit. 2000;5(Suppl 2):S7–S11. doi:10.1097/01.hco.0000126581.89648.10
- Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi:10.1056/NEJM199411103311907
- Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi:10.1161/01.ATV.0000160548.78317.29
- Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348:730–732. doi:10.1038/348730a0
- Du Plooy CS, Martha Cornelia Mels C, Huisman HC, Kruger R. The association of endothelin-1 with markers of arterial stiffness in black South African women: the SABPA Study. J Amino Acids. 2015;2015:8. doi:10.1155/2015/481517
- Kanellis J, Watanabe S, Li JH, et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41:1287–1293. doi:10.1161/01.HYP.0000072820.07472.3B
- Kang DH, Nakagawa T, Feng L, et al. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002;13:2888–2897. doi:10.1097/01.asn.0000034910.58454.fd
- Rao GN, Corson MA, Berk BC. Uric acid stimulates vascular smooth muscle cell proliferation by increasing platelet-derived growth factor A-chain expression. J Biol Chem. 1991;266:8604–8608.
- Liu T, Zhang L, Joo D, Sun S-C. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. doi:10.1038/sigtrans.2017.23
- Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell production of human vascular cells. J Am Soc Nephrol. 2005;16:3553–3562. doi:10.1681/ASN.2005050572
- Netea MG, Kullberg BJ, Blok WL, Netea RT, van der Meer JW. The role of hyperuricemia in the increased cytokine production after lipopolysaccharide challenge in neutropenic mice. Blood. 1997;89:577–582. doi:10.1182/blood.V89.2.577
- Park S, Lakatta EG. Role of inflammation in the pathogenesis of arterial stiffness. Yonsei Med J. 2012;53(2):258–261. doi:10.3349/ymj.2012.53.2.258
- Ko J, Kang H-J, Kim D-A, et al. Uric acid induced the phenotype transition of vascular endothelial cells via induction of oxidative stress and glycocalyx shedding. FASEB J. 2019;25:fj201901148R. doi:10.1096/fj.201901148R
- Yamanaka H, Osaka M, Takayama M, Munakata K, Nejima J, Katayama M. Age-adjusted level of circulating elastin as a cardiovascular risk factor in medical check-up individuals. J Cardiovasc Med (Hagerstown). 2014;15:364–370. doi:10.2459/JCM.0b013e328360940b
- Kushiyama A, Nakatsu Y, Matsunaga Y, et al. Role of uric acid metabolism-related inflammation in the pathogenesis of metabolic syndrome components such as atherosclerosis and nonalcoholic steatohepatitis. Mediators Inlamm. 2016;2016. doi:10.1155/2016/8603164
- Kuwabara M, Kanbay M, Hisatome I. Uric acid and hypertension because of arterial stiffness. Hypertension. 2018;72:582–584. doi:10.1161/HYPERTENSIONAHA.118.11496
- Mishima M, Hamada T, Maharani N, et al. Effects of uric acid on the NO production of HUVECs and its restoration by urate lowering agents. Drug Res (STUTTG.). 2016;66:270–274. doi:10.1055/s-0035-1569405
- Gicquel T, Robert S, Loyer P, et al. IL-1β production is dependent on the activation of purinergic receptors and NLRP3 pathway in human macrophages. FASEB J. 2015;29:4162–4173. doi:10.1096/fj.14-267393
- Moriwaki Y, Yamamoto T, Suda M, et al. Purification and immunohistochemical tissue localization of human xanthine oxidase. Biochim Biophys Acta. 1993;1164(3):327–330. doi:10.1016/0167-4838(93)90266-t
- Nomura J, Busso N, Yves A, et al. Xantine oxidase inhibition by fenbuxostat attenuates experimental atherosclerosis in mice. Sci Reports. 2014;4:4554. doi:10.1038/srep04554
- Lastra G, Manrique C, Jia G, et al. Xanthine oxidase inhibition protects against Western diet-induced aortic stiffness and impaired vasorelaxation in female mice. Am J Physiol Regul Integr Comp Physiol. 2017;313:R67–R77. doi:10.1152/ajpregu.00483.2016
- Aroor AR, Jiaa G, Habibia J, et al. Uric acid promotes vascular stiffness, maladaptive inflammatory responses and proteinuria in western diet fed mice. Metabolism. 2017;74:32–40. doi:10.1016/j.metabol.2017.06.006
- Mehta T, Nuccio E, McFan K, Madero M, Sarnak MJ, Jala D. Association of uric acid with vascular stiffness in the Framingham study. Am J Hypertens. 2015;28(7):877–883. doi:10.1093/ajh/hpu253
- Bae JS, Shin DH, Park PS, et al. The impact of serum uric acid level on arterial stiffness and carotid atherosclerosis: the Korean Multi-Rural Communities Cohort study. Atherosclerosis. 2013;231(1):145–151. doi:10.1016/j.atherosclerosis.2013.08.017
- Liu H, Liu J, Zhao H, Zhou Y, Li L, Wang H, For the BEST Research Group. Relationship between serum uric acid and vascular function and structure markers and gender difference in a real-world population of China-From Beijing Vascular Disease Patients Evaluation Study (BEST) study. J Atheroscler Thromb. 2018;25:254–261. doi:10.5551/jat.39685.
