Abstract
Esophageal adenocarcinoma and its precursor, Barrett’s esophagus, are rapidly rising in incidence. This review serves to highlight the role of pharmacologic, endoscopic, and surgical intervention in the management of Barrett’s esophagus, which requires acid suppression and endoscopic assessment. Treatment with a proton pump inhibitor may decrease acid exposure and delay the progression to dysplasia. Patients who require aspirin for cardioprotection or other indications may also benefit in terms of a protective effect against the development of esophageal cancer. However, without other indications, aspirin is not indicated solely to prevent cancer. A careful endoscopic examination should include assessment of any visible lesions in a Barrett’s segment. An expert gastrointestinal pathologist should confirm neoplasia in the setting of Barrett’s esophagus. For those patients with high-grade dysplasia or intramucosal carcinoma, careful consideration of endoscopic therapy or surgical therapy must be given. All visible lesions in the setting of dysplasia should be targeted with focal endoscopic mucosal resection for both accurate histopathologic diagnosis and treatment. The remainder of the Barrett’s epithelium should be eradicated to address all synchronous and metachronous lesions. This may be done by tissue acquiring or nontissue acquiring means. Radiofrequency ablation has a positive benefit-risk profile for flat Barrett’s esophagus. At this time, endoscopic therapy is not indicated for nondysplastic Barrett’s esophagus. Esophagectomy is still reserved for selected cases with evidence of lymph node metastasis, unsuccessful endoscopic therapy, or with high-risk features of high-grade dysplasia or intramucosal carcinoma.
Introduction
The incidence of esophageal adenocarcinoma has risen dramatically over the last 30 years.Citation1 The mortality rate from esophageal adenocarcinoma is dismal, with 5-year survival rates for advanced-stage adenocarcinoma being <15%.Citation2 The presence of Barrett’s metaplasia is the single most important risk factor for esophageal adenocarcinoma, with a 30-fold increase in risk over the general population.Citation3 Acid reflux is believed to play a critical role in the development of Barrett’s esophagus. The progression to esophageal adenocarcinoma is based on the sequence of changes from normal squamous epithelium to Barrett’s esophagus to dysplasia to cancer. The rate of progression from nondysplastic Barrett’s esophagus to esophageal adenocarcinoma is approximately 0.5% per year.Citation4 The key to prevention and early treatment of esophageal adenocarcinoma is the detection and eradication of dysplasia found in Barrett’s epithelium. High-grade dysplasia is currently the best marker to identify which patients are at risk of progressing to esophageal adenocarcinoma. This review serves to highlight the role of pharmacologic, endoscopic, and surgical intervention in the management of Barrett’s esophagus.
Barrett’s esophagus is diagnosed by both endoscopy and histology. On endoscopy, it is suspected by the presence of salmon-colored columnar mucosa appearing in the esophagus. These areas should be biopsied, which is necessary to identify specialized intestinal metaplasia, which is a histopathologic diagnosis, widely defined by the presence of goblet cells on biopsy. Dysplasia is defined as cytologic and architectural atypia without evidence of invasion past the basement membrane. The distinction between low-grade dysplasia and high-grade dysplasia is based on the severity of atypia and suggestion of neoplastic transformation. Intramucosal carcinoma refers to tumor that has invaded past the basement membrane but is superficial to the lamina propria. Submucosal carcinoma histologically invades past the muscularis mucosa into the submucosa, but does not extend into the muscularis propria. Distinguishing the various stages of progression to cancer as intestinal metaplasia, low-grade dysplasia, high-grade dysplasia, intramucosal carcinoma, and submucosal carcinoma is crucial in guiding therapeutic intervention.
Medical therapy
PPI and H2RA: regression of Barrett’s esophagus
Use of the antisecretory medications, proton pump inhibitors (PPIs) and histamine receptor antagonists (H2RAs), has offered hope of achieving reversal of the metaplastic epithelium characteristics of Barrett’s esophagus. Several observational and prospective studies have assessed for regression of Barrett’s esophagus in response to PPIs. Studies have defined regression as reduction in overall length of the metaplastic segment in the distal esophagus. Partial regression has been demonstrated by appearance of islands of squamous epithelium within the metaplastic columnar lining. It has been surmised that a reduction in the surface area of Barrett’s esophagus would lower the risk of cancer. Studies evaluating regression of Barrett’s esophagus have yielded inconsistent results. In studies of patients treated with H2 blockers, significant regression of Barrett’s esophagus was not noted.Citation5–Citation7 Thus, acid suppression with H2RA has not proved effective in inducing regression in Barrett’s esophagus.
Since the inception of the PPIs in 1989, most studies on Barrett’s esophagus have focused on these agents. Several studies have suggested partial regression, as evidenced by appearance or enlargement of squamous islands. Upwards of half of patients on PPI therapy have demonstrated development of new squamous islands or increasing number and size of islands.Citation6,Citation8–Citation11 The significance of squamous islands remains unclear because metaplasia may still underlie the islands, and the relatively modest reduction in surface area of Barrett’s esophagus at the sites of squamous islands may not decrease the overall risk of cancer in any significant way. Studies of PPI therapy have yielded conflicting data with respect to reduction in length of Barrett’s esophagus. Although some studies have demonstrated a decrease length in the Barrett’s segment,Citation7,Citation11,Citation12 other studies have not demonstrated the same results, including some with high-dose PPIs.Citation10,Citation13,Citation14
The incidence of complete regression in response to PPIs has been reported as approximately 2.4%Citation15 in studies generally including only long-segment Barrett’s esophagus and reported as 7.1% in a study including short-segment Barrett’s esophagus.Citation16 A small number of prospective studies have assessed if normalization of acid exposure leads to regression. Based on a limited number of studies, it appears that control of esophageal pH by PPIs may be associated with regression of Barrett’s esophagus, although control of pH alone may not be sufficient to cause significant regression.Citation12,Citation14
PPI and H2RA: progression to dysplasia and esophageal adenocarcinoma
Studies suggest that acid reflux plays a key role in the progression to dysplasia and esophageal adenocarcinoma, although this role in malignant progression is unclear. When compared with H2RA, PPI therapy has been shown to be more efficacious in preventing the progression of Barrett’s esophagus to both dysplasia and esophageal adenocarcinoma.Citation17,Citation18
In observational studies, PPI therapy has been shown to be associated with a lower risk of developing low-grade dysplasia, high-grade dysplasia, and esophageal adenocarcinoma. A study by El Serag et al found the incidence of any grade dysplasia to be significantly lower amongst those receiving PPIs after a diagnosis of Barrett’s esophagus compared with those not treated by PPIs.Citation18 When considering incidence of high-grade dysplasia or esophageal adenocarcinoma specifically, Nguyen et al demonstrated a lower incidence amongst patients being prescribed versus not being prescribed a PPI (7.4% versus 14.1%).Citation19 It is unclear where in the metaplasia-dysplasia-esophageal adenocarcinoma sequence that PPIs may exert their greatest effect, but there is some evidence that PPIs may delay progression from metaplasia to dysplasia. Studies suggest that initiating PPI therapy soon after the diagnosis of Barrett’s esophagus may prevent this progression.Citation18,Citation20
NSAIDS and aspirin
Nonsteroidal anti-inflammatory drug (NSAID) and aspirin use has previously been associated with a decreased risk of developing colon adenocarcinoma,Citation21,Citation22 and there has been interest in their role in preventing progression of Barrett’s esophagus. NSAIDs and aspirin exert their effects via inhibition of COX-1 and COX-2 enzymes. COX-2 enzyme activity contributes to cancer growth in several ways, such as inhibition of apoptosis and stimulation of angiogenesis.Citation23 COX-2 inhibition has been shown to suppress cell growth and increase apoptosis in Barrett’s esophagus-associated esophageal adenocarcinoma cell lines.Citation24 Overexpression of COX-2 has been demonstrated in patients with reflux esophagitis, Barrett’s esophagus, dysplasia, and esophageal adenocarcinoma.Citation25,Citation26
A study by Vaughan et al showed that current NSAID (including aspirin) users had an 80% reduced risk of esophageal adenocarcinoma compared with never-users of NSAIDs, with the 5-year incidence of esophageal adenocarcinoma being 6.6% versus 14.3% in current versus never-users.Citation27 Additionally, a review of nine studies found a protective association between NSAIDs/aspirin use and esophageal adenocarcinoma, and showed that patients with exposure to NSAIDs or aspirin had a 33% odds reduction of developing esophageal adenocarcinoma.Citation22 Thus, multiple studies demonstrate a protective association between NSAID/aspirin use and development of esophageal adenocarcinoma.
