Abstract
A 65-year-old woman without a history of diabetes mellitus was admitted for elective total knee arthroplasty for osteoarthrosis. There were no specific complaints except for knee flexion contractures, and the results of preoperative tests were unremarkable. On the day of surgery, the patient suffered from a hypoglycemic attack (52 mg/dL) after preoperative overnight fasting. A dextrose infusion immediately corrected the hypoglycemia, and a total knee arthroplasty was then performed. Although a hypoglycemic attack did not recur, further evaluation was required because of nausea that persisted after surgery. The morning serum cortisol level was 0.15 µg/dL with undetectable adrenocorticotropic hormone (ACTH), and the insulin-like growth factor-1 level was 9 ng/mL. An empty sella and bilateral adrenal atrophy were evident in imaging studies. ACTH and growth hormone (GH) did not respond to testing with corticotropin-releasing hormone and GH–releasing peptide-2, respectively. While serum cortisol did not increase on a rapid ACTH stimulation test, urinary free cortisol excretion responded to a prolonged ACTH stimulation test. Finally, the patient was diagnosed as having empty sella syndrome with ACTH and GH deficiencies. After the administration of hydrocortisone as maintenance replacement therapy, the patient’s prolonged postoperative nausea disappeared. Adrenal insufficiency is latent in patients with hypoglycemia episodes. Because patients with adrenal insufficiency require appropriate perioperative corticosteroid supplementation, clinicians should give priority to identifying the underlying etiology of hypoglycemia over non-urgent elective surgery when these co-occur.
Introduction
Hypoglycemic attack is a clinical syndrome whereby a low glucose level results in neurogenic (adrenergic and cholinergic) or neuroglycopenic signs. Although a diverse etiology can lead to hypoglycemia, hypoglycemic agents are the main cause.Citation1 Indeed, patients with diabetes mellitus more frequently suffered from hypoglycemia than patients without diabetes.Citation2,Citation3 Although hypoglycemia can be quickly restored by carbohydrate supplementation in most cases, determining the underlying etiology is a crucial issue especially in patients without diabetes mellitus. This is because adrenal insufficiency, a possibly life-threatening condition, can be latent. Herein, we present a case of secondary adrenal insufficiency where a single hypoglycemic attack just before elective surgery was the only complaint.
Case Description
A 65-year-old woman was admitted for elective total arthroplasty for osteoarthrosis of the right knee. She had taken 50 µg levothyroxine once daily for ten years, but the detailed indication for this was unknown. With the exception of levothyroxine supplementation and osteoarthrosis, the patient had an unremarkable medical record, with no history of diabetes mellitus, head injury, severe headache, stroke, tumor, or radiation therapy. She had undergone three uncomplicated vaginal deliveries when she was 28, 32, and 35 years of age, and regularly menstruated until natural menopause when 52 years old. She was not on any medications, including over-the-counter medications, or supplements except for the levothyroxine. The patient had not received any steroids either orally, by injection or topically. She had no smoking history and only drank alcohol socially.
On physical examination, the patient’s body mass index was 20.6 kg/m2 (height 146 cm, weight 44 kg), body temperature was 35.9°C, blood pressure was 105/63 mmHg, and heart rate was regular at 72 beats per min. The results of preoperative blood tests were unremarkable (). The patient adhered nil per os after midnight before surgery. At 2 pm on the day of surgery, the patient suffered from dizziness just before entering the operating room. A low blood glucose level of 52 mg/dL was detected. A 10-g dextrose intravenous infusion swiftly resolved the dizziness and a hypoglycemic attack was subsequently diagnosed. Total knee arthroplasty was subsequently performed under general anesthesia with desflurane as scheduled, with an uneventful intraoperative course. However, nausea occurred and persisted after surgery. Although the patient was not given intravenous glucose solution after surgery, she did not report further dizziness and four times daily glucose measurements did not detect hypoglycemia.