- Nagayama D, Yamaguchi T, Saiki A, et al. High serum uric acid is associated with increased cardio-ankle vascular index (CAVI) in healthy Japanese subjects: a cross-sectional study. Atherosclerosis. 2015;239(1):163–168. doi:10.1016/j.atherosclerosis.2015.01.011
- Hwang J, Hwang JH, Chung SM, Kwon M-J, Ahn JK. Association between serum uric acid and arterial stiffness in a low-risk, middle-aged, large Korean population A cross-sectional study. Medicine. 2018;97:36. doi:10.1097/MD.0000000000012086
- Choi HY, Kim S-H, Choi AR, et al. Hyperuricemia and risk of increased arterial stiffness in healthy women based on health screening Korean population. PLoS One. 2017;12(6):e0180406. doi:10.1371/journal.pone.0180406
- Zheng X, Wei Q, Long J, et al. Gender-specific association of serum uric acid levels and cardio-ankle vascular index in Chinese adults. Lipids Health Dis. 2018;17:80. doi:10.1186/s12944-018-0712-x
- Bian S, Guo H, Ye P, Luo L, Wu H, Xia W. Serum uric acid level and diverse impacts on regional arterial stiffness and wave reflection. Iranian J Publ Health. 2012;41(8):33–41.
- Ishizaka N, Ishizaka Y, Toda E, Hashimoto H, Nagai R, Yamakado M. Higher serum uric acid is associated with increased arterial stiffness in Japanese individuals. Atherosclerosis. 2007;192:131–137. doi:10.1016/j.atherosclerosis.2006.04.016
- Fang J-I, Wu J-S, Yang Y-C, Wang R-H, Lu F-H, Chang C-J. High uric acid level associated with increased arterial stiffness in apparently healthy women. Atherosclerosis. 2014;236(2):389–393. doi:10.1016/j.atherosclerosis.2014.07.024
- Chou P, Lin KC, Lin HY, Tsai ST. Gender differences in the relationships of serum uric acid with fasting serum insulin and plasma glucose in patients without diabetes. J Rheumatol. 2001;28(3):571–576.
- Hoieggen A, Alderman MH, Kjeldsen SE, et al. The impact of uric acid on cardiovascular outcomes in the LIFE study. Kidney Int. 2004;65:1041–1049. doi:10.1111/j.1523-1755.2004.00484.x
- Heo SH, Lee SH. High levels of serum uric acid are associated with silent brain infarction. J Neurol Sci. 2010;297:6–10. doi:10.1016/j.jns.2010.07.007
- Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2010;62:170–180. doi:10.1002/acr.20065
- Chen X, Li Y, Sheng C-S, Huang Q-F, Zheng Y, Wang J-G. Association of serum uric acid with aortic stiffness and pressure in a Chinese workplace setting. Am J Hypertens. 2010;23(4):387–392. doi:10.1038/ajh.2009.277
- Shin JY, Lee HR, Shim JY. Significance of high-normal serum uric acid level as a risk factor for arterial stiffness in healthy Korean men. Vasc Med. 2012;17(1):37–43. doi:10.1177/1358863X11434197
- Baena CP, Lotufo PA, Mill JG, et al. Serum uric acid and pulse wave velocity among healthy adults: baseline data from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Am J Hypertens. 2015;28:966–970. doi:10.1093/ajh/hpu298
- Liang J, Li Y, Zhou N, et al. Synergistic effects of serum uric acid and cardiometabolic risk factors on early stage atherosclerosis: the cardiometabolic risk in Chinese study. PLoS One. 2012;7(12):e51101. doi:10.1371/journal.pone.0051101
- Sull JW, Koh EN, Cho SK, Bae H-J, Jee SH. Association of uric acid levels with arterial stiffness in Korean women and non-smoking men. Biomed Sci Letters. 2017;23(3):201–207. doi:10.15616/BSL.2017.23.3.201
- Cicero AFG, Salvi P, D’Addato S, Rosticci M, Borghi C, for the Brisighella Heart Study group. Association between serum uric acid, hypertension, vascular stiffness and subclinical atherosclerosis: data from the Brisighella Heart Study. J Hypertens. 2014;32(1):57–64. doi:10.1097/HJH.0b013e328365b916