NSAID/aspirin may protect against esophageal adenocarcinoma either by preventing development of Barrett’s esophagus or by preventing progression from Barrett’s esophagus to esophageal adenocarcinoma. In a retrospective study, NSAID use was not found to be higher in Barrett’s esophagus patients versus esophageal adenocarcinoma patients. However, aspirin and NSAID use was lower in both of these groups compared with controls without Barrett’s esophagus. If there is a true protective effect of NSAIDs, this study suggests it may occur prior to the development of Barrett’s esophagus.Citation28
PPI use is widespread in patients with reflux, and thus associations of NSAID use and malignant progression are often in the setting of concomitant PPI use. In one study of patients with at least one filled NSAID/aspirin prescription, the majority of whom also had at least one filled PPI prescription, a reduced risk of esophageal adenocarcinoma was demonstrated.Citation29 It is possible that a protective effect is due to a combined effect of each medication.
The findings of the majority of these studies are largely associations and observations, because there are significant barriers in conducting a large clinical trial evaluating NSAIDs/aspirin as potential chemoprotective agents. At this time, it is the position of the American Gastroenterological Association to advise against using aspirin solely to prevent esophageal adenocarcinoma in the absence of other cardiovascular indications.Citation30
Celecoxib
COX-1 is responsible for maintaining the integrity of the gastrointestinal mucosa, and COX-1 inhibition is associated with an increased incidence of upper gastrointestinal complications, such as peptic ulcer disease.Citation31 Thus, selective inhibition of COX-2 by celecoxib may be of benefit with fewer side effects. The Chemoprevention for Barrett’s Esophagus Trial, a Phase IIB, multicenter, randomized trial of celecoxib in patients with Barrett’s esophagus and low-grade dysplasia or high-grade dysplasia, randomly assigned 222 patients to treatment with celecoxib or placebo. After 48 weeks, no significant difference was observed in the proportional change of biopsy samples with dysplasia or cancer between the groups.Citation32 This trial does not suggest that celecoxib prevents progression of Barrett’s esophagus, although further studies are needed.
Endoscopic surveillance and detection of neoplasia
Routine surveillance of Barrett’s esophagus is paramount in early detection of neoplasm and institution of appropriate therapies. Standard surveillance protocols call for four quadrant biopsies performed at every 1–2 cm intervals from the proximal gastroesophageal junction extending to the squamocolumnar junction. Guidelines proposed by the American College of Gastroenterology provide intervals for surveillance depending on the pathology.Citation33 If no dysplasia is detected, a second esophagogastroduodenoscopy with biopsies should be performed within a year, and then at 3-yearly intervals. If low-grade dysplasia is found, a repeat esophagogastroduodenoscopy with biopsies should be performed within 6 months, and then at 1-yearly intervals until no dysplasia is found on two consecutive esophagogastroduodenoscopies. In the case of high-grade dysplasia, repeat esophagogastroduodenoscopy with four quadrant biopsies at 1 cm intervals should be performed within 3 months, and then at regular 3-monthly intervals to monitor for esophageal adenocarcinoma if no therapy is performed. Any mucosal irregularity warrants endoscopic mucosal resection. All dysplastic lesions should be confirmed by an expert gastrointestinal pathologist.
The Prague C and M criteria, first introduced in 2004, provide a standardized, validated, and reliable classification system for the endoscopic description of Barrett’s esophagus. Citation35 The key steps involve: identifying the gastroesophageal junction by recognizing key landmarks, such as the proximal margin of the gastric mucosal folds, pinch at the distal esophagus, and diaphragmatic hiatus; defining the proximal extent of circumferential columnar-appearing mucosa in centimeters above the gastroesophageal junction and reporting this distance as the C value, defining the maximum extent of any tongues of columnar-appearing mucosa above the gastroesophageal junction and reporting this distance as the M value.
Surveillance protocols utilizing white light examination have been essential in recognition of lesions, but dysplastic lesions are still often missed on biopsy. A detailed examination of the Barrett’s segment with high-resolution white light endoscopy is critical in the assessment for neoplasia. Additional imaging modalities may offer improved mucosal visualization and assessment of structural changes, thereby allowing for identification of abnormal areas and appropriately targeting biopsies. Narrow band imaging is a technique that utilizes narrowed bandwidths of blue and green light to improve visibility of microstructural and capillary patterns in Barrett’s esophagus, without using dyes. Shorter wave-length light has superficial penetration, and is ideal for mucosal imaging. Narrow band imaging with magnification has been shown to have high sensitivity for diagnosing intestinal metaplasia and high-grade dysplasia on the basis of irregular mucosal pit patterns or irregular microvasculature, but poor specificity.Citation36 Clinically, this may reduce the need for multiple random biopsies and increase the histologic yield of dysplasia. However, addition of narrow band imaging to high-resolution white light endoscopy has not been shown to improve interobserver agreement or increase yield in identifying early neoplasia.Citation37
Additional techniques are being studied and utilized at high-volume centers. Autofluorescence imaging uses endogenous tissue substances (fluorophores), which become excited by light and emit light of longer wave-length than the exciting light. With autofluorescence imaging, normal esophagus and nondysplastic Barrett’s esophagus appears green, whereas areas of high-grade dysplasia/adenocarcinoma appear blue or violet. However, it is associated with a high false-positive rate.Citation38 Confocal laser microendoscopy allows for targeting of suspicious areas for biopsy and real-time histologic assessment of the epithelium. Both the epithelium and subepithelial vascular structures can be imaged at various depths,Citation39 and confocal laser microendoscopy has been shown to increase diagnostic yield of endoscopically inapparent neoplastic epithelium significantly compared with standard endoscopy. Citation40 Finally, in chromoendoscopy, a chemical agent such as methylene blue, indigo carmine, or acetic acid is applied to the esophageal mucosa to distinguish epithelial subtypes based on different pit patterns.
Although the gold standard for diagnosis is histology, there exists high interinterpreter variability in diagnosing dysplasia.Citation41–Citation43 Inherent to the challenge in diagnosing dysplasia is that neoplastic transformation represents a biologic and histologic continuum, but the pathologist must discretely categorize lesions as Barrett’s esophagus, low-grade dysplasia, high-grade dysplasia, intramucosal carcinoma, or submucosal carcinoma. Cytologic features of dysplasia may be confused with inflammatory changes, and often limitations in sample size, depth, and potential crush artifact make it difficult to distinguish reliably between various stages of disease based on a single biopsy specimen. Prior to embarking on any therapy for Barrett’s associated neoplasia, the dysplasia should be confirmed by an expert gastrointestinal pathologist.
