Abstract
Ruxolitinib is an orally bioavailable, selective Janus kinase (JAK) 1 and 2 inhibitor approved for the treatment of myelofibrosis (MF), a bone marrow disease in which the JAK pathway is dysregulated, leading to impaired hematopoiesis and immune function. By inhibiting JAK1 and JAK2, ruxolitinib modulates cytokine-stimulated intracellular signaling. In a phase II clinical trial in patients with MF, ruxolitinib recipients exhibited durable reductions in spleen size, reductions in circulating pro-inflammatory cytokines, improvements in physical activity, weight gain, and alleviation of symptoms (including constitutional symptoms) in patients with and without JAK2 mutation. These findings were confirmed by two phase III clinical MF studies, in which a greater proportion of ruxolitinib recipients achieved a spleen volume reduction of ≥35% from baseline at week 24, compared with placebo in one study (41.9% versus 0.7%; P < 0.0001) and with best available therapy in the other (31.9% versus 0%; P < 0.0001). Alleviation of MF symptoms and improvements in quality of life were also significantly greater in ruxolitinib recipients. Overall survival of patients treated with ruxolitinib was significantly longer than of those receiving the placebo. Owing to risks of potentially serious adverse effects, eg, myelosuppression, ruxolitinib should be used under close physician supervision. Longer follow-up of the phase III MF studies is needed to reach firm conclusions regarding ruxolitinib’s capacity to modify the natural disease course.
Keywords:
Introduction
Myelofibrosis (MF) is a bone marrow disease characterized by excessive production of reticulin and collagen fibers. Although fibrosis can be the outcome of numerous hematologic and nonhematologic conditions,Citation1 the term MF is commonly used in reference either to primary MF (PMF)Citation2 or to the similar disorders evolving from the two other classic Philadelphia-chromosome-negative myeloproliferative neoplasms: polycythemia vera (post-PV MF) and essential thrombocythemia (post-ET MF).Citation3 According to epidemiological studies,Citation4–Citation9 the incidence of PMF may be as high as 1.5 per 100,000. Other studiesCitation10–Citation14 show that by the end of the second decade after PV or ET diagnosis, up to 10%–15% of cases may transform to secondary MF.
In MF, the fibrotic changes appear to be cytokine-stimulated reactions sustained by multilineage clonal cellular proliferation.Citation15–Citation21 The clinical signs of MF include splenomegaly due to extramedullary hematopoiesis; leukocytosis and thrombocytosis, with predisposition to thrombotic events, due to clonal cellular proliferation affecting mainly megakaryocytes and granulocytes; cytopenias, a later finding that worsens with the progression of fibrosis; and constitutional symptoms (eg, fatigue, weight loss, low- grade fever, night sweats), most likely induced by abnormal levels of circulating cytokines.
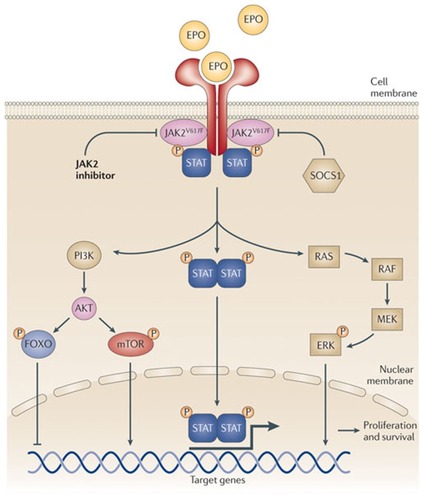
In the past decade, the role of Janus kinases (JAKs) in intracellular pathways has claimed the attention of many myeloproliferative neoplasm researchers. JAKs are non-receptor tyrosine kinases that mediate the transmission of cytokine- and growth-factor-induced intracellular signals (). About 50% of patients with PMF present with the JAK2V617F gain-of-function mutation, resulting in a constitutively activated JAK-signal transducer and activator of transcription (JAK-STAT) pathway.Citation22,Citation23 In turn, the activated JAK-STAT pathway promotes the transcription of numerous genes, eg, for cytokines, fibrogenic factors, and angiogenic factors, among a broad variety of pro-proliferative and anti-apoptotic gene products.Citation24–Citation29 Excessive production of pro-inflammatory cytokines may itself contribute to JAK-STAT activation,Citation30 creating a vicious cycle. Among patients with MF, about 5% are JAK2V617F-negative but instead have a gain-of-function mutation in the thrombopoietin receptor gene (MPLW515 L mutation), resulting in cytokine-independent JAK-STAT activation.Citation31,Citation32 Another small group of patients with MF have neither of these mutations but carry other mutations (eg, in lymphocyte adaptor proteinCitation33 or in the receptor adaptor protein CBL)Citation34 associated with constitutive JAK2 activation. Moreover, patients with MF in the absence of any identified mutation often exhibit JAK2 overactivity. JAK1 also plays a role in MF: a recent studyCitation30 demonstrated JAK1 hyperactivity in MF patients, most likely as a consequence of cytokine hyperstimulation. Collectively, these data implicate JAK1 and JAK2 as important pieces in the puzzle posed by the molecular pathogenesis of MF.
