Abstract
Background
Adherence to antiretroviral treatment (ART) is important to achieve treatment success in human immunodeficiency virus (HIV)-infected patients. Most HIV clinics apply the patient self-report (PSR) method. However, the reliability of this method in experienced HIV patients remains questionable.
Purpose
To validate the PSR method for measuring adherence to ART using lamivudine (3TC) plasma concentrations in experienced HIV patients.
Methods
The study was conducted in Dar Es Salaam and involved 220 patients who were receiving ART services at HIV clinics for more than 12 months. Self-reported adherence information to ART was obtained on the day of HIV clinic visit. The patients were asked to mention the number of doses missed within the past 7 days. In addition, blood samples (2 mL) were collected from each patient on the same day. The blood samples were determined for 3TC plasma concentrations. The target 3TC plasma concentration as indicator concentration for adherent patients was determined in 20 patients who took their evening dose of antiretrovirals under supervision. The blood from these patients was drawn 3 hours after drug administration.
Results
Complete drug levels of 3TC and self-reported adherence data was obtained in 200 treatment-experienced HIV patients. Lamivudine plasma concentrations obtained in these patients ranged between 0.02–17.36 μg/mL. The mean time from dose administration to blood drawing was 3.1 ± 1.2 hours with coefficient of variation >39%. The mean 3TC plasma concentration obtained in 20 patients who took their antiretroviral dose under supervision was found to be 0.67 ± 0.46 μg/mL, range 0.25–2.33 μg/mL. As many as 82.5% of experienced HIV patients had PSRs in agreement with their 3TC plasma concentrations.
Conclusion
PSR adherence is still a valid method for ascertaining adherence to ART in treatment-experienced HIV patients.
Introduction
Adherence to antiretroviral treatment (ART) has been demonstrated to reduce human immunodeficiency virus (HIV)-1 RNA, elevate CD4 cell counts, decrease morbidity and mortality due to acquired immunodeficiency syndrome (AIDS), and improve quality of life.Citation1 Studies have shown that for successful ART, adherence to dosage regimen should be ≥95%.Citation1–Citation3 However, there are many factors which contribute to poor ART adherence, such as complexity of dosing schedule, strict dietary restrictions, drug toxicity, pill burden, forgetting, and poor drug supply chain.Citation4–Citation8 Nonadherence to ART may lead to suboptimal drug levels with possible therapeutic failure, deterioration of the immune system, and/or emergence of medicine-resistant HIV strains.Citation1–Citation3
Estimates of nonadherence to ART have varied widely, depending on the method of measurement, definition of nonadherence, the population studied, and the time over which adherence was monitored. There are various approaches to measure adherence, ranging from patient self-report (PSR), physician assessments, electronic monitoring, pill count, and prescription-refill days.Citation9–Citation11 Determination of antiretroviral drug level has been recently exploited as an alternative method for measuring adherence to ART.Citation9,Citation10 Other methods include visual analog scale; viral load HIV-1 RNA; and macrocytosis, defined as mean corpuscular volume measurement, in patients undergoing stavudine- and zidovudine-containing regimen.Citation12–Citation14
Most HIV clinics in low- and middle-income countries, apply PSR as a routine method for measuring adherence in ART patients. The PSR method is simpler and less costly compared with other subjective and nonsubjective methods. PSR does not require expensive bioanalytical techniques and highly skilled personnel. However, it has been reported that the PSR method can lead to an overestimation of adherence in treatment-experienced HIV patients.Citation6–Citation10 In addition to lacking a standardized instrument for universal use in routine clinical practice,Citation15–Citation19 the validity of PSR in experienced HIV patients in developing countries may be questionable. This study reports the extent of validity of the PSR method in measuring adherence to ART in experienced HIV patients in Tanzania.
Materials and methods
Study site
This was a cross-sectional study involving 220 HIV-infected patients receiving ART at Mwananyamala and Muhimbili HIV clinics located in Dar Es Salaam. The study took place between July and December 2010. Approval to conduct the study was granted by Muhimbili University of Health and Allied Sciences (MUHAS) Ethics Committee.
Enrollment of subjects
Nurse counselors working at the HIV clinics were trained as research assistants. They were also involved in the identification of files of eligible patients for inclusion in the study. Initially, 400 patients were purposely selected and the major inclusion criterion was the receiving of lamivudine (3TC)-based ART for more than 12 months and continued attendance at the HIV clinics for care and treatment. Nurses and counselors at each HIV clinic were involved in identification of those patients who were assessed by HIV clinicians and found to be eligible for the study. Patient selection was based on the “first-in, first-taken” principle, as long as the patient met the inclusion criteria. Out of 400 patients, 220 patients who consented to participate to the study on the day of clinic visitation were selected. The drug combination given to patients was zidovudine/stavudine + 3TC + nevirapine/ efavirenz. The research assistants documented all available data from patients’ files in line with routine measurements.