Table 1 Preoperative Blood Tests
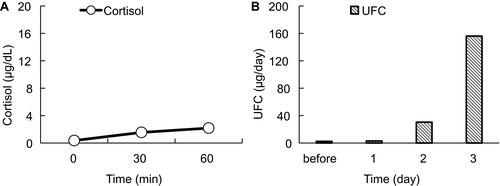
On postoperative day 5, the patient was referred to the Department of Internal Medicine for further investigation of the preoperative hypoglycemic attack and the nausea following surgery. On examination, her body temperature was 36.5°C, blood pressure was 134/70 mmHg, and heart rate was regular at 70 beats per min. No remarkable findings were found for the head, neck, chest, abdomen, and extremities, except for a surgical scar on the right knee and alabaster-like pale skin that was first recognized as a possible sign of adrenal insufficiency. Repeated laboratory tests after surgery showed that the serum sodium concentration remained in the range of 138–142 mEq/L. A 75-g oral glucose tolerance test yielded unremarkable results for glucose and immunoreactive insulin (). Insulin autoantibody was not detected using a radioimmunoassay (Cosmic Co., Tokyo, Japan). shows the results of hormones measured on the morning of fasting. The serum cortisol level was extremely low, with undetectable levels of adrenocorticotropic hormone (ACTH) and dehydroepiandrosterone sulfate. A low insulin-like growth factor-1 level of 9 ng/mL was equivalent to −4.9 SD for 65-year-old Japanese women, but growth hormone (GH) was depleted. Since ACTH deficiency was strongly suggested, levothyroxine supplementation was immediately withdrawn. Magnetic resonance imaging of the pituitary revealed an empty sella (), and computed tomography of the abdomen showed bilateral atrophic adrenal glands but without a mass indicating hemorrhage (). On pituitary stimulation tests, ACTH and cortisol showed no response to exogenous corticotropin-releasing hormone, and GH responded poorly to GH-releasing peptide-2 (). While a conventional-dose rapid ACTH stimulation test using 250 µg synacthen showed a reduced cortisol response, urinary free cortisol excretion substantially increased during a prolonged ACTH stimulation test using 0.5 mg tetracosactide twice daily (). Finally, the patient was diagnosed as having empty sella syndrome with deficits in ACTH and GH.
Table 2 Hormones Measured on the Morning of Fasting
Figure 1 75 g oral glucose tolerance test Horizontal axes indicate time courses. Vertical axes indicate levels of glucose (circles, solid line) and IRI levels (triangles, dotted line).
Abbreviation: IRI, immunoreactive insulin.
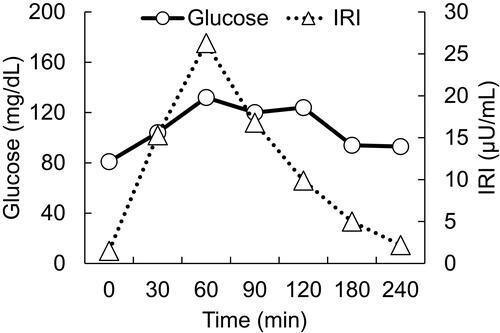
Figure 2 Magnetic resonance imaging of the pituitary. T1-weighted magnetic resonance images, without contrast, are shown. Left, Sagittal plane. Right, coronal plane. Yellow arrows indicate an empty sella.
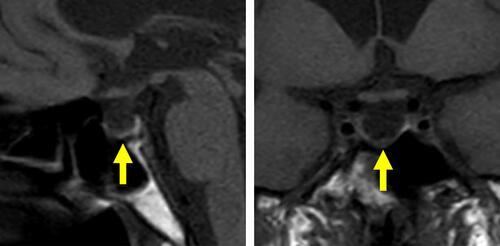
Figure 3 Abdominal computed tomography. Horizontal computed tomography images, without contrast, are shown. Yellow arrows indicate right and left atrophic adrenal glands.