Figure 1 The JAK-STAT intracellular signaling pathway in myelofibrosis. Copyright © 2011, Nature Publishing Group. Reproduced with permission from Quintàs-Cardama A, Kantarjian H, Cortes J, Verstovsek S. Janus kinase inhibitors for the treatment of myeloproliferative neoplasias and beyond. Nat Rev Drug Discov. 2011;10(2):127–140.Citation60
Currently, the only potentially curative treatment for MF is allogeneic hematopoietic stem cell transplantation, an option traditionally feasible only for a small subgroup of patients, the younger and physically fit, although new reports suggest its utility in the older patients as well.Citation35,Citation36 Other treatment modalities (eg, hydroxyurea, anagrelide, splenectomy or splenic irradiation, lenalidomide or thalidomide with or without corticosteroids, transfusions, danazol, androgens) are only palliative and without a substantial influence on survival.Citation37–Citation53 Patients often die from bone marrow failure accompanied by systemic infection or fatal hemorrhage.Citation20,Citation54,Citation55 However, with the discovery of the JAK2V617F mutation,Citation56–Citation59 JAK2 emerged as a potential target for treatment, and several small-molecule, ATP-competitive JAK2 inhibitors were developed (SAR302503 [TG101348], lestaurtinib [CEP-701], XL019, SB1518, CYT387, AZD1480, and ruxolitinib).Citation60–Citation63 Ruxolitinib (formerly known as INCB018424) is the first and currently the only JAK inhibitor approved by the US Food and Drug Administration or any other regulatory agency for treatment of patients with MF;Citation64 and clinical development of several JAK inhibitors (SAR302503 [TG101348], CYT387, and LY278544) is ongoing. Although not as developed as ruxolitinib, available data on the efficacy of the other JAK2 inhibitors suggests similar profiles, mainly reduction in the size of enlarged organs (splenomegaly and hepatomegaly) and elimination of MF-related symptoms. The differences among them so far are mainly seen in relation to their toxicity profiles, eg, a degree of myelosuppression, gastrointestinal and/or neurological side effects.
Preclinical studies of ruxolitinib
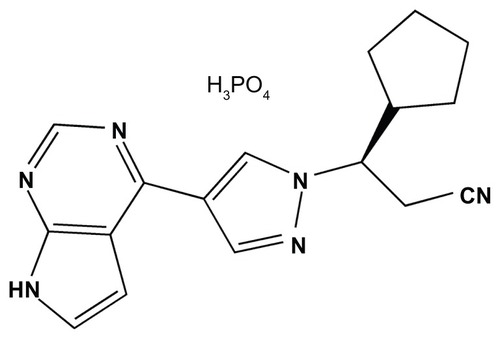
Ruxolitinib phosphate () is an orally administered ATP-competitive cyclopentylpropionitrile derivative. In preclinical studies, it showed inhibitory activity in vitro mainly against JAK1 (IC50 = 3.3 nM) and JAK2 (IC50 = 2.8 nM).Citation30 Moderate to minimal inhibitory activity was observed against nonreceptor tyrosine kinase TYK2 (half-maximal inhibitory concentration [IC50] = 19 nM) and against JAK3 (IC50 = 428 nM), as well as minimal inhibitory activity against multiple other kinases at concentrations about 100-fold higher than the IC50 for JAK1/2.Citation30 Selectivity against JAK1/2 was confirmed by measurements of STAT activity in a cytokine-stimulated whole-blood assay.Citation30
Figure 2 Ruxolitinib-phosphate, a chemical structure of orally available Janus kinase 1 and 2 inhibitor.
In an engineered cell system containing growth-factor- independent JAK2V617F-expressing Ba/F3 cells (Ba/F3-EpoR-JAK2V617F), ruxolitinib demonstrated a dose-dependent reduction of JAK2-mediated downstream phosphorylated proteins with no change in their total levels,Citation30 suggesting that ruxolitinib exerts its effect through achievement of reduced levels of phosphorylated (active) forms. A similar effect was observed in the HEL cell line.Citation30 In these cell lines and in cells from mononuclear PV patients, ruxolitinib demonstrated antiproliferative and proapoptotic effects.Citation30 Analogous effects were not observed on BCR-ABL- 1 signaling or in a cell line expressing an activating mutation in c-KIT.Citation30 The effects of ruxolitinib were attenuated when cells expressing JAK2V617F were cocultured with primary or immortalized human bone marrow mesenchymal stromal cells, probably owing to paracrine activity of the mesenchymal stromal cells.Citation65 Several point mutations identified on a Ba/F3 cell line expressing JAK2V617F may be a cause of resistance to ruxolitinib in experimental in vitro systems.Citation66
Evidence from preclinical studies in mouse models confirmed JAK1 and JAK2 as targets for MF therapy. Balb/c mice injected with Ba/F3-EpoR-JAK2V617F cells had significant reductions in spleen size, tumor burden, and circulating cytokines when treated with ruxolitinib, compared with vehicle treatment.Citation30 In the ruxolitinib-treated mice, the histomorphology of affected organs was normalized, and anemia and lymphopenia were not detected. More than 90% of ruxolitinib-treated mice survived, while by the 22nd day of treatment, more than 90% of vehicle-treated mice died.
Pharmacokinetics and metabolism
The pharmacokinetics and metabolism of ruxolitinib were established in early studies in healthy volunteers who received single doses of 25 mgCitation67 or single or multiple ascending doses of 5 mg to as much as 200 mg.Citation68 After a single oral dose, >95% of the drug is absorbed, and >97% of the absorbed drug becomes bound to plasma proteins. Plasma concentrations peak 1–3 hours after administration, with monophasic or biphasic decline. The terminal half-life is 2–3 hours. Administration of doses of up to 200 mg demonstrated dose-proportional exposure.
