Abstract
Fabry disease (FD) is an X-linked lysosomal storage disorder caused by absence or deficient activity of α-galactosidase A (α-Gal A) due to mutations in the α-galactosidase A gene (GLA), leading to progressive accumulation of globotriaosylceramide (Gb3) in tissues and organs including heart, kidney, the eyes, vascular endothelium, the nervous system and the skin. Cardiac involvement is leading to fatal complications and reduced life expectancy. FD is treatable with disease-specific treatment (enzyme replacement therapy (ERT) or with chaperone therapy). Therefore, the early diagnosis of FD is crucial for reducing the morbidity and mortality. Screening of high-risk populations (eg, patients with unexplained left ventricular hypertrophy (LVH), young patients with unexplained stroke, and patients with unexplained renal failure proteinuria or microalbuminuria) yields good results. The diagnostic algorithm is gender-specific. Initially, the measurement of α-Gal A activity is recommended in males, and optionally in females. In males with non-diagnostic residual activity (5–10%) activity, genetic testing is afterwards done for confirming the diagnosis. In fact, diagnosis of FD is not possible without genetic testing for both males and females. Globotriaosysphingosine (lyso-Gb3) for identification of atypical FD variants and high- sensitive troponin T (hsTNT) for identification of cardiac involvement are also important diagnostic biomarkers. The aim of this review was to provide an update on diagnosis and screening of patients with FD.
Introduction
Fabry disease (FD) is an X-linked rare multisystemic lysosomal storage disorder resulting from α-galactosidase A (α-Gal A) deficiency and is disease-specific, but not curatively treatable with enzyme replacement therapy (ERT) since 2001 and chaperone therapy with Migalastat (Galafold®, Amicus Therapeutics, Cranbury, NJ, USA) since 2016.Citation1–Citation4 The recombinant enzyme is available as agalsidase-alpha (Replagal®, Shire Human Genetic Therapies AB, Stockholm, Sweden) and agalsidase-beta (Fabrazyme®, Sanofi Genzyme, Cambridge, MA, USA).Citation5 However, early diagnosis of FD is crucial to reduce morbidity and mortality. Cardiovascular complications are the major cause of death in patients with FD.Citation6,Citation7
Epidemiology and Pathogenesis of Fabry Disease
The prevalence of FD has been estimated between 1:40,000 and 1:117,000 individuals.Citation8 However, this prevalence is underestimated. In newborn screenings, higher prevalence values were described, for example in Spain, where the prevalence of FD in males was estimated at 1:7575 (0.013%).Citation9 In genetic screening studies of high-risk populations, the overall prevalence of individuals with α-galactosidase A (GLA) gene variants, including genetic variants of unknown significance (GVUS), was 0.62%; prevalence of definitive FD diagnosis was 0.12%.Citation10 In patients (male and female) receiving hemodialysis treatment in Brazil, the estimated prevalence for FD was 0.87%, close to that reported in other trials.Citation11–Citation14 In young patients with stroke a prevalence of 0–4% was found.Citation15–Citation19 In patients with unexplained hypertrophic cardiomyopathy (HCM) FD should be considered. In two cohorts of men with unexplained LVH (≥ 13mm) the prevalence of decreased α-gal A activity was 6.3% and 3.0%, respectively.Citation20,Citation21 In a cohort of females with unexplained HCM, up to 12% of these patients were affected by FD.Citation22 In large cohorts of patients with unexplained LVH, α-gal A mutations were described in 0.5% and 1.0%, respectively.Citation23,Citation24 Doheny et al reanalyzed all published screening studies for FD from 1995 through 2017 and provided more valid prevalence estimates of previously unrecognized FD in high-risk cohorts, ranging from 0% in female renal transplant patients to about 0.9% among both male and female cardiac cohorts.Citation25 Previous prevalence values from 1.6-fold in male renal transplant patients to 33-fold and 15-fold in male and female patients with stroke, respectively, may be due to benign variants.Citation25 Capuano et al reanalyzed the prevalence of GLA mutations in dialysis patients in FD screening studies published between 1995 and 2019 after assigning their correct phenotype. They found an overall prevalence recalculated on the basis of only pathogenetic mutations of 0.14%.Citation26