Collection of adherence information from patients
Eligible patients were asked by the research assistants to report the exact number and date of the doses they had missed in the past 7 days, as is done routinely in HIV clinics in Tanzania. They were also asked to indicate the hour at which they ingested the last ARV dose before presenting to the clinic. In addition, blood samples were collected from each patient. A single venous blood sample (2 mL) was drawn into an ethylenediaminetetraacetic acid tube. The time interval between the last ARV dose intake and blood sampling was documented. The patients were not aware of the study before attending their consultation and therefore did not know that they were having blood drawn to measure 3TC plasma levels. All blood samples were single samples obtained just after undergoing medical examinations. The blood samples were centrifuged immediately at 1000 g to obtain plasma. The obtained plasma samples were kept at −80°C until drug assay.
The study determined the indicator (target) 3TC plasma concentration in 20 separate patients who had also been on ART for more than 12 months. Since on average, a patient spent 3 hours at the HIV clinic before blood withdrawal, analogously, blood was drawn from these patients 3 hours after taking their ARV evening dose under supervision. The blood samples were processed to obtain plasma and stored until drug assay, as described above.
Determination of ARV in blood samples
Lamivudine plasma concentrations were determined by using a high-performance liquid chromatography method published by Zhou and Sommadossi with minor modifications.Citation19 Sample analysis was carried out in the MUHAS–Sida (Swedish International Development Cooperation Agency) Bioanalytical Laboratory, Unit of Pharmacology and Therapeutics, School of Pharmacy, MUHAS in Dar Es Salaam, Tanzania. Prior to running the samples from the study patients, the method was validated with respect to intra- and interday precision and accuracy. The intra- and interday precision rates were always <10% and sample recovery was consistently between 90%–110% of the spiked samples, thus indicating the accuracy and suitability of the analytical method used.
Data analysis
To verify the accuracy of the PSR method in treatment-experienced HIV patients, the proportion of patients whose self-reported responses were in agreement with the determined plasma concentrations was calculated. reflects the plasma concentrations obtained in 20 patients who took their evening ARV dose under supervision. The mean 3TC plasma concentration determined from blood samples drawn 3 hours after drug intake in these supervised patients was 0.67 ± 0.46 μg/mL (0.21–1.13 μg/mL) and ranged from 0.25 to 2.33 μg/mL. Therefore, all patients with 3TC plasma concentrations ≥0.21–2.33 μg/mL were taken as adherent as long as they stated so when interviewed by the research assistant. To confirm the existence of a correlation between PSR and drug levels in some patients, the following information was sought: those who reported missing some doses and had 3TC plasma concentrations <0.21 μg/mL; those who had plasma concentrations within the predeter-mined range and reported adhering well to the recommended dosing regimen; and those who had higher levels of 3TC and reported taking two ARV doses on the morning of the day they attended the HIV clinic. These groups were regarded as having PSR corresponding with obtained plasma drug levels.
Table 1 Predetermined lamivudine (3TC) plasma concentrations in 20 patients who took lamivudine-based adherence to antiretroviral treatment under supervision and blood collected 3 hours after drug intake
Results
The patient population was predominantly (65%) female with a mean age of 49 ± 5 years.
The mean time (data not shown) from dose administration to blood drawing was 3.1 ± 1.2 hours with coefficient of variation (CV) 39%. Some demographic data, such as age, sex, weight, height, and concomitant therapy, were included in patient files, but in most files these data were missing.
reflects the 3TC plasma concentrations obtained from 20 patients who took 3TC-based antiretroviral therapy (ART) in the evening under supervision and had their blood drawn 3 hours after drug intake. The mean 3TC plasma concentrations obtained from these patients was 0.67 ± 0.46 μg/ mL (0.21–1.13 μg/mL) and ranged from 0.25 to 2.33 μg/mL with CV 69%.
Complete drug levels of 3TC and PSR-adherence data were obtained in 200 out of 220 study patients. shows the distribution of 200 patients with respect to 3TC plasma concentrations. The concentrations have been categorized into three groups: (1) those below the cut-off point of <0.21 μg/mL; (2) those within the target predetermined range –0.21 to 2.33 μg/mL; (3) and those >2.33 μg/mL.
Figure 1 Percentage distribution of patients in relation to obtained lamivudine (3TC) plasma concentrations.
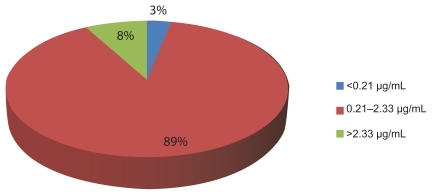
Lamivudine plasma concentrations obtained in these patients ranged between 0.02 and 17.36 μg/mL (CV > 60%). Self-reported adherence indicated that, out of 200 patients whose plasma samples were analyzed for 3TC concentrations, 5% (10/200) reported missing two to three doses in the past 7 days, including the day they visited the clinic, and their plasma concentrations were <0.21 μg/mL. There were six other patients who reported not missing any dose in the past 7 days but had 3TC plasma concentrations below the level required for adherent patients. Three patients reported taking two doses of ARV in the morning and had 3TC plasma concentrations >2.33 μg/mL.