Ruxolitinib is metabolized predominantly in the liver, as a substrate of cytochrome P450 3A4 (CYP3A4), and its metabolites are mainly excreted in urine.Citation67,Citation68 There is no evidence of accumulation of either ruxolitinib or its metabolites. Citation67 Factors that may influence the pharmacokinetics of ruxolitinib have been evaluated. A high-fat meal reduced the maximum plasma concentration by 24%, but had no substantial influence on bioavailability.Citation68 Because of the routes by which ruxolitinib is metabolized and excreted, exposure may be increased in patients with impaired renal or hepatic function.Citation69,Citation70 When coadministered with rifampin (a CYP3A4 inducer), or erythromycin (a moderate CYP3A4 inhibitor), no changes in ruxolitinib pharmacokinetics were observed in healthy subjects.Citation71 However, in healthy subjects who received ruxolitinib concomitantly with ketoconazole (a strong CYP3A4 inhibitor), the area under the curve increased by 91% and the half-life increased from 3.7 to 6.0 hours. There is a possibility of similar effects when ruxolitinib is coadministered with drugs that are strong inhibitors of CYP3A4 ().Citation72,Citation73
Table 1 Examples of strong cytochrome P450 3A4 inhibitors with potential influence on the pharmacokinetics of coadministered ruxolitinibCitation71,Citation72
Safety across clinical trials
In healthy volunteers and in patients with MF, myelosuppression, and in particular thrombocytopenia, was the dose-limiting toxicity of ruxolitinib. The maximum tolerated dose was established as 25 mg twice daily (bid) and 100 mg once daily (qd).Citation68,Citation74 One healthy volunteer receiving a ruxolitinib dose of 50 mg/bid developed high-grade neutropenia and recovered 12 days after ruxolitinib discontinuation.Citation68 In phase I/II and III clinical trials in patients with MF, the most common hematologic adverse effects were thrombocytopenia and anemia ().Citation74–Citation78 Myelosuppression was dose-dependent and was not a frequent reason for withdrawal.Citation74–Citation76 Dose-dependent myelosuppression was not observed in a study of healthy volunteers.Citation68 In the blinded, placebo-controlled phase III trial, the most frequent nonhematologic adverse events reported more commonly for ruxolitinib treatment than for placebo were ecchymosis, dizziness, and fatigue (mostly grade 1 or 2). Given the mechanism of action of ruxolitinib (JAK1/JAK2 inhibition), immunosuppression may be a possible adverse event; however, this was not observed to an appreciable extent in the clinical trials so far.
Table 2 Observed incidence of thrombocytopenia and anemia in clinical trials of ruxolitinib
In a phase I/II clinical trial, investigators described clinical symptoms and signs suggesting development of systemic inflammatory response syndrome in two patients (1.3%) following sudden cessation of ruxolitinib.Citation74 A similar reaction was not described among patients in two phase III clinical trials.Citation75–Citation77 Nevertheless, recently published phase I/II data from one centerCitation78,Citation79 describe similar effects of abrupt cessation in four patients, and 2 weeks after cessation a fifth patient developed a syndrome similar to disseminated intravascular coagulation with sequential severe polyarticular arthritis. Cytokine-rebound phenomena were suggested as mechanisms leading to “ruxolitinib discontinuation syndrome.” Apart from this one-center experience, such events have not been observed by other investigators in any other study (~170 clinical centers). However, to avoid any possibility of such complications, it is advisable to taper the dose when discontinuing ruxolitinib.Citation78,Citation79
Efficacy in the phase I/II clinical trial of ruxolitinib in MF
A phase I/II clinical trialCitation74 of open-label ruxolitinib in MF (INCB018424-251; ClinicalTrials.gov, NCT00509899) was conducted at two United States centers: the MD Anderson Cancer Center in Houston, Texas and the Mayo Clinic in Rochester, Minnesota. In all, 153 patients (PMF 53%, post-PV MF 32%, and post-ET MF 15%) were enrolled, with a median age of 65 years (range, 40–84 years). On the Lille scoring system,Citation80 65% of patients were at high risk, 28% at intermediate-2 risk, 7% at undetermined risk, and 82% were JAK2V617F-positive. In phase I of the study, a maximum tolerated dose and dose-limiting toxicity were identified.
In phase II, several dosing regimens, all below the maximum tolerated dose, were investigated. Among them, 15 mg/bid and 25 mg/bid regimens were identified as the most appropriate for optimal efficacy and minimal adverse effects. In 52% and 49% of the patients on these regimens (15 mg/bid and 25 mg/bid, respectively), ruxolitinib reduced palpable splenomegaly by ≥50% from baseline (the study’s predefined measure of clinical improvement) after three cycles of treatment (one cycle = 4 weeks of daily ruxolitinib). Among patients exhibiting this response, the response was maintained after 12 months of treatment in 73% of those on 15 mg/bid and 78% of those on 25 mg/bid. The 15 mg/bid regimen was associated with a lower incidence of grade 3 or 4 thrombocytopenia. In a subset of 24 patients in the 15 mg/bid group, change in spleen volume was evaluated by magnetic resonance imaging (MRI); the median reduction after six cycles of treatment was 33%, corresponding to a median 52% reduction in palpable spleen length. In the same MRI substudy, hepatomegaly decreased by 14% in six patients with hepatomegaly at baseline.