- Fu S, Luo L, Ye P, Xiao W. Multimarker analysis for new biomarkers in relation to central arterial stiffness and hemodynamics in a Chinese community-dwelling population. Angiology. 2015;66:950–956. doi:10.1177/0003319715573910
- Li Y, Lu J, Wu X, Yang C. Serum uric acid concentration and asymptomatic hyperuricemia with subclinical organ damage in general population. Angiology. 2014;65:634–640. doi:10.1177/0003319713513143
- Lim JH, Kim YK, Kim YS, Na SH, Rhee MY, Lee MM. Relationship between serum uric acid levels, metabolic syndrome, and arterial stiffness in Korean. Korean Circ J. 2010;40:314–320. doi:10.4070/kcj.2010.40.7.314
- Canepa M, Viazzi F, Strait JB, et al. Longitudinal association between serum uric acid and arterial stiffness: results from the Baltimore longitudinal study of aging. Hypertension. 2017;69(2):228–235. doi:10.1161/HYPERTENSIONAHA.116.08114
- Nagano S, Takahashi M, Miyai N, et al. Association of serum uric acid with subsequent arterial stiffness and renal function in normotensive subjects. Hypertension. 2017;40:620–624. doi:10.1038/hr.2017.10
- Ding X, Ye P, Wang X, et al. Peripheral arterial stiffness is associated with basal plasma uric acid: a prospective cohort study. Saudi J Biol Sci. 2017;24:574–581. doi:10.1016/j.sjbs.2017.01.028
- Feig DI, Kang DH, Nakagawa T, Mazzali M, Johnson RJ. Uric acid and hypertension. Curr Hypertens Rep. 2006;8:111–115. doi:10.1007/s11906-006-0005-z
- Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension. 2003;42:247–252. doi:10.1161/01.HYP.0000085858.66548.59
- Kuwabara M. Hyperuricemia, cardiovascular disease, and hypertension. Pulse (Basel). 2016;3:242–252. doi:10.1159/000443769
- Mazzali M, Hugues J, Kim YG, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38:1101–1106. doi:10.1161/hy1101.092839
- Tsai WC, Huang YY, Lin CC, et al. Uric acid is an independent predictor of arterial stiffness in hypertensive patients. Heart Vessels. 2009;24(5):371–375. doi:10.1007/s00380-008-1127-9
- Ramirez AJ, Christen AI, Sanchez RA. Serum acid uric elevation is associated to arterial stiffness in hypertension patients with metabolic disturbances. Curr Hypertens Rev. 2018;14(2):154–160. doi:10.2174/1573402114666180413143312
- Vlachopoulos C, Xaplanteris P, Vyssoulis G, et al. Association of serum uric acid level with aortic stiffness and arterial wave reflections in newly diagnosed, never-treated hypertension. Am J Hypertens. 2011;24:33–39. doi:10.1038/ajh.2010.111
- Hsu P-F, Chuang S-Y, Cheng H-M, et al. Associations of serum uric acid levels with arterial wave reflections and central systolic blood pressure. Int J Cardiol. 2013;168(3):2057–2063. doi:10.1016/j.ijcard.2013.01.164
- Mulè G, Riccobene R, Castiglia A, et al. Relationship between mild hyperuricemia and aortic stiffness in untreated hypertensive patients. Nutr Metab Cardiovasc Dis. 2014;24:744–750. doi:10.1016/j.numecd.2014.01.014
- Tsioufis C, Chatzis D, Vezali E, et al. The controversial role of serum uric acid in essential hypertension: relationships with indices of target organ damage. J Hum Hypertens. 2005;19:211–217. doi:10.1038/sj.jhh.1001810
- Tsioufis C, Kyvelou S, Dimitriadis K, et al. The diverse associations of uric acid with low-grade inflammation, adiponectin and arterial stiffness in never-treated hypertensives. J Human Hypertens. 2011;25:554–559. doi:10.1038/jhh.2010.98
- Saladini F, Benetti E, Fania C, et al. Association between uric acid, metabolic variables and arterial stiffness in the early phase of hypertension. J Hypertens. 2016; e208. doi:10.1097/01.hjh.0000467906.49825.ad.