The number of patients whose self-reported responses were in agreement with the determined plasma concentrations was calculated as follows:
Patients whose 3TC plasma concentrations were found to lie within the predetermined target concentration (0.21–1.13 μg/mL) obtained in 20 supervised patients.
Patients who reported not missing any dose and their 3TC plasma concentrations were found to be >1.13 μg/mL but <2.33 μg/mL (1.13–2.33 μg/mL).
Patients who reported missing ARV doses and also had 3TC plasma concentrations <0.21 μg/mL.
summarizes the distribution of the 200 study patients in relation to PSR adherence and 3TC plasma concentration. The proportion of HIV-treated patients whose self-reported responses were in agreement with the determined plasma concentrations is also demonstrated in . As many as 82.5% of patients (165) had PSR responses in agreement with their 3TC plasma concentrations ().
Figure 2 Proportion of patients whose determined lamivudine (3TC) plasma concentrations was in agreement with patient self-report.
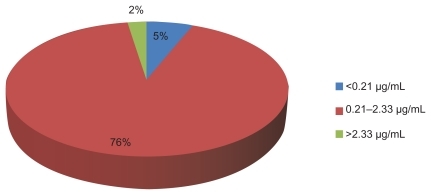
Table 2 Proportion of patients (number in brackets) in relation to patient self-report (PSR) and lamivudine (3TC) plasma concentration agreement (n = 200)
Discussion
The study has determined the validity of PSR adherence to ART in experienced HIV patients in a low-income country. Lamivudine plasma concentrations were used as indicator of adherence in the study patients. The quantified 3TC plasma levels are grouped as reflected in . Lamivudine plasma concentrations obtained in these patients ranged between 0.02 and 17.36 μg/mL (CV > 60%). There was high variation of 3TC plasma concentrations obtained among the study patients. The results are consistent with those obtained by Moore et al and Panhard et al.Citation20,Citation21 The large variation of 3TC plasma concentrations among patients may be partly explained by the different hours at which the patients took their morning ARV doses before blood was drawn by the phlebotomist. It could also be explained by variability of body weights and heights (body mass index) and renal conditions among patients. Unfortunately, the study clinic did not register the body mass index of most patients, and none of the patients had a serum creatinine assessment to determine renal performance. The study used only the data available in patient files in line with what is done routinely in Tanzanian clinics. Lamivudine is a prodrug that undergoes phosphorylation when catalyzed by intracellular kinases to form 3TC-5′-triphosphate, the active metabolite that prevents viral replication.Citation23 Variation in 3TC disposition and, subsequently, plasma concentrations among patients could therefore depend on a patient’s individual capacity to phosphorylate the parent drug. Nevertheless, 3TC is renally excreted and, recently, a low 3TC renal clearance was demonstrated in patients who had impaired renal functions.Citation24,Citation25 Lamivudine has very short elimination half-life of 3–4 hoursCitation21,Citation22 and this may also have contributed to the large variation in the drug plasma concentrations among individual patients.
In this study, it was shown that there was discrepancy between the obtained drug plasma concentrations and patient self-reported drug intake in about 18% of patients. Although the proportion of nonadherent patients in the study was found to be low, it is still important to validate the PSR method regularly to reinforce its effectiveness, particularly in treatment-experienced HIV patients in low- and middle-income countries. Overall results show that there was good adherence in the patients studied. Several factors could explain the good adherence obtained during the study; for instance, free access to ARV drugs, the then-effective ARV procurement system, and adequate counseling of eligible patients.
The study applied 3TC plasma concentrations as a validity marker of PSR. However, it is still debatable whether the best drug of choice, as a marker of adherence, should have short or long half-life. Those in support of short half-life drugs argue that missing a single dose of such a drug will lead to a detectable change in drug plasma concentration, as opposed to drugs with long half-life. As for drugs with a long half-life, a single missed dose may not be detectable, especially if the steady state plasma concentration had already been attained. Recently, Segeral et al applied plasma concentrations of efevirenz and nevirapine (both have long half-lives) to determine adherence in Cambodian patients undergoing first-line World Health Organization-recommended HAART; the results obtained were consistent with those obtained by other methods.Citation12
Conclusion
This study has demonstrated good agreement between PSR adherence and 3TC plasma concentrations in over 80% of the study patients. It can be concluded that the PSR-adherence method is still reliable for measuring adherence in patients undergoing ART, including experienced HIV patients in HIV clinics in Tanzania.