Patients also demonstrated improvement in other measures of disease burden. On a 6-minute walk test,Citation81 as performed in 27 patients after 1, 3, and 6 months of treatment, median distances walked were 34, 57, and 71 m, respectively. Moreover, after a year of treatment, patients on 15 mg/bid and 25 mg/bid regimens gained weight: a median 9.4 and 7.1 kg, respectively. Ruxolitinib recipients with a body mass index in the lowest quartile at baseline had the most prominent weight gain. In general, improvements in performance status were maintained with therapy.
Ruxolitinib treatment also led to decreases in peripheral blood cell counts, including CD34-positive cells. In addition, peripheral blood cytokine levels (of interleukin-1 and tumor necrosis factor-alfa) decreased in association with improvement of symptoms, while plasma levels of leptin and erythropoietin increased. Thirty-four patients were available for evaluation of JAK2V617F allele burden reduction; the mean maximal suppression was modest (13% from baseline). However, a dose-dependent reduction of constitutively phosphorylated STAT3 and STAT5 was observed.
Recently, the two centers participating in this phase I/II clinical trial have published separate reportsCitation78,Citation82,Citation83 of their long-term experience in the treatment of patients with MF. For 51 ruxolitinib-treated patients enrolled in the trial between October 2007 and February 2009 inclusive, the Mayo Clinic in Rochester reported a high discontinuation rate: 51%, 72%, and 89% at 1, 2, and 3 years, respectively.Citation78 As of October 2011, 18 patients (35%) had died and five patients (10%) had developed transformation to leukemia. Survival rate showed no significant difference between the ruxolitinib recipients and a cohort of 410 recipients of standard PMF treatment at their center during the past decade (P = 0.43).
In contrast, the MD Anderson Cancer Center reported that of 107 patients enrolled in the phase I/II trial, 58 (54%) were still receiving ruxolitinib at a median of 32 months.Citation82 As of December 2011, 33 patients (31%) had died, 19 of them off-study and none for therapy-related reasons, and nine patients (8%) had developed transformation to leukemia, four of them off-study. By log-rank analysis, the survival of patients receiving ruxolitinib was significantly longer than in a historical cohort of 310 patients treated with standard or investigational therapy who would have met the phase I/II trial enrollment criteria (hazard ratio = 0.61, 95% CI: 0.41–0.89; P = 0.02).Citation83 Survival of high-risk ruxolitinib recipients (of whom 21 of 63, or 33%, died) was also significantly longer (P = 0.006) than that of high-risk patients from the control group (of whom 112 of 165, or 68%, died). Patients continue to be followed. The outcome differences between the cohorts at the two centers are possibly related to the inferior efficacy of therapy at the Mayo Clinic in Rochester due to lower dosage and shorter duration (higher discontinuation rate) of therapy.Citation83
Phase III clinical trials of ruxolitinib in MF
Two phase III clinical trials, the Controlled Myelofibrosis Study with Oral JAK1/JAK2 Inhibitor Treatment I and II (COMFORT-ICitation76,Citation77 AND COMFORT-II;Citation75 ClinicalTrials.gov, NCT00952289 and NCT00934544, respectively), have been conducted and are still ongoing.
COMFORT-I is a double-blind, placebo-controlled study that enrolled 309 adults with MF in the United States, Canada, and Australia. Patients were randomized (1:1) to receive ruxolitinib or placebo. Based on baseline peripheral blood platelet count (Plt), the ruxolitinib was initiated at 15 mg/bid (Plt = 100–200 × 109/L) or 20 mg/bid (Plt > 200 × 109/L). Dose adjustment was allowed in accordance with efficacy and safety observations during the study, as defined by the protocol. At week 24, 41.9% and 0.7% of patients receiving ruxolitinib and placebo, respectively, achieved a spleen volume reduction ≥ 35% from baseline (the primary endpoint), as evaluated by MRI or computed tomography.Citation76,Citation77 Changes in symptoms were measured by the modified Myelofibrosis Symptom Assessment Form v2.0 Total Symptom Score (TSS).Citation84 In the ruxolitinib and placebo arms, respectively, 45.9% and 5.3% (P < 0.0001) of patients had at least a 50% improvement in TSS; mean TSS improved by 46.1% in the ruxolitinib and worsened by 41.8% in the placebo group. All individual symptoms assessed in the Myelofibrosis Symptom Assessment Form improved in ruxolitinib recipients and worsened in placebo recipients.Citation76,Citation77 The same trends of improvements in TSS and reductions in spleen volume were observed in subgroup analyses based on MF type (PMF, post-PV MF, or post-ET MF), IPSS risk group (intermediate-2 or high), age (≤65 or >65 years), JAK2V617F mutation status (presence or absence), baseline palpable spleen length (≤10 or >10 cm), and baseline hemoglobin level (≥10 or <10 g/dL).Citation85
Quality of life (QoL) was measured by European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30).Citation86 Improvements in QoL correlated with the alleviation of symptoms.Citation76–Citation87 Patients with spleen size reductions of at least 10% realized meaningful improvements in symptoms and QoL.Citation87,Citation88
At a median follow-up of 52 weeks in the ruxolitinib and 51 weeks in the placebo arm, there had been 13 and 24 deaths, respectively, with a hazard ratio of 0.50 (95% CI: 0.25–0.98; P = 0.04), which provided evidence that ruxolitinib may prolong the life of patients with advanced MF.Citation85
COMFORT-II is a double-blind phase III study of 219 patients with MF, conducted in nine European countries. Patients were randomized (2:1) to ruxolitinib or best available therapy (BAT). The ruxolitinib dose was 15 mg/bid or 20 mg/bid, based on the same Plt values as in COMFORT-I, and was subject to adjustment within the range of 5 mg/bid to 25 mg/bid. The BAT could be oral, parenteral, or no therapy. Spleen volume reductions of ≥35% (by MRI or computed tomography) at weeks 48 and 24 were the primary and key secondary endpoints, respectively. The primary endpoint was reached by 28.5% of ruxolitinib and 0% of BAT recipients (P < 0.0001), and the key secondary endpoint by 31.9% and 0% (P < 0.0001).Citation75 Response rates were also higher for ruxolitinib than for BAT in subgroups based on JAK2V617F mutational status, risk group, MF type, hydroxyurea pretreatment, baseline spleen size or volume, age, and sex.Citation89
Symptoms measured by the EORTC QLQ-C30 showed significant improvements in the ruxolitinib group, starting at week 8, with continued improvement through week 48 versus BAT (P < 0.05).Citation90 Similarly, mean subscores in the Functional Assessment of Cancer Therapy–Lymphoma System (FACT-LymS)Citation91 improved with ruxolitinib treatment. No significant difference was found between risk-based subgroups of ruxolitinib recipients.