- Cipolli JAA, Ferreira-Sae MC, Martins RP, et al. Relationship between serum uric acid and internal carotid resistive index in hypertensive women: a cross-sectional study. BMC Cardiovasc Disord. 2012;12:52. doi:10.1186/1471-2261-12-52
- Frauchiger B, Schmid HP, Roedel C, Moosmann P, Staub D. Comparison of carotid arterial resistive indices with intima-media thickness as sonographic markers of atherosclerosis. Stroke. 2001;32:836–841. doi:10.1161/01.STR.32.4.836
- Gomez-Marcos MA, Recio-Rodríguez JI, Patino-Alonso MC, et al.; Vasorisk group. Relationship between uric acid and vascular structure and function in hypertensive patients and sex-related differences. Am J Hypertens. 2013;26(5):599–607. doi:10.1093/ajh/hps097.
- Alper AB Jr, Chen W, Yau L, Srinivasan SR, Berenson GS, Hamm LL. Childhood uric acid predicts adult blood pressure: the Bogalusa Heart Study. Hypertension. 2005;45:34–38. doi:10.1161/01.HYP.0000150783.79172.bb
- Sundström J, Sullivan L, D’Agostino RB, Levy D, Kannel WB, Vasan RS. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension. 2005;45:28–33. doi:10.1161/01.HYP.0000150784.92944.9a
- Perlstein TS, Gumieniak O, Williams GH, et al. Uric acid and the development of hypertension: the normative aging study. Hypertension. 2006;48:1031–1036. doi:10.1161/01.HYP.0000248752.08807.4c
- Mellen PB, Bleyer AJ, Erlinger TP, et al. Serum uric acid predicts incident hypertension in a biethnic cohort: the atherosclerosis risk in communities study. Hypertension. 2006;48:1037–1042. doi:10.1161/01.HYP.0000249768.26560.66
- Nakanishi N, Okamoto M, Yoshida H, Matsuo Y, Suzuki K, Tatara K. Serum uric acid and risk for development of hypertension and impaired fasting glucose or type II diabetes in Japanese male office workers. Eur J Epidemiol. 2003;18:523–530. doi:10.1023/a:1024600905574
- Masuo K, Kawaguchi H, Mikami H, Ogihara T, Tuck ML. Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension. 2003;42:474–480. doi:10.1161/01.HYP.0000091371.53502.D3
- Kuwabara M, Niwa K, Hisatome I, et al. Asymptomatic hyperuricemia without comorbidities predicts cardiometabolic diseases: five year Japanese cohort study. Hypertension. 2017;69:1036–1044. doi:10.1161/HYPERTENSIONAHA.116.08998
- Kuwabara M, Hisatome I, Niwa K, et al. Uric acid is a strong risk marker for developing hypertension from prehypertension: a 5-year Japanese cohort study. Hypertension. 2018;71:78–86. doi:10.1161/HYPERTENSIONAHA.117.10370
- Tomiyama H, Shiina K, Vlachopoulos C, et al. Involvement of arterial stiffness and inflammation in hyperuricemia-related development of hypertension. Hypertension. 2018;72:739–745. doi:10.1161/HYPERTENSIONAHA.118.11390
- Tanaka A, Kawaguchi A, Tomiyama H, et al. Cross-sectional and longitudinal associations between serum uric acid and endothelial function in subjects with treated hypertension. Int J Cardiol. 2018;272:308–313. doi:10.1016/j.ijcard.2018.06.017