Acknowledgments
The study was funded by the Swedish International Development Cooperation Agency (Sida) through the Directorate of Research and Publications at MUHAS. We thank Eliford Ngaimisi and Lema Aggrey for running high-performance liquid chromatography and data analysis. We also thank the patients and the health workers at the HIV clinics for their participation in the study.
Disclosure
The authors report no conflicts of interest in this work.
References
- MannheimerSFriedlandGMattsJThe consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trialsClin Infect Dis20023481115112111915001
- PatersonDLSwindellsSMohrJAdherence to protease inhibitor therapy and outcomes in patients with HIV infectionAnn Intern Med200013310213010877736
- GarciaRSchooleyRTBadaróRAn adherence trilogy is essential for long-term HAART successBraz J Infect Dis20037530731414552740
- ReadTMijchAFairleyCKAdherence to antiretroviral therapy: are we doing enoughIntern Med J2003335–625425612752896
- MunakataJBennerJSBeckerSClinical and economic outcomes of nonadherence to highly active antiretroviral therapy in patients with human immunodeficiency virusMed Care2006441089389917001259
- TsengALCompliance issues in the treatment of HIV infectionAm J Health Syst Pharm19985517181718249775345
- RosenbachKAAllisonRNadlerJPDaily dosing of highly active antiretroviral therapyClin Infect Dis200234568669211823957
- RuddPAhmedSZacharyVImproved compliance measures: application in an ambulatory hypertensive drug trialClin Pharmacol Ther19904866766852147405
- KastrissiosHSuárezJRHammerSThe extent of non-adherence in a large AIDS clinical trial using plasma dideoxynucleoside concentrations as a markerAIDS19981217230523119863873
- YasudaJMMillerCCurrierJSThe correlation between plasma concentrations of protease inhibitors, medication adherence and virological outcome in HIV-infected patientsAntivir Ther20049575376115535413
- ArnstenJHDemasPAFarzadeganHAntiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoringClin Infect Dis20013381417142311550118
- SegeralOMadecYBanBSimplified assessment of antiretroviral adherence and prediction of virological efficacy in HIV-infected patients in CambodiaAIDS Res Treat2010201014207621490902
- RomanelliFEmpeyKPomeroyCMacrocytosis as an indicator of medication (zidovudine) adherence in patients with HIV infectionAIDS Patient Care STDS200216940541112396692
- RouetFEkoueviDKChaixMLTransfer and evaluation of an automated, low-cost real-time reverse transcription-PCR test for diagnosis and monitoring of human immunodeficiency virus type 1 infection in a West African resource-limited settingJ Clin Microbiol20054362709271715956387
- NieuwkerkPTSprangersMABurgerDMLimited patient adherence to highly active antiretroviral therapy for HIV-1 infection in an observational cohort studyArch Intern Med2001161161962196811525698
- ChesneyMAFactors affecting adherence to antiretroviral therapyClin Infect Dis200030Suppl 2S17117610860902
- MurriRAmmassariAGallicanoKPatient-reported nonadherence to HAART is related to protease inhibitor levelsJ Acquir Immune Defic Syndr200024212312810935687
- AmmassariAMurriRPezzottiPSelf-reported symptoms and medication side effects influence adherence to highly active antiretroviral therapy in persons with HIV infectionJ Acquir Immune Defic Syndr200128544544911744832
- ZhouXJSommadossiJPRapid quantitation of (-)-2′-deoxy-3′-thiacytidine in human serum by high-performance liquid chromatography with ultraviolet detectionJ Chromatogr B Biomed Sci Appl199769124174249174279
- MooreKHYuenGJHusseyEKPopulation pharmacokinetics of lamivudine in adult human immunodeficiency virus-infected patients enrolled in two phase III clinical trialsAntimicrob Agents Chemother199943123025302910582904
- PanhardXLegrandMTaburetAMPopulation pharmacokinetic analysis of lamivudine, stavudine and zidovudine in controlled HIV-infected patients on HAARTEur J Clin Pharmacol200763111019102917694300
- YuenGJLouYBumgarnerNFEquivalent steady-state pharmacokinetics of lamivudine in plasma and lamivudine triphosphate within cells following administration of lamivudine at 300 milligrams once daily and 150 milligrams twice dailyAntimicrob Agents Chemother200448117618214693537
- HealdAEHsyuPHYuenGJPharmacokinetics of lamivudine in human immunodef iciency virus-infected patients with renal dysfunctionAntimicrob Agents Chemother1996406151415198726029
- JohnsonMAMooreKHYuenGJClinical pharmacokinetics of lamivudineClin Pharmacokinet199936141669989342
- WagnerGJRabkinJGMeasuring medication adherence: are missed doses reported more accurately then perfect adherence?AIDS Care200012440540811091773