A post-hoc comparison of the COMFORT-I placebo and COMFORT-II BAT groups showed no significant difference in symptoms and QoL. In the placebo group, median spleen volume increased at week 24 by 8.5% (range, −46.4% to +48.8%) and in the BAT group by 5.1% (range, −33.3% to +29.7%).Citation92
Conclusion
In clinical trials, ruxolitinib alleviated the burdensome manifestations of MF, namely splenomegaly and disease core symptoms. Patients experienced reductions in spleen size, decreases in circulating pro-inflammatory cytokines, increases in weight, and substantial improvements in symptoms and QoL. Based on the efficacy and tolerability reported in clinical trials, ruxolitinib became the first drug approved by the US Food and Drug Administration, in mid-November 2011, for the treatment of MF, and now has an important place among available treatment options. The reported data suggests that its effects are independent of patient characteristics including age, MF subtype, risk group, JAK2V617F mutation status, baseline palpable spleen length, and baseline hemoglobin level. Although data from the COMFORT-I phase III clinical trial provides evidence that ruxolitinib prolongs the life of patients with advanced MF, ruxolitinib does not have a curative potential in the disease. On the other hand, ruxolitinib seems to offer significant and clinically meaningful benefit over other treatment modalities currently used when allogeneic hematopoietic stem cell transplantation is not an option. Also, it may become useful in pretreating patients deemed unfit for allogeneic hematopoietic stem cell transplantation, perhaps aiding them in becoming clinically fit for the transplant procedure. However, this would have to be proved in clinical trials. Due to potentially serious adverse effects, ruxolitinib should be used under close supervision of a physician. Follow-up data from the ruxolitinib phase III clinical trials, especially concerning long-term effects and survival, are needed to draw any stronger conclusions about its enduring benefits in MF. The next wave of clinical studies will explore the combination strategies in MF, by combining ruxolitinib with other active agents in this disease, eg, lenalidomide, danazol, erythropoietin, interferon, and others, with a goal to bring additional benefits to the JAK2 inhibitor therapy, like improvement in blood cell count and decrease in bone marrow fibrosis.
Disclosure
Professor S. Verstovsek has received research support from Incyte for the conduct of clinical studies. The other authors state no conflicts of interest.
References
- KuterDJBainBMuftiGBaggAHasserjianRPBone marrow fibrosis: pathophysiology and clinical significance of increased bone marrow stromal fibresBr J Haematol2007139335136217910625
- VardimanJWThieleJArberDAThe 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changesBlood2009114593795119357394
- BarosiGMesaRAThieleJProposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a consensus statement from the International Working Group for Myelofibrosis Research and TreatmentLeukemia200822243743817728787
- ChaiterYBrennerBAghaiETatarskyIHigh incidence of myeloproliferative disorders in Ashkenazi Jews in northern IsraelLeuk Lymphoma1992732512551477653
- DouganLEMatthewsMLArmstrongBKThe effect of diagnostic review on the estimated incidence of lymphatic and hematopoietic neoplasms in Western AustraliaCancer19814838668727248915
- JohanssonPKuttiJAndreassonBTrends in the incidence of chronic Philadelphia chromosome negative (Ph−) myeloproliferative disorders in the city of Goteborg, Sweden, during 1983–1999J Intern Med2004256216116515257729
- KuttiJRidellBEpidemiology of the myeloproliferative disorders: essential thrombocythaemia, polycythaemia vera and idiopathic myelofibrosisPathol Biol (Paris)200149216416611317963
- MesaRASilversteinMNJacobsenSJWollanPCTefferiAPopulation-based incidence and survival figures in essential thrombocythemia and agnogenic myeloid metaplasia: an Olmsted County Study, 1976–1995Am J Hematol1999611101510331505
- WoodliffHJDouganLMyelofibrosis in Western Australia: an epidemiological study of 29 casesMed J Aust1976115523525933939
- Alvarez-LarránACervantesFBellosilloBEssential thrombocythemia in young individuals: frequency and risk factors for vascular events and evolution to myelofibrosis in 126 patientsLeukemia20072161218122317519959
- NajeanYRainJDDreschCRisk of leukaemia, carcinoma, and myelofibrosis in 32P- or chemotherapy-treated patients with polycythaemia vera: a prospective analysis of 682 cases. The “French Cooperative Group for the Study of Polycythaemias.”Leuk Lymphoma199622Suppl 11111198951781