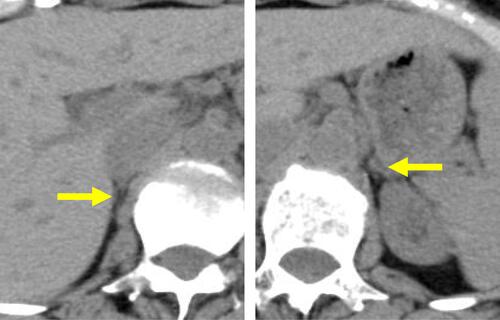
Figure 4 Pituitary stimulation tests. (A) Corticotropin-releasing hormone test. ACTH, circles and solid line. Cortisol, triangles and dotted line. (B) Thyrotropin-releasing hormone test. TSH, circles and solid line. PRL, triangles and dotted line. (C) Gonadotropin-releasing hormone test. LH, circles and solid line. FSH, triangles and dotted line. (D) Growth hormone-releasing peptide-2. GH, circles and solid line. Horizontal axes indicate time courses. Vertical axes indicate hormone levels.
Abbreviations: ACTH, adrenocorticotropic hormone; FSH, follicle-stimulating hormone; GH, growth hormone; LH, luteinizing hormone; PRL, prolactin; TSH, thyroid-stimulating hormone.
Figure 5 ACTH stimulation tests. (A) Conventional dose rapid ACTH stimulation test using 250 µg synacthen. Circles and a solid line represent the serum cortisol level. (B) Prolonged ACTH stimulation test using 0.5 mg tetracosactide twice daily. Vertical gray columns represent UFC. Horizontal axes indicate time courses. Vertical axes indicate values.
Abbreviations: ACTH, adrenocorticotropic hormone; UFC, urinary free cortisol.
After hydrocortisone was orally administered at a dose of 5 mg/day, the nausea resolved. Hydrocortisone was gradually escalated to 15 mg/day, with the dose split three times a day. However, polyuria did not develop, indicating normal posterior pituitary function. Finally, the patient was uneventfully transferred to a rehabilitation hospital. The patient’s GH deficiency was left untreated because she refused GH replacement therapy. Thyroid function tests on a follow-up outpatient visit showed free triiodothyronine at 2.07 pg/mL, free thyroxine at 1.33 ng/dL and thyroid-stimulating hormone at 5.60 µIU/mL, all being unremarkable; levothyroxine was thus not restarted.
Discussion
Hypoglycemia among hospitalized patients is associated with an adverse clinical outcome, and can be classified into three levels: level 1, glucose concentration 54–70 mg/dL; level 2, glucose concentration <54 mg/dL; and level 3, altered mental and/or physical status requiring assistance.Citation4 Hypoglycemia is common in hospitalized patients with diabetes mellitus and in those in a critical-care setting under a tight glycemic control protocol.Citation2,Citation5 However, non-diabetic patients rarely show hypoglycemia during inpatient care outside of intensive care units; in one study, estimated frequencies were 50, 36, 13, 11, and 8 per 10,000 admissions, using cut-off values of 59, 54, 49, 45, and 40 mg/dL, respectively.Citation3
While hypoglycemic agents are a representative cause of hypoglycemia, the present case showed level 2 hypoglycemia despite the absence of the use of hypoglycemic agents.Citation1 Considering the completely depleted serum cortisol level identified later, this hypoglycemic attack may have been a warning sign of adrenal insufficiency. Diverse etiologies result in hypoglycemia.Citation1 For example, inborn errors of metabolism can be an underlying etiology especially in cases of hypoglycemia occurring when a patient is in a fasting state.Citation6 However, such errors do not explain this present case because these are basically transmitted as autosomal recessive traits and develop in infants or children.Citation6 A cortisol deficit gives rise to various clinical symptoms and signs comprising fatigue, appetite loss, weight loss, nausea, vomiting, and abdominal pain, as well as laboratory abnormalities such as hyponatremia and normochromic anemia.Citation7 Because these are non-specific, patients with adrenal insufficiency are often misdiagnosed as having a psychotic, psychiatric, or gastrointestinal disease.Citation8 Cortisol is a catabolic hormone influencing carbohydrate, lipid, and protein metabolism. Depleted cortisol increases insulin sensitivity in patients with adrenal insufficiency and is thought to involve hypoglycemia.Citation9 The hypoglycemia related to adrenal insufficiency is thought to be more common in neonates and children than in adults.Citation10,Citation11 Nevertheless, a recent study using a continuous glucose monitoring system reported a number of cases of nocturnal hypoglycemia in adult patients with adrenal insufficiency despite glucocorticoid replacement.Citation12–Citation14 In Islamic culture, patients with adrenal insufficiency can suffer from hypoglycemia during Ramadan fasting, despite the continuance of glucocorticoid replacement therapy.Citation15 These reports indicate adrenal-insufficient adults are also prone to hypoglycemia, especially during fasting, and that the frequency may have been underestimated. For an early diagnosis, adrenal insufficiency must be considered if hypoglycemia due to an uncertain etiology is observed.