FD is caused by a deficiency or absence of α-Gal A activity due to mutations in the GLA gene (located on Xq22.1), resulting in intracellular accumulation of globotriaosylceramide (Gb3) and multiorgan damage.Citation27,Citation28 Heterozygous female may have clinical manifestations of FD, too, but usually on average 10 years later than male patients. Phenotypic expression and penetrance varies among families with the same variant and, also, within the same family. There are various phenotypes, with early severe classic presentations, and the later, milder presentation.Citation28 Two phenotypes of FD are relevant: classic FD and variant FD. Males with the classic FD develop early signs and symptoms in childhood or adolescence (e.g., periodic crisis of severe pain in the extremities (acroparesthesias), neuropathic pain, telangiectasias and vascular lesions called angiokeratomas (in the groin, hip and periumbilical areas), sweating abnormalities (anhidrosis and hypohidrosis), gastrointestinal symptoms, corneal (cornea verticillate) and lenticular opacities).Citation29–Citation31 Vascular complications, renal failure with unknown etiology, including unexplained proteinuria or microalbuminuria, unexplained LVH mimicking sarcomeric HCM, or neurological manifestations (e.g., cryptogenic stroke, transient ischemic attack [TIA], sensoneuronal hearing loss, migraine) may be clinical features in adults.Citation10,Citation30,Citation32–Citation34 Patients with atypical variants may develop late-onset renal failure, LVH, cerebrovascular disease, or a combination thereof.Citation30,Citation35 Symptoms in female subjects vary. Presentation ranges from asymptomatic to severely affected, but subjects are typically older compared to male subjects.Citation30,Citation34–Citation36 Gb3-deposition and its deacylated metabolite globotriaosysphingosine (lyso-Gb3) are associated with the cellular involvement.Citation37 This may lead to alterations of tissue (interstitial fibrosis and replacement fibrosis, inflammation, apoptosis, hypertrophy).Citation38 The cellular damage is multifactorial and partly caused by cellular signaling or microvascular ischemia via affected endothelial cells or both, leading to organ dysfunction, renal and heart failure, for example.Citation39 Plasma lyso-Gb3 concentrations appear to correlate with disease stages, the mutation severity and decreasing during ERT.Citation37 There is still debate about which patients or variants really do show correlation of LysoGb3 levels as treatment response (or both ERT and chaperone).Citation5,Citation40
Diagnosis of Fabry Disease
Diagnosis of classic FD in males may be straightforward, whereas in females and in individuals with genetic variants the diagnosis can be challenging.Citation41
A diagnostic approach involving a detailed history, family history, physical examination, clinical and biochemical findings, genetic testing, various imaging procedures, and expert opinion is recommended.Citation10,Citation42,Citation43 Some clinical findings and signs, e.g., acroparesthesia, angiokeratomas and cornea verticillate may have a high specificity.Citation10
In males suspected to have FD, α-Gal A activity should be measured. Alpha Gal A activity < 1% is highly suggestive for the diagnosis of classic FD.Citation44 Even in the presence of clinical signs, the α-Gal A activity is variable and can be within the normal range in females.Citation45,Citation46 Most of these females develop clinically significant disease.Citation27,Citation47
Genetic confirmatory testing is mandatory in males and females.Citation10 Mutations in the GLA gene may be associated with the classic FD, a variant phenotype or a GVUS.Citation10 We present an updated diagnostic algorithm in .Citation10,Citation27,Citation31,Citation43,Citation48,Citation49 At the diagnosis of FD, determination of amenability for pharmacological chaperone therapy with migalastat is possible with good laboratory practice (GLP)-validated human embryogenic kidney cell-based in vitro assay.Citation50,Citation51 This in vitro assay is currently the only approved method for this evaluation.Citation52