- PassamontiFRumiEArcainiLPrognostic factors for thrombosis, myelofibrosis, and leukemia in essential thrombocythemia: a study of 605 patientsHaematologica200893111645165118790799
- RandiMLBarboneEFabrisFVarottoLMacriCGirolamiAPost- polycythemia myeloid metaplasia: experience with a large cohort of patientsJ Med19942563633697769374
- WolanskyjAPSchwagerSMMcClureRFLarsonDRTefferiAEssential thrombocythemia beyond the first decade: life expectancy, long-term complication rates, and prognostic factorsMayo Clin Proc200681215916616471068
- VannucchiAMBianchiLCellaiCDevelopment of myelofibrosis in mice genetically impaired for GATA-1 expression (GATA-1(low) mice)Blood200210041123113212149188
- Le Bousse-KerdilèsMCMartyréMCFrench INSERM research network on idiopathic myelofibrosis. Involvement of the fibrogenic cytokines, TGF-beta and bFGF, in the pathogenesis of idiopathic myelofibrosisPathol Biol (Paris)200149215315711317961
- ReillyJTPathogenesis of idiopathic myelofibrosis: role of growth factorsJ Clin Pathol19924564614641624590
- ChagraouiHKomuraETulliezMGiraudierSVainchenkerWWendlingFProminent role of TGF-beta 1 in thrombopoietin-induced myelofibrosis in miceBlood2002100103495350312393681
- JacobsonRJSaloAFialkowPJAgnogenic myeloid metaplasia: a clonal proliferation of hematopoietic stem cells with secondary myelofibrosisBlood1978512189194620081
- TefferiAMyelofibrosis with myeloid metaplasiaN Engl J Med2000342171255126510781623
- ReederTLBaileyRJDewaldGWTefferiABoth B and T lymphocytes may be clonally involved in myelofibrosis with myeloid metaplasiaBlood200310151981198312406879
- IhleJNKerrIMJaks and Stats in signaling by the cytokine receptor superfamilyTrends Genet199511269747716810
- RawlingsJSRoslerKMHarrisonDAThe JAK/STAT signaling pathwayJ Cell Sci2004117Pt 81281128315020666
- DrachmanJGGriffinJDKaushanskyKThe c-Mpl ligand (thrombopoietin) stimulates tyrosine phosphorylation of Jak2, Shc, and c-MplJ Biol Chem199527010497949827534285
- NeubauerHCumanoAMullerMWuHHuffstadtUPfefferKJak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesisCell19989333974099590174
- ParganasEWangDStravopodisDJak2 is essential for signaling through a variety of cytokine receptorsCell19989333853959590173
- RadosevicNWintersteinDKellerJRNeubauerHPfefferKLinnekinDJAK2 contributes to the intrinsic capacity of primary hematopoietic cells to respond to stem cell factorExp Hematol200432214915615102475
- SilvennoinenOWitthuhnBAQuelleFWClevelandJLYiTIhleJNStructure of the murine Jak2 protein-tyrosine kinase and its role in interleukin 3 signal transductionProc Natl Acad Sci U S A19939018842984338378315
- WitthuhnBAQuelleFWSilvennoinenOJAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietinCell19937422272368343951
- Quintás-CardamaAVaddiKLiuPPreclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasmsBlood2010115153109311720130243
- PikmanYLeeBHMercherTMPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasiaPLoS Med200637e27016834459
- PardananiADLevineRLLashoTMPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patientsBlood2006108103472347616868251
- OhSTSimondsEFJonesCNovel mutations in the inhibitory adaptor protein LNK drive JAK-STAT signaling in patients with myeloproliferative neoplasmsBlood2010116698899220404132
- GrandFHHidalgo-CurtisCEErnstTFrequent CBL mutations associated with 11q acquired uniparental disomy in myeloproliferative neoplasmsBlood2009113246182619219387008
- BarbuiTBarosiGBirgegardGPhiladelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNetJ Clin Oncol201129676177021205761
- SamuelsonSSandmaierBMHeslopHEAllogeneic haematopoietic cell transplantation for myelofibrosis in 30 patients 60–78 years of ageBr J Haematol20111531768221323890
- LöfvenbergEWahlinAManagement of polycythaemia vera, essential thrombocythaemia and myelofibrosis with hydroxyureaEur J Haematol19884143753813197824
- Martínez-TrillosAGayaAMaffioliMEfficacy and tolerability of hydroxyurea in the treatment of the hyperproliferative manifestations of myelofibrosis: results in 40 patientsAnn Hematol201089121233123720567824
- SteurerMGastlGJedrzejczakWWAnagrelide for thrombocytosis in myeloproliferative disorders: a prospective study to assess efficacy and adverse event profileCancer2004101102239224615476273
- BirgegardGBjorkholmMKuttiJAdverse effects and benefits of two years of anagrelide treatment for thrombocythemia in chronic myeloproliferative disordersHaematologica200489552052715136214