- Kodama S, Saito K, Yachi Y, et al. Association between serum uric acid and development of type 2 diabetes. Diabetes Care. 2009;32:1737–1742. doi:10.2337/dc09-0288
- Lv Q, Meng XF, He FF, et al. High serum uric acid and increased risk of type 2 diabetes: a systemic review and meta-analysis of prospective cohort studies. PLoS One. 2013;8:e56864. doi:10.1371/journal.pone.0056864
- Xu Y, Zhu J, Gao L, et al. Hyperuricemia as an independent predictor of vascular complications and mortality in type 2 diabetes patients: a meta-analysis. PLoS One. 2013;8(10):e78206. doi:10.1371/journal.pone.0078206
- Rizzo M, Obradovic M, Labudovic-Borovic M, et al. Uric acid metabolism in pre-hypertension and the metabolic syndrome. Curr Vasc Pharmacol. 2014;12:572–585. doi:10.2174/1570161111999131205160756
- Tsushima Y, Nishizawa H, Tochinoet Y, et al. Uric acid secretion from adipose tissue and its increase in obesity. J Biol Chem. 2013;288:27138–27149. doi:10.1074/jbc.M113.485094
- Stehouwer CDA, Henry RMA, Ferreira I. Arterial stiffness in diabetes and the metabolic syndrome: a pathway to cardiovascular disease. Diabetologia. 2008;51:527–539. doi:10.1007/s00125-007-0918-3
- Zhang J, Xiang G, Xiang L, Sun H. Serum uric acid is associated with arterial stiffness in men with newly diagnosed type 2 diabetes mellitus. J Endocrinol Investig. 2014;37(5):441–447. doi:10.1007/s40618-013-0034-9
- Sun N, Zhang Y, Tian J-L WH. Relationship between uric acid and arterial stiffness in the elderly with metabolic syndrome components. Chin Med J (Engl). 2013;126(16):3097–3102. doi:10.3760/cma.j.issn.0366-6999.20130215
- Wijnands JM, Boonen A, van Sloten TT, et al. Association between serum uric acid, aortic, carotid and femoral stiffness among adults aged 40–75 years without and with type 2 diabetes mellitus: the Maastricht Study. J Hypertens. 2015;33(8):1642–1650. doi:10.1097/HJH.0000000000000593
- Wang MC, Tsai WC, Chen JY, Huang JJ. Stepwise increase in arterial stiffness corresponding with the stages of chronic kidney disease. Am J Kidney Dis. 2005;45:494–501. doi:10.1053/j.ajkd.2004.11.011
- Nemcsik J, Kiss I, Tisler A. Arterial stiffness, vascular calcification and bone metabolism in chronic kidney disease. World J Nephrol. 2012;1(1):25–34. doi:10.5527/wjn.v1.i1.25
- Briet M, Boutouyrie P, Laurent S, London GM. Arterial stiffness and pulse pressure in CKD and ESRD. Kidney Int. 2012;82:388–400. doi:10.1038/ki.2012.131
- Xia X, He F, Wu X, Peng F, Huang F, Yu X. Relationship between serum uric acid and all-cause and cardiovascular mortality in patients treated with peritoneal dialysis. Am J Kidney Dis. 2014;64:257–264. doi:10.1053/j.ajkd.2013.08.027
- Bellomo G. Asymptomatic hyperuricemia following renal transplantation. World J Nephrol. 2015;4(3):324–329. doi:10.5527/wjn.v4.i3.324.