Figure 1 Updated diagnostic algorithm for FD. Adapted with permission from van der Tol L, Smid BE, Poorthuis BJ, et al. A systematic review on screening for Fabry disease: prevalence of individuals with genetic variants of unknown significance. J Med Genet. 2014;51:1–9. Copyright © 2014, BMJ Publishing Group Ltd.Citation10 Adapted with permission from Yogasundaram H, Kim D, Oudit O, Thompson RB, Weidemann F, Oudit GY. Clinical Features, Diagnosis, and Management of Patients With Anderson-Fabry Cardiomyopathy. Can J Cardiol. 2017;33:883–897. © 2017 Canadian Cardiovascular Society.Citation27 Adapted with permission from Putko BN, Wen K, Thompson RB, et al. Anderson-Fabry cardiomyopathy: prevalence, pathophysiology, diagnosis and treatment. Heart Fail Rev. 2015;20:179–191. Copyright © 2014, Springer Nature.Citation49 Adapted with permission from Laney DA, Bennett RL, Clarke V, et al. Fabry disease practice guidelines: recommendations of the National Society of Genetic Counselors. J Genet Couns. 2013;22:555–564. © 2013 National Society of Genetic Counselors, Inc.Citation43 Data from Niemann et al Citation48 and Putko et al.Citation49 α-Galactosidase A (α-Gal A), α-galactosidase A gene (GLA gene), globotriaosysphingosine (Lyso-Gb3), left ventricular (LV). *Punch biopsy, taken from proximal (thigh: 15 cm above the patella) and distal (leg: 10 cm above the lateral malleolus) hairy skin sites or from other lesions to evaluate Gb3 deposits, and for other histopathological evaluations.Citation31
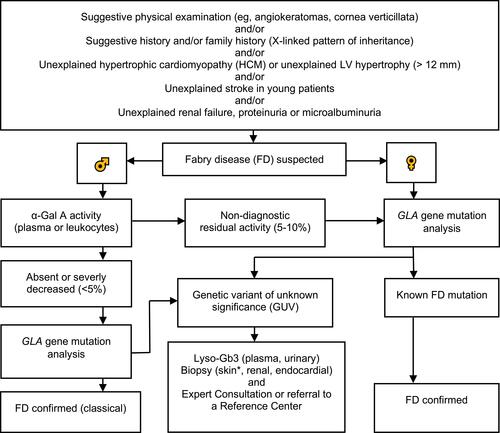
Lyso-Gb3 has also clinical relevance and emerged a powerful biomarker for FD, particularly the variable α-Gal A activity and mutations.Citation37,Citation48 In patients with GVUS, to determine if the mutation is possibly clinically significant, and if the diagnosis of FD should be made, the lyso-Gb3 test can be used. Lyso-Gb3 can be used for stratifying patients as classic and variant phenotypes, even before clinical manifestation is present.Citation48 Lyso-Gb3 is especially helpful as a phenotype discriminative marker because of phenotypic variability, most prominently in females.Citation42
Diagnosis of Cardiac Involvement
The first step to assess the Fabry cardiomyopathy is transthoracic echocardiography.Citation53,Citation54 Typical signs for FD are concentric LVH and a prominent papillary muscle. In addition, most patients show signs of diastolic dysfunction. Global left ventricular function assessed by ejection fraction (EF) during echocardiography is only reduced in end-stage patients.Citation53,Citation54
Patients with FD suspected to have cardiac involvement should undergo cardiac magnetic resonance imaging (CMR) imaging, echocardiography, a 24-h Holter ECG to assess cardiac structure and function, as early as possible.Citation55
The diagnostic gold standard for cardiac fibrosis in cardiomyopathy patients with FD is CMR imaging examination with late gadolinium enhancement (LGE-CMR).Citation55 In cases of patients with contraindications, e.g., in patients with an implanted cardioverter or pacemaker, or with end-stage renal disease, echocardiographic modalities (e.g., speckle-tracking echocardiography [STE]), are available for indirect evaluation of cardiac involvement.Citation56,Citation57 Starting ERT is recommended as soon as clinical signs of organ involvement are seen, e.g., when detection of LGE first becomes apparent.Citation42,Citation55 T1-MRI mapping has been proposed to discriminate FD from other causes of LVH.Citation58 In FD patients with LVH, abnormal native myocardial T1 values are highly prevalent.Citation59,Citation60 Cardiac MRI-derived myocardial mapping may have a high sensitivity and specificity to discriminate FD patients.Citation58,Citation59 Nappi et al showed the feasibility of PET-MR imaging for the early detection of cardiac involvement, even in patients with non-hypertrophic stage.Citation61 PET-MR examinations can provide unique and simultaneous results to detect myocardial inflammation.Citation58
In cardiology, high-sensitive Troponin T (hsTNT) is established to detect myocardial tissue damage. However, for Fabry cardiomyopathy only few data are available regarding biomarkers to detect cardiac involvement.