- SirhanSLashoTLHansonCAMesaRAPardananiATefferiAThe presence of JAK2V617F in primary myelofibrosis or its allele burden in polycythemia vera predicts chemosensitivity to hydroxyureaAm J Hematol200883536336518266209
- MesaRANagorneyDSSchwagerSAllredJTefferiAPalliative goals, patient selection, and perioperative platelet management: outcomes and lessons from 3 decades of splenectomy for myelofibrosis with myeloid metaplasia at the Mayo ClinicCancer2006107236137016770787
- ElliottMAChenMGSilversteinMNTefferiASplenic irradiation for symptomatic splenomegaly associated with myelofibrosis with myeloid metaplasiaBr J Haematol199810325055119827926
- BesaECNowellPCGellerNLGardnerFHAnalysis of the androgen response of 23 patients with agnogenic myeloid metaplasia: the value of chromosomal studies in predicting response and survivalCancer19824923083137053830
- ShimodaKShideKKamezakiKThe effect of anabolic steroids on anemia in myelofibrosis with myeloid metaplasia: retrospective analysis of 39 patients in JapanInt J Hematol200785433834317483079
- CervantesFAlvarez-LarránADomingoAArellano-RodrigoEMontserratEEfficacy and tolerability of danazol as a treatment for the anaemia of myelofibrosis with myeloid metaplasia: long-term results in 30 patientsBr J Haematol2005129677177515953003
- MerupMKuttiJBirgergårdGNegligible clinical effects of thalidomide in patients with myelofibrosis with myeloid metaplasiaMed Oncol2002192798612180484
- BarosiGElliottMCanepaLThalidomide in myelofibrosis with myeloid metaplasia: a pooled-analysis of individual patient data from five studiesLeuk Lymphoma200243122301230712613516
- MesaRAElliottMASchroederGTefferiADurable responses to thalidomide-based drug therapy for myelofibrosis with myeloid metaplasiaMayo Clin Proc200479788388915244384
- AbgrallJFGuibaudIBastieJNThalidomide versus placebo in myeloid metaplasia with myelofibrosis: a prospective, randomized, double- blind, multicenter studyHaematologica20069181027103216885042
- ThomasDAGilesFJAlbitarMThalidomide therapy for myelofibrosis with myeloid metaplasiaCancer200610691974198416583431
- MesaRASteensmaDPPardananiAA phase 2 trial of combination low-dose thalidomide and prednisone for the treatment of myelofibrosis with myeloid metaplasiaBlood200310172534254112517815
- JabbourEThomasDKantarjianHComparison of thalidomide and lenalidomide as therapy for myelofibrosisBlood2011118489990221622644
- SilversteinMNGomesMRReMineWHElvebackLRAgnogenic myeloid metaplasia. Natural history and treatmentArch Intern Med196712055465506054588
- BarosiGMyelofibrosis with myeloid metaplasia: diagnostic definition and prognostic classification for clinical studies and treatment guidelinesJ Clin Oncol19991792954297010561375
- KralovicsRPassamontiFBuserASA gain-of-function mutation of JAK2 in myeloproliferative disordersN Engl J Med2005352171779179015858187
- JamesCUgoVLe CouèdicJPA unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia veraNature200543470371144114815793561
- LevineRLWadleighMCoolsJActivating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosisCancer Cell20057438739715837627
- BaxterEJScottLMCampbellPJAcquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disordersLancet200536594641054106115781101
- Quintàs-CardamaAKantarjianHCortesJVerstovsekSJanus kinase inhibitors for the treatment of myeloproliferative neoplasias and beyondNat Rev Drug Discov201110212714021283107
- HedvatMHuszarDHerrmannAThe JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumorsCancer Cell200916648749719962667
- TynerJWBummTGDeiningerJCYT387, a novel JAK2 inhibitor, induces hematologic responses and normalizes inflammatory cytokines in murine myeloproliferative neoplasmsBlood2010115255232524020385788
- VerstovsekSOdenikeOScottBPhase I dose-escalation trial of SB1518, a novel JAK2/FLT3 inhibitor, in acute and chronic myeloid diseases, including primary or post-essential thrombocythemia/polycythemia vera myelofibrosis [abstract]Blood2009114223905
- US Food and Drug AdministrationSilver SpringUS Food and Drug Administration [updated November 16, 2001; cited November 18, 2011]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm280102.htmAccessed December 21, 2011
- ManshouriTEstrovZKnezLCreightonCKantarjianHVerstovsekSBone marrow stroma-mediated paracrine inhibition of ruxolitinib (INCB018424)-induced apoptosis of JAK2V617F mutated cells: protective effect of myeloproliferative neoplasm patient-derived but not healthy donnor-derived stroma [abstract]Haematologica2011966 Suppl 20316
- DeshpandeAReddyMMSchadeGOKinase domain mutations confer resistance to novel inhibitors targeting JAK2V617F in myeloproliferative neoplasmsLeukemiaSeptember 162011 [Epub ahead of print.]