Furthermore, endoscopic evaluation should include specific attention to any visible lesions. Rigorous assessment of the remainder of the Barrett’s epithelium should be done to evaluate for any occult neoplasia. Visible lesions, including mucosal irregularities, in the setting of dysplasia should be targeted with endoscopic mucosal resection for accurate histopathologic staging. Larger and more intact specimens from endoscopic resection may allow pathologists to make a more reliable staging diagnosis. The effect of staging by endoscopic mucosal resection specimens is demonstrated by our center’s complete Barrett’s eradication endoscopic mucosal resection study, which upstaged seven of 49 (14%) and downstaged 15 of 49 (31%) cases by endoscopic mucosal resection compared with pretreatment endoscopic biopsies.Citation44 Additionally, in a large study comparing Barrett’s esophagus patients undergoing endoscopic mucosal resection versus biopsy, significantly greater interobserver agreement on the diagnosis of dysplasia was demonstrated for endoscopic mucosal resection specimens.Citation45
Endoscopic therapy
Endoscopic therapy is now an established form of treatment at referral centers for neoplasia associated with Barrett’s esophagus. These modalities offer an organ-sparing and safer approach compared with esophagectomy, and patients with high-grade dysplasia or intramucosal carcinoma may be appropriate candidates for treatment (see ). Endoscopic therapy does not confer the same benefit for low-grade dysplasia or nondysplastic Barrett’s esophagus, given the potential complications of therapy and the lower risk of malignant transformation in these patients. Future risk stratification may enable us to select which patients may benefit from intervention in these populations.
Figure 1 Management of patients with Barrett’s esophagus with neoplasia.Citation34
Abbreviations: EMR, endoscopic mucosal resection; GI, gastrointestinal; PPI, proton pump inhibitor; HGD, high-grade dysplasia; IMC, intramucosal carcinoma; SMI, submucosal invasion; LNM, lymph node metastasis.
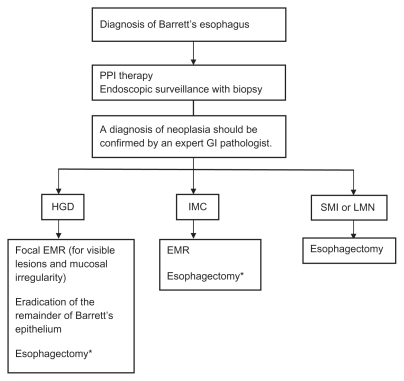
The risk of Barrett’s esophagus patients with high-grade dysplasia harboring occult invasive esophageal adenocarcinoma is estimated at around 40%,Citation46 and this has previously supported prophylactic esophagectomy over endoscopic monitoring. A review of the literature reporting rates of invasive cancer in 441 patients who underwent prophylactic esophagectomy for high-grade dysplasia demonstrated a pooled average rate of 39.9%. This rate was decreased to 12.7% when applying the strict definition of invasive cancer as being tumor invading the submucosa, and most patients were actually found to have intramucosal carcinoma.Citation47 Intramucosal carcinoma carries a ≤3% risk of nodal metastasis,Citation47–Citation51 and thus may be amenable to local endoscopic treatment.Citation44,Citation52 By comparison, submucosal invasion carries a higher risk of nodal metastasis, thus surgery and/or systemic therapy is required.Citation48,Citation53 With accurate distinction between high-grade dysplasia and intramucosal carcinoma from invasive cancer, endoscopic therapy may instead be a more appropriate alternative than esophagectomy.
Endoscopic therapy for high-grade dysplasia or intramucosal carcinoma is performed with the goal of preserving the esophagus while ablating all Barrett’s esophagus epithelium to prevent neoplastic progression. This includes ablating both dysplastic and nondysplastic epithelium due to the risk of developing synchronous or metachronous lesions in remaining metaplastic Barrett’s esophagus tissue.Citation54 Endoscopic therapies include tissue acquiring and nontissue acquiring modalities. Endoscopic resection, including both endoscopic mucosal resection and endoscopic mucosal dissection is a tissue-acquiring modality, and allows for histopathologic diagnosis. Nontissue acquiring modalities include photodynamic therapy, radiofrequency ablation, and cryotherapy, which treat by tissue destruction.
Endoscopic resection
Endoscopic mucosal resection and endoscopic mucosal dissection allow for removal of tissue and establishing a histopathologic diagnosis. Endoscopic mucosal resection can be performed using the band ligation or cap technique, both of which involve endoscopic suction with a friction fitted cap to create a pseudopolyp. Endoscopic mucosal resection can be performed focally or for the entirety of the Barrett’s epithelium. Focal endoscopic mucosal resection can be performed on suspicious-appearing lesions, but is associated with high recurrence rates, ranging from 14%–47% in the studies.Citation55–Citation62 These high rates are due in part to multifocal synchronous lesions previously missed by biopsy, as well as the metachronous development of new lesions.Citation63 Five-year follow-up data for 231 Barrett’s esophagus patients with intramucosal carcinoma demonstrated a 95.7% complete response rate in one study.Citation64
Complete Barrett’s eradication endoscopic mucosal resection also known as circumferential endoscopic mucosal resection, stepwise radical endoscopic resection, or wide area endoscopic mucosal resection, involves resecting the entire Barrett’s segment. This minimizes the risk of potential development of synchronous or metachronous lesions. Complete response from complete Barrett’s eradication endoscopic mucosal resection has been in the range of 76%–100% in studies.Citation44,Citation52,Citation65–Citation67 In a multicenter European cohort study, 169 Barrett’s esophagus patients with high-grade dysplasia or early carcinoma underwent complete endoscopic mucosal resection. After 27 months of follow-up, remission of neoplasia was demonstrated in 97.5% of patients, and complete elimination of metaplasia in 85%. The recurrence rate for metachronous lesions was 1.8%.Citation67
Endoscopic submucosal dissection involves marking the boundary of a lesion with cautery, injecting a viscous fluid into the submucosal space to provide a cushion under the lesion, followed by dissection using a cutting device.Citation68 Endoscopic mucosal dissection is utilized for suspicious-appearing lesions larger than 1.5 cm, and employs an en bloc dissection. This approach may be considered for lesions at the gastroesophageal junction or gastric cardia, where it is often difficult to create a pseudopolyp.Citation63 The recurrence rate was 0% in one study of 15 Barrett’s esophagus patients who underwent endoscopic mucosal dissection at the gastroesophageal junction for esophageal adenocarcinoma.Citation69 One potential barrier to this approach is reflux-induced submucosal fibrosis in the distal esophagus.Citation63 Complications of endoscopic mucosal resection include stricture formation in nearly half of cases, bleeding, and rarely, perforation.Citation44,Citation52,Citation65–Citation67,Citation70 However, stenoses and bleeding are complications that can be endoscopically treated.