- Madero M, Sarnak MJ, Wang X, et al. Uric acid and long term outcomes in CKD. Am J Kidney Dis. 2009;53:796–803. doi:10.1053/j.ajkd.2008.12.021
- Elsurer R, Afsar B. Serum uric acid and arterial stiffness in hypertensive chronic kidney disease patients: sex-specific variations. Blood Press Monit. 2014;19:271–279. doi:10.1097/MBP.0000000000000056
- Liu X, Wu J, Wu H, et al. Association of serum uric acid with arterial stiffness in peritoneal dialysis patients. Kidney Blood Res. 2018;43:1451–1458. doi:10.1159/000493659
- Bauer F, Pagonas N, Seibert FS, et al. Serum uric acid and arterial function after renal transplantation. Ann Transplant. 2017;14(22):431–439. doi:10.12659/aot.901657
- Tanindi A, Erkan AF, Alhan A, Tör HF. Arterial stiffness and central arterial wave reflection are associated with serum uric acid, total bilirubin, and neutrophil-to-lymphocyte ratio in patients with coronary artery disease. Anatol J Cardiol. 2015;15:396–403. doi:10.5152/akd.2014.5447
- Khan F, George J, Wong K, McSwiggan S, Struthers AD, Belch JJF. The association between serum urate levels and arterial stiffness/endothelial function in stroke survivors. Atherosclerosis. 2000;200(2):374–379. doi:10.1016/j.atherosclerosis.2007.12.023
- Park JS, Kang S, Ahn CW, Cha BS, Kim KR, Lee HC. Relationships between serum uric acid, adiponectin and arterial stiffness in postmenopausal women. Maturitas. 2012;73:344–348. doi:10.1016/j.maturitas.2012.09.009
- Lee H, Jung Y-H, Kwon Y-J, Park B. Uric acid level has a J-shaped association with arterial stiffness in Korean postmenopausal women. Korean J Fam Med. 2017;38:333–337. doi:10.4082/kjfm.2017.38.6.333
- McDonagh EM, Thorn CF, Callaghan JT, Altman RB, Klein TE. PharmGKB summary: uric acid-lowering drugs pathway, pharmacodynamics. Pharmacogenet Genomics. 2014;24(9):464–476. doi:10.1097/FPC.0000000000000058
- Grimaldi-Bensouda L, Alperovitch A, Aubrun E, et al. Impact of allopurinol on risk of myocardial infarction. Ann Rheum Dis. 2015;74:836–842. doi:10.1136/annrheumdis-2012-202972
- MacIsaac RL, Salatzki J, Higgins P, et al. Allopurinol and cardiovascular outcomes in adults with hypertension. Hypertension. 2016;67:535–540. doi:10.1161/HYPERTENSIONAHA.115.06344
- Struthers AD, Donnan PT, Lindsay P, McNaughton D, Broomhall J, MacDonald TM. Effect of allopurinol on mortality and hospitalisations in chronic heart failure: a retrospective cohort study. Heart. 2002;87:229–234. doi:10.1136/heart.87.3.229
- Luk AJ, Levin GP, Moore EE, Zhou XH, Kestenbaum BR, Choi HK. Allopurinol and mortality in hyperuricaemic patients. Rheumatology (Oxford). 2009;48:804–806. doi:10.1093/rheumatology/kep069
- Bredemeier M, Lopes LM, Eisenreich MA, et al. Xanthine oxidase inhibitors for prevention of cardiovascular events: a systematic review and meta-analysis of randomized controlled trial. BMC Cardiovasc Disord. 2018;18:24. doi:10.1186/s12872-018-0757-9
- Deng G, Qiu Z, Li D, Fang Y, Zhang S. Effects of allopurinol on arterial stiffness: a meta-analysis of randomized controlled trials. Med Sci Monit. 2016;22:1389–1397. doi:10.12659/msm.898370
- Alem MM, Alshehri AM, Cahusac PMB, Walters MR. Effect of xanthine oxidase inhibition on arterial stiffness in patients with chronic heart failure. Clin Med Insights Cardiol. 2018;12:1–10. doi:10.1177/1179546818779584
- George J, Carr E, Davies J, Belch JJ, Struthers A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation. 2006;114:2508–2516. doi:10.1161/CIRCULATIONAHA.106.651117
- Higgins P, Dawson J, Lees KR, McArthur K, Quinn TJ, Walters MR. Xanthine oxidase inhibition for the treatment of cardiovascular disease: a systematic review and meta-analysis. Cardiovasc Ther. 2012;30(4):217–226. doi:10.1111/j.1755-5922.2011.00277
- Rajendra NS, Ireland S, George J, Belch JJ, Lang CC, Struthers AD. Mechanistic insights into the therapeutic use of high-dose allopurinol in angina pectoris. J Am Coll Cardiol. 2011;58:820–828. doi:10.1016/j.jacc.2010.12.052
- Kanbay M, Ozkara A, Selcoki Y, et al. Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearance, and proteinuria in patients with normal renal functions. Int Urol Nephrol. 2007;39:1227–1233. doi:10.1007/s11255-007-9253-3