- ShillingADNedzaFMEmmTMetabolism, excretion, and pharmacokinetics of [14C]INCB018424, a selective Janus tyrosine kinase 1/2 inhibitor, in humansDrug Metab Dispos201038112023203120699411
- ShiJGChenXMcGeeRFThe pharmacokinetics, pharmacodynamics, and safety of orally dosed INCB018424 phosphate in healthy volunteersJ Clin Pharmacol201151121644165421257798
- ChenXShiJMcGeeRThe effect of various degrees of hepatic dysfunction on the pharmacokinetics of INCB018424 [abstract]Clin Pharmacol Ther2010872 Suppl 1PIIIP59
- Jakafi [package insert]Wilmington DEIncyte Corporation2011
- ShiJGChenXEmmTThe effect of CYP3A4 inhibition or induction on the pharmacokinetics and pharmacodynamics of orally administered ruxolitinib (INCB018424 phosphate) in healthy volunteersJ Clin PharmacolMay 202011 [Epub ahead of print.]
- OstojicAVrhovacRVerstovsekSRuxolitinib: a new JAK1/2 inhibitor that offers promising options for treatment of myelofibrosisFuture Oncol2011791035104321919691
- OstojicAVrhovacRVerstovsekSRuxolitinib for the treatment of myelofibrosisDrugs Today (Barc)2011471181782922146225
- VerstovsekSKantarjianHMesaRASafety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosisN Engl J Med2010363121117112720843246
- HarrisonCNKiladjianJAl-AliHKResults of a randomized study of the JAK inhibitor ruxolitinib (INC424) versus best available therapy (BAT) in primary myelofibrosis (PMF), post-polycythemia vera-myelofibrosis (PPV-MF) or post-essential thrombocythemia-myelofibrosis (PET-MF) [abstract]J Clin Oncol20112915 Suppl 1LBA6501
- VerstovsekSMesaRGotlibJLevyRGuptaVResults of COMFORT-I, a randomized double-blind phase III trial of JAK 1/2 inhibitor INCB18424 (424) versus placebo (PB) for patients with myelofibrosis (MF) [abstract]J Clin Oncol20112915 Suppl 16500
- VerstovsekSMesaRGotlibJResults of COMFORT-I, a randomized, double-blind phase III trial of the JAK1 and JAK2 inhibitor ruxolitinib (INCB018424) versus placebo for patients with myelofibrosis [abstract]Haematologica2011966 Suppl 20505
- TefferiALitzowMRPardananiALong-term outcome of treatment with ruxolitinib in myelofibrosisN Engl J Med2011365151455145721995409
- TefferiAPardananiASerious adverse events during ruxolitinib treatment discontinuation in patients with myelofibrosisMayo Clin Proc201186121188119122034658
- DupriezBMorelPDemoryJLPrognostic factors in agnogenic myeloid metaplasia: a report on 195 cases with a new scoring systemBlood1996883101310188704209
- MesaRKantarjianHTefferiAFunctional assessment of performance status in patients with myelofibrosis (MF): Utility and feasibility of the 6-minute walk test (6MWT) [abstract]J Clin Oncol200996Suppl 15s7083
- VerstovsekSEstrovZCortesJThe MD AndersonCancer Center (MDACC) experience with ruxolitinib, an oral JAK1 and JAK2 inhibitor, in myelofibrosis: long-term follow-up outcomes of 107 patients from a phase I/II study [abstract]Blood2011118213851
- VerstovsekSKantarjianHEstrovZComparison of outcomes of advanced myelofibrosis patients treated with ruxolitinib (INCB018424) to those of a historical control group: survival advantage of ruxolitinib therapy [abstract]Blood201111821793
- MesaRAKantarjianHTefferiAEvaluating the serial use of the Myelofibrosis Symptom Assessment Form for measuring symptomatic improvement: performance in 87 myelofibrosis patients on a JAK1 and JAK2 inhibitor (INCB018424) clinical trialCancer2011117214869487721480207
- VerstovsekSMesaRGotlibJConsistent benefit of ruxolitinib over placebo in spleen volume reduction and symptom improvement across subgroups and overall survival advantage: results from COMFORT-I [abstract]Blood201111821278
- AaronsonNKAhmedzaiSBergmanBThe European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncologyJ Natl Cancer Inst19938553653768433390
- MesaRKantarjianHShieldsAResults using the Modified Myelofibrosis Symptom Assessment Form (MFSAF v2.0) in COMFORT- I: a randomized, double-blind phase III trial of JAK1/2 inhibitor ruxolitinib vs placebo in myelofibrosis (MF) [abstract]Haematologica2011966 Suppl 20912
- MesaRGotlibJGuptaVAssociations between improvements in myelofibrosis (MF) symptoms and quality of life measures with splenomegaly reduction in COMFORT-I: a randomized, double-blind, phase III trial of the JAK1 and JAK2 inhibitor ruxolitinib versus placebo in patients with MF [abstract]Blood201111821384221828130
- HarrisonCKiladjianJGisslingerHRuxolitinib provides reductions in splenomegaly across subgroups: an analysis of spleen response in the COMFORT-II study [abstract]Blood201111821279
- HarrisonCKiladjianJKathrin Al-AliHHealth-related quality of life and symptoms in myelofibrosis patients treated with ruxolitinib versus best available therapy [abstract]Blood20111182179521642596
- CellaDWebsterKCashyJDevelopment of a measure of health-related quality of life for non-Hodgkin’s lymphoma clinical research: the Functional Assessment of Cancer Therapy – Lymphoma (FACT-Lym) [abstract]Blood200510611750
- MesaRVerstovsekSCervantesFComparison of the efficacy of placebo and best available therapy for the treatment of myelofibrosis in the COMFORT studies [abstract]Blood2011118211753