Strong increases in urinary free cortisol excretion during the prolonged ACTH stimulation test in this case suggests a central adrenal insufficiency. The observed alabaster-like pale skin also indicates a pituitary insult.Citation16 Because pro-opiomelanocortin–derived peptides released from the pituitary stimulate the melanocortin-1 receptor leading to skin pigmentation, patients with central adrenal insufficiency have pale skin, while almost all patients with primary adrenal insufficiency show hyperpigmentation.Citation16 A serum sodium concentration that was within normal range in this present case also indicates a pituitary insult rather than a primary adrenal insufficiency. Hyponatremia can occur in patients with secondary adrenal insufficiency due to a weakened glucocorticoid inhibition of vasopressin secretion.Citation17,Citation18 Because aldosterone secretion is preserved in patients with secondary adrenal insufficiency, however, the prevalence was reported to be less common compared with primary adrenal insufficiency.Citation19,Citation20
An adrenal crisis is a life-threatening event associated with a high mortality rate in patients with adrenal insufficiency.Citation21 Surgery is one of the representative causes of an adrenal crisis and accounts for 6–16% of cases.Citation21 To avoid the risk of this harmful condition, perioperative glucocorticoid supplementation for patients with adrenal insufficiency is vital.Citation22 Thus, a precise etiological diagnosis of hypoglycemia takes priority over non-urgent elective surgery, such as arthroplasty for osteoarthrosis. In addition, perioperative glucocorticoid coverage must be undertaken if adrenal insufficiency exists.
Conclusion
Determining the underlying etiology of hypoglycemia takes priority over non-urgent elective surgery when these co-occur because adrenal insufficiency, possibly a life-threatening condition, may be latent.
Abbreviations
ACTH, adrenocorticotropic hormone; GH, growth hormone.
Ethics and Consent for Publication
The patient described in this report provided permission to publish data and accompanying images, and written informed consent was obtained. A formal ethical review by an institutional review board was not required because this is a case report.
Data Sharing Statement
The data described in this article are openly available in the Open Science Framework at DOI:10.17605/OSF.IO/TPA6U.
Disclosure
Professor Masanori Abe reports grants from Eli Lilly Japan K.K, Kyowa Kirin Co., Ltd, Mitsubishi Tanabe Pharma Corporation, Otsuka Pharmaceutical Co., Ltd, Daiichi Sankyo Co., Ltd, Torii Pharmaceutical Co., Ltd, Chugai Pharmaceutical Co., Ltd, Nikkiso Co., Ltd, NIPRO Corporation, Ono Pharmaceutical Co., Ltd, Terumo Corporation, and Toray Medical Co., Ltd, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009;94(3):709–728. doi:10.1210/jc.2008-1410
- Turchin A, Matheny ME, Shubina M, Scanlon JV, Greenwood B, Pendergrass ML. Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care. 2009;32(7):1153–1157. doi:10.2337/dc08-2127
- Nirantharakumar K, Marshall T, Hodson J, et al. Hypoglycemia in non-diabetic in-patients: clinical or criminal? PLoS One. 2012;7(7):e40384. doi:10.1371/journal.pone.0040384
- American Diabetes Association. 15. Diabetes care in the hospital: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S193–S202. doi:10.2337/dc20-S015
- Anabtawi A, Hurst M, Titi M, Patel S, Palacio C, Rajamani K. Incidence of hypoglycemia with tight glycemic control protocols: a comparative study. Diabetes Technol Ther. 2010;12(8):635–639. doi:10.1089/dia.2010.0009