Radiofrequency ablation
Radiofrequency ablation of a Barrett’s esophagus segment can be done circumferentially using a balloon-based catheter or locally using a focal device. Lesions are treated by application of direct thermal energy with electrodes embedded in the circumferential balloon or focal device. There are two separate applications of thermal energy, with scraping of treated tissue between the first and second ablation to ensure adequate and uniform thermal contact. Potential complications of radiofrequency ablation include noncardiac chest pain, nontransmural lacerations, and stricture formation.Citation44
Nondysplastic Barrett’s esophagus has demonstrated regression in response to radiofrequency ablation in large clinical studies. In the Ablation of Intestinal Metaplasia (AIM) study, a 70% complete remission rate of Barrett’s metaplasia was demonstrated in the circumferentially treated group at 1-year follow-up. Stricture formation did not occur, and no buried Barrett’s esophagus was found in the 4306 biopsy specimens.Citation71 The AIM-II trial at 2.5-year follow-up demonstrated complete eradication in 98.4% of patients treated with circumferential radiofrequency ablation followed by focal radiofrequency ablation of remaining Barrett’s esophagus.Citation72 Five-year outcomes from the AIM-II trial demonstrated complete remission in 92% of the participating patients, with no buried glands, dysplasia, strictures, or serious adverse events reported.Citation73
Radiofrequency ablation has been shown to be efficacious in the treatment of dysplastic Barrett’s esophagus as well. In a recent landmark randomized trial, patients with high-grade dysplasia demonstrated complete eradication in 81.0% of cases treated with radiofrequency ablation compared with 19.0% in controls. Overall, 77.4% of patients in the ablation group demonstrated complete eradication of intestinal metaplasia, compared with 2.3% in controls. Disease progression was lower in the ablation group (3.6% versus 16.3%) and fewer cancers were noted (1.2% versus 9.3%). The rate of esophageal stricture in the radiofrequency ablation group was 6%, which is much lower than the rate associated with endoscopic mucosal resection.Citation74 Similarly, 1-year follow-up in another study of 142 patients with highgrade dysplasia demonstrated a remission rate of 90.2%.Citation75
The efficacy of utilizing both endoscopic mucosal resection and radiofrequency ablation in patients with dysplastic Barrett’s esophagus has been evaluated. In a multicenter European trial of 24 patients with Barrett’s esophagus, visible lesions were endoscopically resected, followed by serial radiofrequency ablation. Neoplasia and metaplasia was eradicated in 95% and 88% of cases, respectively, and recurrence of neoplasia after 22 months of median follow-up was 0%.Citation76 Efficacy of focal endoscopic mucosal resection followed by radiofrequency ablation was also demonstrated in a trial of patients with longer Barrett’s esophagus segments (> 10 cm), with complete response rates of 83% and 79% for neoplasia and intestinal metaplasia, respectively, and no recurrence of neoplasia during 9.1 months of mean follow-up.
The above studies each evaluated the efficacy of focal endoscopic mucosal resection followed by radiofrequency ablation. Whether this approach or stepwise radical endoscopic resection alone is more efficacious and has fewer complications was evaluated by van Vilsteren et al in a randomized trial. Patients in the stepwise radical endoscopic resection group underwent focal endoscopic mucosal resection followed by serial endoscopic mucosal resection, whereas patients in the endoscopic mucosal resection/ radiofrequency ablation group underwent focal endoscopic mucosal resection followed by radiofrequency ablation. Rates of complete remission were similar between the groups (100% for stepwise radical endoscopic resection versus 96% for endoscopic mucosal resection/radiofrequency ablation). However, the stepwise radical endoscopic resection group had a much higher rate of stenosis (88%) and required more endoscopic dilations.Citation70
Photodynamic therapy
Photodynamic therapy employs a light-sensitizing reaction to produce oxygen radicals, which destroy targeted cells. A photosensitizer such as porfimer sodium or 5-aminolevulinic acid is administered intravenously 48–72 hours prior to endoscopy and accumulates in esophageal tissue prior to light activation. Subsequently, a bare cylinder, or balloon-based diffusing light fibers, are introduced endoscopically into the esophagus, and activation of the photosensitizer occurs in the dysplastic cells. Free oxygen radicals are generated which eventually induce cellular apoptosis.Citation77
Studies suggest that photodynamic therapy is an effective treatment modality for eradication of Barrett’s esophagus with high-grade dysplasia and intramucosal carcinoma. Overholt et al retrospectively evaluated 103 patients with low-grade dysplasia, high-grade dysplasia, and intramucosal carcinoma, and intention-to-treat success rates were 92.9%, 77.5%, and 44.4% for each respective group after a mean of 50 months. Stricture formation overall occurred in 30% of cases.Citation78 Pech et al prospectively followed 66 patients with high-grade dysplasia or intramucosal carcinoma treated with 5-aminolevulinic acid photodynamic therapy and followed for a median of 37 months. Complete response was demonstrated in 97% of the high-grade dysplasia group and 100% of the intramucosal carcinoma group.Citation79
Limitations of photodynamic therapy include additional time required to administer photosensitizers 2–3 days prior to therapy. Given the systemic distribution of the photosensitizers, patients must also avoid direct sunlight up to 6 weeks due to potential photosensitivity reactions.Citation77 Other adverse effects include noncardiac chest pain and symptomatic stricture formation. Stricture formation was reported in one study to occur in 27% of patients, with significant risk factors being endoscopic mucosal resection therapy before photodynamic therapy, a prior history of an esophageal stricture, and the number of photodynamic therapy applications.Citation80 Another concern is the potential development of subsquamous Barrett’s esophagus glands, because cases of esophageal adenocarcinoma arising from such glands after photodynamic therapy have been reported.Citation78,Citation81 Given these limitations, photodynamic therapy has fallen out of favor in recent years, and referral centers have adopted endoscopic mucosal resection and radiofrequency ablation as the preferred endoscopic treatment modalities for Barrett’s esophagus.
Cryotherapy
The latest endoscopic treatment modality is cryotherapy, which utilizes a spray catheter introduced via the endoscope and directed at targeted abnormal tissue. Sprayed liquid nitrogen or carbon dioxide is applied, which produces freeze-thaw cycles that result in tissue destruction by intracellular disruption or tissue ischemia, while minimizing fibrosis.Citation82 Orogastric decompression tube placement allows for expulsion of excess gas and prevention of inadvertent perforation. Dumot et al enrolled patients with Barrett’s esophagus and high-grade dysplasia or intramucosal carcinoma who were deemed inoperable or had refused esophagectomy into a trial. Thirty patients underwent cryotherapy, and afterwards 27 (90%) demonstrated pathologic downstaging. After 1-year median follow-up, elimination of cancer or downstaging of dysplasia was demonstrated in 68% of high-grade dysplasia and 80% of intramucosal carcinoma patients.Citation83 In a more recent trial, patients with high-grade dysplasia (n = 17) demonstrated a 94% regression rate while patients with intramucosal carcinoma (n = 4) and early esophageal adenocarcinoma (n = 3) demonstrated a 100% regression rate. Complete response to intestinal metaplasia occurred in 53% with high-grade dysplasia, 75% with intramucosal carcinoma, and 67% with esophageal adenocarcinoma.Citation84 In a third study of 60 patients with high-grade dysplasia, 97% had complete eradication of high-grade dysplasia, 87% had complete eradication of all dysplasia with persistent metaplasia, and 57% had complete eradication of all metaplasia.Citation85
The safety profile of cryotherapy appears to be favorable. From the above series, the overall incidence of stricture formation was 9/114 patients (7.9%),Citation83–Citation85 which is lower than the reported rates for endoscopic mucosal resection and photodynamic therapy. Perforation occurred in a patient with Marfan syndrome. Other less serious adverse effects included chest pain, dysphagia, odynophagia, and sore throat.Citation84 While cryotherapy appears safe, well tolerated, and efficacious for the treatment of high-grade dysplasia and intramucosal carcinoma in Barrett’s esophagus, there are no randomized trials comparing it with other modalities. Furthermore, unlike the long-term complete response rates demonstrated for endoscopic mucosal resection, data on long-term efficacy of cryotherapy is lacking.