Seydelmann et al showed, that hsTNT is the most promising biomarker for cardiac involvement in FD, with Values >14ng/mL, suggesting pathological late enhancement in MRI.Citation62 This biomarker was correlated with the progression of FD, and patients with elevation of hsTNT during observation showed the progress of myocardial fibrosis, and a decrease of EF.Citation62 Elevation of hsTNT can predict an increment in the magnitude of fibrosis.Citation63
In patients with FD and cardiac involvement, assessed by echocardiographic findings, NT-proBNP levels are elevated and correlate with electrocardiographic findings, but not with myocardial fibrosis.Citation62,Citation64 However, NT-proBNP levels are not correlated with disease progression.Citation64,Citation65 The evaluation of myocardial fibrosis defines the stage of the cardiomyopathy and is associated with a poor prognosis.Citation53,Citation66
The noninvasive biochemical markers, imaging strategies and genetic tests for the diagnosis of FD are sufficient, therefore the place value for endocardial biopsy has diminished.Citation27
Diagnosis of Renal Involvement
General diagnostic tools for the baseline assessment of renal function, e.g., serum creatinine, calculated GFR and urine protein diagnostics (24 hour collection would be ideal, but spot urine total protein/creatinine and albumin/creatinine ratios has been established) can be used initially in FD patients.Citation67 Additionally, the creatinine and cystatin C based GFR-calculation is recommended for the assessment of renal function.Citation63 Serum cystatin C concentration is a more sensitive marker than creatinine to detect early renal dysfunction.Citation65 Urine lyso-GB3 showed no correlation to renal involvement in FD.Citation68,Citation69 Proteinuria and albuminuria is the most important indicator for early renal involvement in FD; regular measurement is essential.Citation70 Measurement of albumin-creatinine ratio in spot urine is highly recommended for early detection of microalbuminuria. Glomerular hyperfiltration (age-corrected eGFR > 130 mL/min/1.73/m2 corrected for age) may be an early marker for renal involvement.Citation69 Furthermore, a kidney ultrasound should be carried out.Citation71
In FD patients with persistent albuminuria and/or proteinuria a renal biopsy should be performed.Citation72
Diagnosis of Neurologic Involvement
Unfortunately there are no specific blood biomarkers available for the assessment of neurological signs in FD patients. In cases of suggestive physical examination and/or history (and/or) family history (e.g., paresthesias/dysesthesias, hypohidrosis/anhidrosis, chronic burning pain, attacks of excruciating pain, sensory losses, tinnitus, hearing loss, nausea, dizziness, abdominal cramp, [post-prandial] diarrhea, bloating) further neurological diagnostics should be initiated.Citation73–Citation75 Neurological involvement is usually assessed by brain MRI, audiometry, etc. White matter hyperintensities, dolichoectasia, and infarcts are characteristic signs found on brain MR imaging in classic FD.Citation76,Citation77
Screening Strategies for Fabry Disease
In epidemiology, screening is defined
as the examination of asymptomatic people in order to classify them as likely or unlikely to have the disease that is the object of screening. People who appear likely to have the disease are investigated further to arrive at a final diagnosis. Those people who are then found to have the disease are treated.Citation78
To be suitable for general use, a procedure for early detection and treatment should meet many criteria in addition to reducing morbidity or mortality.Citation78 For cost-effectiveness, a high positive predictive value (PVP) is required in the population screened.Citation78 Therefore, screening for FD is currently only recommended in high-risk populations and in families of index patients.Citation11,Citation79 Due to various reasons, systematic newborn screening for FD was not introduced.Citation9 Several screening strategies are possible. For males, initially, biomarker-based screening methods (α-Gal A or lyso-Gb3) are used.Citation49,Citation80 Enzyme-based screening methods are not appropriate for females.Citation19 Vascular lesions, especially the retinal vessel diameter can help during screening for FD.Citation81
High-Risk Screening
Screening of the following high-risk populations could increase the diagnostic rate of FD:Citation82 1) Patients with unexplained HCM or with unexplained LVH (>12 mm);Citation20,Citation23 2) Patients with end-stage renal disease (receiving hemodialysis treatment), patients after kidney transplantation or patients with unexplained proteinuria or microalbuminuria;Citation80 3) Individuals (aged 15–55 years) with unexplained stroke.Citation15,Citation16,Citation18,Citation19
Many screening studies in high-risk populations revealed an unexpectedly large number of mutations or GVUS in the galactosidase alpha gene (GLA) and/or impaired α-gal A activity.Citation10 In contrast to the absent or near absent α-gal A activity in classically affected males, most male patients with GVUS have residual activity of α-gal A.10
The diagnostic algorithm () may also be adapted for screening for FD.
Family Screening
Pedigree analysis and genetic counseling is crucial for patients with FD, relatives may need to be genetically tested.Citation43
Conclusion
FD is a rare, progressive multi-system disease with a reduction in life expectancy. Therefore, early diagnosis of FD is essential, using diagnostic and screening procedures adapted to consensus guidelines, which we present in this review.
Disclosure
Dr. Vardarli and Dr. Rischpler report no conflicts of interest in this work. Dr. Herrmann reports personal fees from Bayer, stock options (less than 1%) from Sofie Biosciences, personal fees from SIRTEX, non-financial support from ABX, personal fees from Adacap, personal fees from Curium, personal fees from Endocyte, grants and personal fees from BTG, personal fees from IPSEN, personal fees from Siemens Healthineers, personal fees from GE Healthcare, personal fees from Amgen, personal fees from Novartis, personal fees from Y-mAbs, outside the submitted work. Dr. Weidemann has received research grants from Genzyme and Shire and speaker honoraria from Amicus, Genzyme, and Shire.