- Weinstein DA, Steuerwald U, De Souza CFM, Derks TGJ. Inborn errors of metabolism with hypoglycemia: glycogen storage diseases and inherited disorders of gluconeogenesis. Pediatr Clin North Am. 2018;65(2):247–265. doi:10.1016/j.pcl.2017.11.005
- Bancos I, Hahner S, Tomlinson J, Arlt W. Diagnosis and management of adrenal insufficiency. Lancet Diabetes Endocrinol. 2015;3(3):216–226. doi:10.1016/S2213-8587(14)70142-1
- Bleicken B, Hahner S, Ventz M, Quinkler M. Delayed diagnosis of adrenal insufficiency is common: a cross-sectional study in 216 patients. Am J Med Sci. 2010;339(6):525–531. doi:10.1097/MAJ.0b013e3181db6b7a
- Christiansen JJ, Djurhuus CB, Gravholt CH, et al. Effects of cortisol on carbohydrate, lipid, and protein metabolism: studies of acute cortisol withdrawal in adrenocortical failure. J Clin Endocrinol Metab. 2007;92(9):3553–3559. doi:10.1210/jc.2007-0445
- Patti G, Guzzeti C, Di Iorgi N, et al. Central adrenal insufficiency in children and adolescents. Best Pract Res Clin Endocrinol Metab. 2018;32(4):425–444. doi:10.1016/j.beem.2018.03.012
- Cambiaso P, Schiaffini R, Pontrelli G, et al. Nocturnal hypoglycaemia in ACTH and GH deficient children: role of continuous glucose monitoring. Clin Endocrinol. 2013;79(2):232–237. doi:10.1111/cen.2013.79.issue-2
- Watanabe T, Ozawa A, Ishii S, et al. Usage of continuous glucose monitoring (CGM) for detecting an unrecognized hypoglycemia and management of glucocorticoid replacement therapy in adult patients with central hypoadrenalism. Endocr J. 2018;65(5):547–556. doi:10.1507/endocrj.EJ16-0387
- Meyer G, Hackemann A, Reusch J, Badenhoop K. Nocturnal hypoglycemia identified by a continuous glucose monitoring system in patients with primary adrenal insufficiency (Addison’s Disease). Diabetes Technol Ther. 2012;14(5):386–388. doi:10.1089/dia.2011.0158
- Petersen KS, Rushworth RL, Clifton PM, Torpy DJ. Recurrent nocturnal hypoglycaemia as a cause of morning fatigue in treated Addison’s disease–favourable response to dietary management: a case report. BMC Endocr Disord. 2015;15:61. doi:10.1186/s12902-015-0058-6
- Chihaoui M, Chaker F, Yazidi M, et al. Ramadan fasting in patients with adrenal insufficiency. Endocrine. 2017;55(1):289–295. doi:10.1007/s12020-016-1186-0
- Charmandari E, Nicolaides NC, Chrousos GP. Adrenal insufficiency. Lancet. 2014;383(9935):2152–2167. doi:10.1016/S0140-6736(13)61684-0
- Erkut ZA, Pool C, Swaab DF. Glucocorticoids suppress corticotropin-releasing hormone and vasopressin expression in human hypothalamic neurons. J Clin Endocrinol Metab. 1998;83(6):2066–2073. doi:10.1210/jcem.83.6.4881
- Oelkers W. Hyponatremia and inappropriate secretion of vasopressin (antidiuretic hormone) in patients with hypopituitarism. N Engl J Med. 1989;321(8):492–496. doi:10.1056/NEJM198908243210802
- Sert M, Tetiker T, Kirim S, Kocak M. Clinical report of 28 patients with Sheehan’s syndrome. Endocr J. 2003;50(3):297–301. doi:10.1507/endocrj.50.297
- Tucci V, Sokari T. The clinical manifestations, diagnosis, and treatment of adrenal emergencies. Emerg Med Clin North Am. 2014;32(2):465–484. doi:10.1016/j.emc.2014.01.006
- Puar TH, Stikkelbroeck NM, Smans LC, Zelissen PM, Hermus AR. Adrenal crisis: still a deadly event in the 21st century. Am J Med. 2016;129(3):339.e331–e339. doi:10.1016/j.amjmed.2015.08.021
- Coursin DB, Wood KE. Corticosteroid supplementation for adrenal insufficiency. JAMA. 2002;287(2):236–240. doi:10.1001/jama.287.2.236