Post-therapy surveillance
Current clinical practice requires lifelong surveillance with periodic histologic sampling of eradicated Barrett’s esophagus to monitor for recurrence, the presence of buried (subsquamous) glands/metaplasia, and progression to neoplasia. As mentioned, photodynamic therapy has been associated with subsquamous Barrett’s esophagus glands that possess neoplastic potential,Citation78–Citation81 thus necessitating sufficient depth of biopsy specimens to assess for this risk. Recent studies suggest that biopsy specimens are adequate to assess for buried glands and intestinal metaplasia. Secondary analysis of the AIM dysplasia trial looked at 5648 biopsy specimens from 113 patients who underwent radiofrequency ablation or sham procedures. Biopsy specimens demonstrated a similar rate of subepithelium between both groups (78.4% for radiofrequency ablation group versus 79.1% for sham group).Citation86 Follow-up of the AIM-II trial demonstrated that biopsies at 5 years revealed focal metaplasia in 8% of patients, demonstrating the importance of surveillance in identifying persistent or recurrent disease.
Surgical management
Esophagectomy
Until the past decade, esophagectomy for Barrett’s esophagus with high-grade dysplasia and intramucosal carcinoma had been the traditional standard. Surgery allows for accurate staging and assessment of adequacy by looking for negative margins and lymph nodes. Complete resection of the entire Barrett’s segment is done in cases of unsuspected multifocal disease and to minimize the risk of metachronous lesion development in residual Barrett’s.Citation87 Recently, there has been a shift to treating high-grade dysplasia and intramucosal carcinoma with endoscopic therapies, but submucosal invasion remains an indication for esophagectomy. Esophagectomy is still reserved for select cases with evidence of lymph node metastasis, unsuccessful endoscopic therapy, or with high-risk features of high-grade dysplasia or intramucosal carcinoma.Citation88 High-risk features to consider include high-grade dysplasia obtained from multiple levels, protruding or excavated lesions, poorly differentiated tumors, and invasion into lymphatic channels, blood vessels, or neural structures.Citation88 A patient’s age, comorbidities, and willingness to undergo surgery should also be taken into account.
Compared with endoscopic treatment, there is significant morbidity and mortality associated with esophagectomy, with overall morbidity rates as high as 50% and mortality as high as 10%.Citation89 Adverse outcomes include pulmonary complications, hemorrhage, anastomotic leak, infection, postoperative arrythmias, and nerve palsy. When considering the mortality rates for esophagectomy, it is important to recognize that most studies describe outcomes after surgery for cancer and not high-grade dysplasia, and that high-volume centers tend to have better outcomes. At high-volume centers with greater surgical expertise, the mortality rate from esophagectomy for cancer is 2%–3%.Citation90,Citation91 Furthermore, patients with cancers tend to be more debilitated preoperatively, and comorbid diseases are less frequent in patients with high-grade dysplasia alone.Citation92 Esophagectomy performed specifically for high-grade dysplasia has a pooled mortality rate of 1%,Citation92 making it a lower-risk surgery than when performed for cancer. The best surgical option for high-grade dysplasia or intramucosal carcinoma should produce the least morbidity balanced against best long-term survival.
Several studies provide evidence that esophagectomy prevents cancer-related mortality in patients with high-grade dysplasia and intramucosal carcinoma. In a study at the Cleveland Clinic, pathologic review of resection specimens revealed high-grade dysplasia in 59 patients and invasive cancer in 50 patients. Operative mortality was 0%, and 10-year survival was 86% for both groups.Citation93 Overall 5-year survival was 83% for all resected high-grade dysplasia patients (91% for those without invasion and 68% for those with invasion). Operative mortality occurred in one patient (2% mortality rate).Citation94 Similar 5-year survival rates of 88% and 86% have been reported in similar studies.Citation95,Citation96
Recurrence rates of Barrett’s esophagus or adenocarcinoma following esophagectomy have been assessed in a limited number of trials. In a study by Chang et al of 34 Barrett’s esophagus patients with high-grade dysplasia or esophageal adenocarcinoma, the cure rate after esophagectomy and 2-year surveillance was 85%, demonstrated by having no clinical, radiographic, or endoscopic evidence of recurrent esophageal adenocarcinoma or metastasis.Citation97 In a study by Wolfsen et al the cure rate for dysplasia or localized esophageal adenocarcinoma was reported to be lower at 77.8%.Citation98 This highlights the need for continued endoscopic surveillance following resection.
Conventional approaches for esophagectomy are transhiatal and transthoracic resection. Transhiatal resection without thoractomy can be done to prevent respiratory compromise, with the incidence of atelectasis or pneumonia being low (2% and <1% of cases, respectively).Citation99 A randomized trial comparing 106 patients undergoing transhiatal esophagectomy and 114 patients undergoing transthoracic esophagectomy with lymphadenectomy demonstrated a significantly lower rate of postoperative respiratory complications with the transhiatal approach (27% versus 57%). A trend towards greater survival was shown for the transthoracic approach at 5 years (39% versus 27%). No significant difference was noted for inhospital mortality.Citation100 One potential limitation of the transhiatal approach is the inability to retrieve lymph nodes required for nodal staging;Citation101 more extensive resection using a transthoracic approach with thoracotomy likely improves staging accuracy. The needs of the patient (accurate staging versus functional status and operative risk) as well as the surgeon’s expertise ultimately determine the preferred approach.
Minimally invasive esophagectomy utilizes video-assisted or laparoscopic techniques and is a promising approach. Minimally invasive esophagectomy has potential advantages over open esophagectomy because of a lower incidence of pulmonary complications, faster postoperative recovery, and decreased length of hospital stay.Citation102 Minimally invasive esophagectomy avoids the thoracotomy and laparotomy, thus limiting the extent of surgical trauma and presumably reducing postoperative morbidity and increasing quality of life.Citation103 However, lymph node retrieval is largely inferior to standards of open surgery, and morbidity and mortality of minimally invasive esophagectomy is not proven to be lower when compared with open esophagectomy at experienced centers.Citation104 Recommendations favoring minimally invasive esophagectomy over open esophagectomy cannot be made due to a lack of randomized trials comparing the two approaches.
In patients with few comorbidities and an otherwise long life expectancy, a vagal-sparing esophagectomy can be considered to improve outcomes and quality of life. Peyre et al demonstrated lower infectious, respiratory, and anastomotic complications in patients with high-grade dysplasia or intramucosal carcinoma undergoing this procedure compared with transhiatal esophagectomy. Quality of life advantages were also demonstrated, with the potential for reduced morbidity owing to avoidance of postvagotomy dumping and diarrhea, as well as a shorter hospital stay.Citation105 However, lymphadenectomy is not performed with this procedure, so it is not an option for patients with submucosal cancer given the need for nodal staging. Long-term outcomes data are lacking, thus its overall utility remains to be seen.