- Ng KP, Stringer SJ, Jesky MD, et al. Allopurinol is an independent determinant of improved arterial stiffness in chronic kidney disease: a cross sectional study. PLoS One. 2014;9(3):e91961. doi:10.1371/journal.pone.0091961
- Agarwal V, Hans N, Messerli FH. Effect of allopurinol on blood pressure: a systematic review and meta-analysis. J Clin Hypertens (Greenwich). 2013;15:435–442. doi:10.1111/j.1751-7176.2012.00701.x
- Kamatani N, Fujimori S, Hada T, et al. An allopurinol-controlled, randomized, double-dummy, double-blind, parallel between-group, comparative study of febuxostat (TMX-67), a non-purine-selective inhibitor of xanthine oxidase, in patients with hyperuricemia including those with gout in Japan: Phase 3 clinical study. J Clin Rheumatol. 2011;17:S13–S18. doi:10.1097/RHU.0b013e31821d36cc
- Malik UZ, Hundley NJ, Romero G, et al. Febuxostat inhibition of endothelial-bound XO: implications for targeting vascular ROS production. Free Radic Biol Med. 2011;51:179–184. doi:10.1016/j.freeradbiomed.2011.04.004
- Sezai A, Soma M, Nakata K, et al. Comparison of febuxostat and allopurinol for hyperuricemia in cardiac surgery patients (NU‑FLASH Trial). Circ J. 2013;77:2043–2049. doi:10.1253/circj.cj-13-0082
- Sezai A, Soma M, Nakata K, et al. Comparison of febuxostat and allopurinol for hyperuricemia in cardiac surgery patients with chronic kidney disease (NU‑FLASH trial for CKD). J Cardiol. 2015;66:298–303. doi:10.1016/j.jjcc.2014.12.017
- Tausche AK, Christoph M, Forkmann M, et al. As compared to allopurinol, urate-lowering therapy with febuxostat has superior effects on oxidative stress and pulse wave velocity in patients with severe chronic tophaceous gout. Rheumatol Int. 2014;34(1):101–109. doi:10.1007/s00296-013-2857-2
- Zhang T, Pope JE. Cardiovascular effects of urate-lowering therapies in patients with chronic gout: a systematic review and meta-analysis. Rheumatology. 2017;56:1144–1153. doi:10.1093/rheumatology/kex065
- White WB, Saag KG, Becker MA, et al.; for the CARES Investigators. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med. 2018;378:1200–1210. doi:10.1056/NEJMoa1710895
- Cuenca JA, Balda J, Palacio A, Young L, Pillinger MH, Tamari L. Febuxostat and cardiovascular events: a systematic review and meta-analysis. Int J Rheumatol. 2019;2019:1–10. doi:10.1155/2019/1076189
- Pancholia AK. Sodium-glucose cotransporter-2 inhibition for the reduction of cardiovascular events in high-risk patients with diabetes mellitus. Indian Heart J. 2018;70(6):915–921. doi:10.1016/j.ihj.2018.08.022
- Sanchez RA, Sanchez MJ, Ramirez AJ. Canagliflozin ameliorates arterial stiffness by reducing serum uric acid in type 2 diabetic patients. Diabetes. 2018;67(Supplement 1):1216–P. doi:10.2337/db18-1216-P
- Bekki M, Tahara N, Tahara A, et al. Switching dipeptidyl peptidase-4 inhibitors to tofogliflozin, a selective inhibitor of sodium-glucose cotransporter 2 improves arterial stiffness evaluated by cardio-ankle vascular index in patients with type 2 diabetes: a pilot study. Curr Vasc Pharmacol. 2018. doi:10.2174/1570161116666180515154555
- Chilton R, Tikkanen I, Cannon CP, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes, Obes Metab. 2015;17:1180–1193. doi:10.1111/dom.12572
- Aroor AR, Das NA, Carpenter AJ, et al. Glycemic control by the SGLT2 inhibitor empagliflozin decreases aortic stiffness, renal resistivity index and kidney injury. Cardiovasc Diabetol. 2018;17:108. doi:10.1186/s12933-018-0750-8
- Striepe K, Jumar A, Ott C, et al. Effects of the selective sodium-glucose cotransporter 2 inhibitor empagliflozin on vascular function and central hemodynamics in patients with type 2 diabetes mellitus. Circulation. 2017;136(12):1167–1169. doi:10.1161/CIRCULATIONAHA.117.029529
- Singh M, Kumar A. Risks associated with SGLT2 inhibitors: an overview. Curr Drug Saf. 2018;13(2):84–91. doi:10.2174/1574886313666180226103408
- Waring WS, McKnight JA, Webb DJ, Maxwell SR. Lowering serum urate does not improve endothelial function in patients with type 2 diabetes. Diabetologia. 2007;50(12):2572–2579. doi:10.1007/s00125-007-0817-7