Antireflux surgery
Antireflux surgery using fundoplication theoretically eliminates acid reflux and provides better control of gastroesophageal reflux disease than PPIs. The concept that adequate reflux control following antireflux surgery is necessary to reduce the rate of progression of disease is supported by studies that suggest progression is significantly more likely to occur with a failed fundoplication and persistent reflux.Citation4,Citation106,Citation107 In the only randomized trial to date comparing antireflux surgery and PPIs, 43 patients treated medically and 58 treated with antireflux surgery were compared. No significant difference between the two groups was found with respect to preventing progression to dysplasia and adenocarcinoma.Citation108 Optimal candidates for antireflux surgery include those who lack major comorbidities and demonstrate some response to PPI therapy.Citation33 Antireflux surgery has not been proven to reduce progression of nondysplastic Barrett’s esophagus to high-grade dysplasia or adenocarcinoma significantly, and does not obviate the need for continued surveillance. The decision to pursue antireflux surgery should depend on patient preference and the severity of reflux symptoms despite PPI therapy, rather than for definitive management of intestinal metaplasia.
Conclusion
In summary, the management of Barrett’s esophagus requires acid suppression and endoscopic assessment. For patients with nondysplastic Barrett’s esophagus, treatment with a PPI may decrease acid exposure and delay the progression to dysplasia. A careful endoscopic examination should include assessment of any visible lesions in a Barrett’s segment. An expert gastro-intestinal pathologist should confirm neoplasia in the setting of Barrett’s esophagus. For those patients with high-grade dysplasia or intramucosal carcinoma, careful consideration of endoscopic therapy or surgical therapy must be given. All visible lesions in the setting of dysplasia should be targeted with focal endoscopic mucosal resection for both accurate histopathologic diagnosis and for treatment. The remainder of the Barrett’s epithelium should also be eradicated to address all synchronous and metachronous lesions. This may be done with tissue acquiring or nontissue acquiring means. Radiofrequency ablation has a positive benefit-risk profile for flat Barrett’s esophagus. At this time, endoscopic therapy is not indicated for nondysplastic Barrett’s esophagus. Esophagectomy is still reserved for selected cases with evidence of lymph node metastasis, unsuccessful endoscopic therapy, or with high-risk features of high-grade dysplasia or intramucosal carcinoma.
Disclosure
VJAK has received honoraria and consulting fees from Manua Kea Technologies, Paris, France.
References
- PohlHWelchHGThe role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidenceJ Natl Cancer Inst200597214214615657344
- EnzingerPCMayerRJEsophageal cancerN Engl J Med2003349232241225214657432
- FitzgeraldRCLascarRTriadafilopoulosGReview article: Barrett’s oesophagus, dysplasia and pharmacologic acid suppressionAliment Pharmacol Ther200115326927611207503
- ZehetnerJDeMeesterSRAyaziSLong-term follow-up after anti-reflux surgery in patients with Barrett’s esophagusJ Gastrointest Surg201014101483149120824377
- CooperBTBarbezatGOTreatment of Barrett’s esophagus with H2 blockersJ Clin Gastroenterol1987921391412883213
- NeumannCSIqbalTHCooperBTLong term continuous omeprazole treatment of patients with Barrett’s oesophagusAliment Pharmacol Ther1995944514548527623
- PetersFTGaneshSKuipersEJEndoscopic regression of Barrett’s oesophagus during omeprazole treatment; a randomised double blind studyGut199945448949410486353
- GoreSHealeyCJSuttonRRegression of columnar lined (Barrett’s) oesophagus with continuous omeprazole therapyAliment Pharmacol Ther1993766236288161668
- SamplinerREEffect of up to 3 years of high-dose lansoprazole on Barrett’s esophagusAm J Gastroenterol19948910184418487942680
- CooperBTNeumannCSCoxMAIqbalTHContinuous treatment with omeprazole 20 mg daily for up to 6 years in Barrett’s oesophagusAliment Pharmacol Ther19981298938979768533
- WilkinsonSPBiddlestoneLGoreSShepherdNARegression of columnar-lined (Barrett’s) oesophagus with omeprazole 40 mg daily: Results of 5 years of continuous therapyAliment Pharmacol Ther19991391205120910468702
- MalesciASavarinoVZentilinPPartial regression of Barrett’s esophagus by long-term therapy with high-dose omeprazoleGastrointest Endosc19964467007058979061
- CooperBTChapmanWNeumannCSGeartyJCContinuous treatment of Barrett’s oesophagus patients with proton pump inhibitors up to 13 years: Observations on regression and cancer incidenceAliment Pharmacol Ther200623672773316556174
- SharmaPSamplinerRECamargoENormalization of esophageal pH with high-dose proton pump inhibitor therapy does not result in regression of Barrett’s esophagusAm J Gastroenterol19979245825859128303
- SamplinerREReduction of acid exposure and regression of Barrett’s esophagusDig Dis200018420320711356991
- WestonAPBadrASHassaneinRSProspective multivariate analysis of factors predictive of complete regression of Barrett’s esophagusAm J Gastroenterol199994123420342610606297
- GatenbyPARamusJRCaygillCPCharlettAWinsletMCWatsonATreatment modality and risk of development of dysplasia and adenocarcinoma in columnar-lined esophagusDis Esophagus200922213314219018855
- El-SeragHBAguirreTVDavisSKuebelerMBhattacharyyaASamplinerREProton pump inhibitors are associated with reduced incidence of dysplasia in Barrett’s esophagusAm J Gastroenterol200499101877188315447744
- NguyenDMEl-SeragHBHendersonLSteinDBhattacharyyaASamplinerREMedication usage and the risk of neoplasia in patients with Barrett’s esophagusClin Gastroenterol Hepatol20097121299130419523538
- HillmanLCChiragakisLShadboltBKayeGLClarkeACProton-pump inhibitor therapy and the development of dysplasia in patients with Barrett’s oesophagusMed J Aust2004180838739115089728
- HeathCWJrThunMJGreenbergERLevinBMarnettLJNonsteroidal antiinflammatory drugs and human cancer. Report of an interdisciplinary research workshopCancer19947410288528887954252
- CorleyDAKerlikowskeKVermaRBufflerPProtective association of aspirin/NSAIDs and esophageal cancer: A systematic review and meta-analysisGastroenterology20031241475612512029
- FosslienEMolecular pathology of cyclooxygenase-2 in neoplasiaAnn Clin Lab Sci200030132110678579
- SouzaRFShewmakeKBeerDGCryerBSpechlerSJSelective inhibition of cyclooxygenase-2 suppresses growth and induces apoptosis in human esophageal adenocarcinoma cellsCancer Res200060205767577211059772
- MorrisCDArmstrongGRBigleyGGreenHAttwoodSECyclooxygenase-2 expression in the Barrett’s metaplasia-dysplasia-adenocarcinoma sequenceAm J Gastroenterol200196499099611316217
- ButtarNSWangKKAndersonMAThe effect of selective cyclooxygenase-2 inhibition in Barrett’s esophagus epithelium: An in vitro studyJ Natl Cancer Inst200294642242911904314
- VaughanTLDongLMBlountPLNon-steroidal anti-inflammatory drugs and risk of neoplastic progression in Barrett’s oesophagus: A prospective studyLancet Oncol200561294595216321762
- AndersonLAJohnstonBTWatsonRGNonsteroidal anti-inflammatory drugs and the esophageal inflammation-metaplasia-adenocarcinoma sequenceCancer Res20066694975498216651456
- NguyenDMRichardsonPEl-SeragHBMedications (NSAIDs, statins, proton pump inhibitors) and the risk of esophageal adenocarcinoma in patients with Barrett’s esophagusGastroenterology201013872260226620188100
- SpechlerSJSharmaPSouzaRFInadomiJMShaheenNJAmerican Gastroenterological Association medical position statement on the management of Barrett’s esophagusGastroenterology201114031084109121376940
- HenryDLimLLGarcia RodriguezLAVariability in risk of gastrointestinal complications with individual non-steroidal anti-inflammatory drugs: Results of a collaborative meta-analysisBMJ1996312704615631566
- HeathEICantoMIPiantadosiSSecondary chemoprevention of Barrett’s esophagus with celecoxib: Results of a randomized trialJ Natl Cancer Inst200799754555717405999
- WangKKSamplinerREUpdated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagusAm J Gastroenterol2008103378879718341497
- ChennatJSKondaVJWaxmanIEndotherapy for Barrett’s esophagus: Which, how, when and who?Gastrointest Endosc Clin N Am201121111913321112502
- SharmaPDentJArmstrongDThe development and validation of an endoscopic grading system for Barrett’s esophagus: The Prague C and M criteriaGastroenterology200613151392139917101315
- MannathJSubramanianVHawkeyCJRagunathKNarrow band imaging for characterization of high grade dysplasia and specialized intestinal metaplasia in Barrett’s esophagus: A meta-analysisEndoscopy201042535135920200809
- CurversWLBohmerCJMallant-HentRCMucosal morphology in Barrett’s esophagus: Interobserver agreement and role of narrow band imagingEndoscopy2008401079980518828075
- KaraMAPetersFPTenKateVanFJDeventerSJFockensPBergmanJJEndoscopic video autofluorescence imaging may improve the detection of early neoplasia in patients with Barrett’s esophagusGastrointest Endosc200561667968515855971
- WaxmanIKondaVJEndoscopic techniques for recognizing neoplasia in Barrett’s esophagus: Which should the clinician use?Curr Opin Gastroenterol201026435236020571387
- DunbarKBOkoloP3rdMontgomeryECantoMIConfocal laser endomicroscopy in Barrett’s esophagus and endoscopically inapparent Barrett’s neoplasia: A prospective, randomized, double-blind, controlled, crossover trialGastrointest Endosc200970464565419559419
- MontgomeryEBronnerMPGoldblumJRReproducibility of the diagnosis of dysplasia in Barrett esophagus: A reaffirmationHum Pathol200132436837811331953
- OrmsbyAHPetrasREHenricksWHObserver variation in the diagnosis of superficial oesophageal adenocarcinomaGut200251567167612377805
- KerkhofMvan DekkenHSteyerbergEWGrading of dysplasia in Barrett’s oesophagus: Substantial interobserver variation between general and gastrointestinal pathologistsHistopathology200750792092717543082
- ChennatJKondaVJRossASComplete Barrett’s eradication endoscopic mucosal resection: An effective treatment modality for high-grade dysplasia and intramucosal carcinoma – an American single-center experienceAm J Gastroenterol2009104112684269219690526
- WaniSMathurSCCurversWLGreater interobserver agreement by endoscopic mucosal resection than biopsy samples in Barrett’s dysplasiaClin Gastroenterol Hepatol20108978378820472096
- FergusonMKNaunheimKSResection for Barrett’s mucosa with high-grade dysplasia: Implications for prophylactic photodynamic therapyJ Thorac Cardiovasc Surg199711458248299375613
- KondaVJRossASFergusonMKIs the risk of concomitant invasive esophageal cancer in high-grade dysplasia in Barrett’s esophagus overestimated?Clin Gastroenterol Hepatol20086215916418096439
- ParafFFlejouJFPignonJPFeketeFPotetFSurgical pathology of adenocarcinoma arising in Barrett’s esophagus. Analysis of 67 casesAm J Surg Pathol19951921831917832278
- FeithMSteinHJSiewertJRPattern of lymphatic spread of Barrett’s cancerWorld J Surg20032791052105712917758
- SteinHJFeithMBruecherBLNaehrigJSarbiaMSiewertJREarly esophageal cancer: Pattern of lymphatic spread and prognostic factors for long-term survival after surgical resectionAnn Surg2005242456657316192817
- RiceTWZuccaroGJrAdelsteinDJRybickiLABlackstoneEHGoldblumJREsophageal carcinoma: Depth of tumor invasion is predictive of regional lymph node statusAnn Thorac Surg19986537877929527214
- LopesCVHelaMPesentiCCircumferential endoscopic resection of Barrett’s esophagus with high-grade dysplasia or early adenocarcinomaSurg Endosc200721582082417294308
- van SandickJWvan LanschotJJten KateFJPathology of early invasive adenocarcinoma of the esophagus or esophagogastric junction: Implications for therapeutic decision makingCancer200088112429243710861416
- WaxmanIKondaVJMucosal ablation of Barrett esophagusNat Rev Gastroenterol Hepatol20096739340119488071
- EllCMayAGossnerLEndoscopic mucosal resection of early cancer and high-grade dysplasia in Barrett’s esophagusGastroenterology2000118467067710734018
- LarghiALightdaleCJMemeoLBhagatGOkparaNRotterdamHEUS followed by EMR for staging of high-grade dysplasia and early cancer in Barrett’s esophagusGastrointest Endosc2005621162315990814
- MayAGossnerLPechOLocal endoscopic therapy for intraepithelial high-grade neoplasia and early adenocarcinoma in Barrett’s oesophagus: Acute-phase and intermediate results of a new treatment approachEur J Gastroenterol Hepatol200214101085109112362099
- MayAGossnerLPechOIntraepithelial high-grade neoplasia and early adenocarcinoma in short-segment Barrett’s esophagus (SSBE): Curative treatment using local endoscopic treatment techniquesEndoscopy200234860461012173079
- Mino-KenudsonMBruggeWRPuricelliWPManagement of superficial Barrett’s epithelium-related neoplasms by endoscopic mucosal resection: Clinicopathologic analysis of 27 casesAm J Surg Pathol200529568068615832094
- NijhawanPKWangKKEndoscopic mucosal resection for lesions with endoscopic features suggestive of malignancy and high-grade dysplasia within Barrett’s esophagusGastrointest Endosc200052332833210968845
- PechOMayAGossnerLEllCBarrett’s esophagus: Endoscopic resectionGastrointest Endosc Clin N Am200313350551214629106
- ButtarNSWangKKSeboTJExtent of high-grade dysplasia in Barrett’s esophagus correlates with risk of adenocarcinomaGastroenterology200112071630163911375945
- SeewaldSAngTLGotodaTSoehendraNTotal endoscopic resection of Barrett esophagusEndoscopy200840121016102019065485
- PechOBehrensAMayALong-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagusGut20085791200120618460553
- PetersFPKaraMARosmolenWDStepwise radical endoscopic resection is effective for complete removal of Barrett’s esophagus with early neoplasia: A prospective studyAm J Gastroenterol200610171449145716863545
- SeewaldSAkaraviputhTSeitzUCircumferential EMR and complete removal of Barrett’s epithelium: A new approach to management of Barrett’s esophagus containing high-grade intraepithelial neoplasia and intramucosal carcinomaGastrointest Endosc200357785485912776032
- PouwRESeewaldSGondrieJJStepwise radical endoscopic resection for eradication of Barrett’s oesophagus with early neoplasia in a cohort of 169 patientsGut20105991169117720525701
- WangKKPrasadGTianJEndoscopic mucosal resection and endoscopic submucosal dissection in esophageal and gastric cancersCurr Opin Gastroenterol201026545345820703112
- YoshinagaSGotodaTKusanoCOdaINakamuraKTakayanagiRClinical impact of endoscopic submucosal dissection for superficial adenocarcinoma located at the esophagogastric junctionGastrointest Endosc200867220220918226681
- van VilsterenFGPouwRESeewaldSStepwise radical endoscopic resection versus radiof-requency ablation for Barrett’s oesophagus with high-grade dysplasia or early cancer: a multicentre randomised trialGut201160676577321209124
- SharmaVKWangKKOverholtBFBalloon-based, circumferential, endoscopic radiofrequency ablation of Barrett’s esophagus: 1-year follow-up of 100 patientsGastrointest Endosc200765218519517258973
- FleischerDEOverholtBFSharmaVKEndoscopic ablation of Barrett’s esophagus: A multicenter study with 2.5-year follow-upGastrointest Endosc200868586787618561930
- FleischerDEOverholtBFSharmaVKEndoscopic radiofrequency ablation for Barrett’s esophagus: 5-year outcomes from a prospective multicenter trialEndoscopy2010421078178920857372
- ShaheenNJSharmaPOverholtBFRadiofrequency ablation in Barrett’s esophagus with dysplasiaN Engl J Med2009360222277228819474425
- GanzRAOverholtBFSharmaVKCircumferential ablation of Barrett’s esophagus that contains high-grade dysplasia: A US Multicenter RegistryGastrointest Endosc2008681354018355819
- PouwREWirthsKEisendrathPEfficacy of radiofrequency ablation combined with endoscopic resection for Barrett’s esophagus with early neoplasiaClin Gastroenterol Hepatol201081232919602454
- GrossSAWolfsenHCThe role of photodynamic therapy in the esophagusGastrointest Endosc Clin N Am20102013553vi19951793
- OverholtBFPanjehpourMHalbergDLPhotodynamic therapy for Barrett’s esophagus with dysplasia and/or early stage carcinoma: Long-term resultsGastrointest Endosc200358218318812872083
- PechOGossnerLMayALong-term results of photodynamic therapy with 5-aminolevulinic acid for superficial Barrett’s cancer and high-grade intraepithelial neoplasiaGastrointest Endosc2005621243015990815
- PrasadGAWangKKButtarNSWongkeesongLMLutzkeLSBorkenhagenLSPredictors of stricture formation after photodynamic therapy for high-grade dysplasia in Barrett’s esophagusGastrointest Endosc2007651606617185080
- Van LaethemJLPenyMOSalmonICremerMDeviereJIntramucosal adenocarcinoma arising under squamous re-epithelialisation of Barrett’s oesophagusGut200046457457710716690
- KuflikEGCryosurgery updatedJ Am Acad Dermatol19943169259447962774
- DumotJAVargoJJ2ndFalkGWFreyLLopezRRiceTWAn openlabel, prospective trial of cryospray ablation for Barrett’s esophagus high-grade dysplasia and early esophageal cancer in high-risk patientsGastrointest Endosc200970463564419559428
- GreenwaldBDDumotJAHorwhatJDLightdaleCJAbramsJASafety, tolerability, and efficacy of endoscopic low-pressure liquid nitrogen spray cryotherapy in the esophagusDis Esophagus2010231131919515183
- ShaheenNJGreenwaldBDPeeryAFSafety and efficacy of endoscopic spray cryotherapy for Barrett’s esophagus with high-grade dysplasiaGastrointest Endosc201071468068520363409
- ShaheenNJPeeryAFOverholtBFBiopsy depth after radiofrequency ablation of dysplastic Barrett’s esophagusGastrointest Endosc2010723490e.1496e.120598302
- BozioGBaulieuxJMabrutJYThe role of surgery in the management of Barrett’s esophagus (from dysplasia to cancer)J Visc Surg20111481192621310681
- KondaVJFergusonMKEsophageal resection for high-grade dysplasia and intramucosal carcinoma: When and how?World J Gastroenterol201016303786379220698041
- BaileySHBullDAHarpoleDHOutcomes after esophagectomy: A ten-year prospective cohortAnn Thorac Surg200375121722212537219
- BirkmeyerJDStukelTASiewersAEGoodneyPPWennbergDELucasFLSurgeon volume and operative mortality in the United StatesN Engl J Med2003349222117212714645640
- LawSEsophagectomy without mortality: What can surgeons do?J Gastrointest Surg201014Suppl 1S101S10719774427
- FernandoHCMurthySCHofstetterWThe Society of Thoracic Surgeons practice guideline series: Guidelines for the management of Barrett’s esophagus with high-grade dysplasiaAnn Thorac Surg20098761993200219463651
- RiceTWPro: Esophagectomy is the treatment of choice for high-grade dysplasia in Barrett’s esophagusAm J Gastroenterol2006101102177217917032178
- ReedMFTolisGJrEdilBHSurgical treatment of esophageal high-grade dysplasiaAnn Thorac Surg20057941110111515797034
- TsengEEWuTTYeoCJHeitmillerRFBarrett’s esophagus with high grade dysplasia: Surgical results and long-term outcome – an updateJ Gastrointest Surg20037216417012600440
- HeadrickJRNicholsFC3rdMillerDLHigh-grade esophageal dysplasia: Long-term survival and quality of life after esophagectomyAnn Thorac Surg20027361697170212078755
- ChangLCOelschlagerBKQuirogaELong-term outcome of esophagectomy for high-grade dysplasia or cancer found during surveillance for Barrett’s esophagusJ Gastrointest Surg200610334134616504878
- WolfsenHCHemmingerLLDeVaultKRRecurrent Barrett’s esophagus and adenocarcinoma after esophagectomyBMC Gastroenterol200441815327696
- OrringerMBMarshallBIannettoniMDTranshiatal esophagectomy: Clinical experience and refinementsAnn Surg1999230339240010493486
- HulscherJBvan SandickJWde BoerAGExtended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagusN Engl J Med2002347211662166912444180
- WolffCSCastilloSFLarsonDRIvorLewisapproach is superior to transhiatal approach in retrieval of lymph nodes at esophagectomyDis Esophagus2008214328333
- BiereSSCuestaMAvan der PeetDLMinimally invasive versus open esophagectomy for cancer: A systematic review and meta-analysisMinerva Chir200964212113319365313
- BiereSSMaasKWBonavinaLTraditional invasive vs minimally invasive esophagectomy: a multi-center, randomized trial (TIME-trial)BMC Surg201111221226918
- DeckerGCoosemansWDe LeynPMinimally invasive esophagectomy for cancerEur J Cardiothorac Surg2009351132018952454
- PeyreCGDeMeesterSRRizzettoCVagal-sparing esophagectomy: The ideal operation for intramucosal adenocarcinoma and Barrett with high-grade dysplasiaAnn Surg2007246466567117893503
- LagergrenJViklundPIs esophageal adenocarcinoma occurring late after antireflux surgery due to persistent postoperative reflux?World J Surg200731346546917171490
- O’RiordanJMByrnePJRaviNKeelingPWReynoldsJVLong-term clinical and pathologic response of Barrett’s esophagus after antireflux surgeryAm J Surg20041881273315219481
- ParrillaPMartinez de HaroLFLong-term results of a randomized prospective study comparing medical and surgical treatment of Barrett’s esophagusAnn Surg2003237329129812616111