References
- Brady RO, Gal AE, Bradley RM, Martensson E, Warshaw AL, Laster L. Enzymatic defect in Fabry’s disease. Ceramidetrihexosidase deficiency. N Engl J Med. 1967;276(21):1163–1167. doi:10.1056/NEJM196705252762101
- Desnick RJ, Brady R, Barranger J, et al. Fabry disease, an under-recognized multisystemic disorder: expert recommendations for diagnosis, management, and enzyme replacement therapy. Ann Intern Med. 2003;138:338–346. doi:10.7326/0003-4819-138-4-200302180-00014
- Eng CM, Guffon N, Wilcox WR, et al. Safety and efficacy of recombinant human alpha-galactosidase A replacement therapy in Fabry’s disease. N Engl J Med. 2001;345:9–16. doi:10.1056/NEJM200107053450102
- Kramer J, Lenders M, Canaan-Kuhl S, et al. Fabry disease under enzyme replacement therapy-new insights in efficacy of different dosages. Nephrol Dial Transplant. 2018;33:1362–1372. doi:10.1093/ndt/gfx319
- Muntze J, Gensler D, Maniuc O, et al. Oral chaperone therapy migalastat for treating fabry disease: enzymatic response and serum biomarker changes after 1 year. Clin Pharmacol Ther. 2019;105:1224–1233. doi:10.1002/cpt.1321
- Mehta A, Clarke JT, Giugliani R, et al. Natural course of Fabry disease: changing pattern of causes of death in FOS - fabry outcome survey. J Med Genet. 2009;46:548–552. doi:10.1136/jmg.2008.065904
- Waldek S, Patel MR, Banikazemi M, Lemay R, Lee P. Life expectancy and cause of death in males and females with Fabry disease: findings from the Fabry registry. Genet Med. 2009;11:790–796. doi:10.1097/GIM.0b013e3181bb05bb
- Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281:249–254. doi:10.1001/jama.281.3.249
- Colon C, Ortolano S, Melcon-Crespo C, et al. Newborn screening for Fabry disease in the north-west of Spain. Eur J Pediatr. 2017;176:1075–1081. doi:10.1007/s00431-017-2950-8
- van der Tol L, Smid BE, Poorthuis BJ, et al. A systematic review on screening for Fabry disease: prevalence of individuals with genetic variants of unknown significance. J Med Genet. 2014;51(1):1–9. doi:10.1136/jmedgenet-2013-101857
- Sodre LSS, Huaira R, Bastos MG, Colugnati FAB, Coutinho MP, Fernandes N. Screening for fabry disease in kidney disease: a cross-sectional study in males and females. Kidney Blood Press Res. 2017;42(6):1258–1265. doi:10.1159/000485929
- Saito O, Kusano E, Akimoto T, et al. Prevalence of Fabry disease in dialysis patients: japan Fabry disease screening study (J-FAST). Clin Exp Nephrol. 2016;20(2):284–293. doi:10.1007/s10157-015-1146-7
- Herrera J, Miranda CS. Prevalence of Fabry’s disease within hemodialysis patients in Spain. Clin Nephrol. 2014;81:112–120. doi:10.5414/CN108053
- Kleinert J, Kotanko P, Spada M, et al. Anderson-Fabry disease: a case-finding study among male kidney transplant recipients in Austria. Transpl Int. 2009;22:287–292. doi:10.1111/j.1432-2277.2008.00791.x
- Rolfs A, Bottcher T, Zschiesche M, et al. Prevalence of Fabry disease in patients with cryptogenic stroke: a prospective study. Lancet. 2005;366(9499):1794–1796. doi:10.1016/S0140-6736(05)67635-0
- Brouns R, Sheorajpanday R, Braxel E, et al. Middelheim Fabry Study (MiFaS): a retrospective Belgian study on the prevalence of Fabry disease in young patients with cryptogenic stroke. Clin Neurol Neurosurg. 2007;109:479–484. doi:10.1016/j.clineuro.2007.03.008
- Lanthier S, Saposnik G, Lebovic G, et al. Prevalence of fabry disease and outcomes in young canadian patients with cryptogenic ischemic cerebrovascular events. Stroke. 2017;48:1766–1772. doi:10.1161/STROKEAHA.116.016083
- Kinoshita N, Hosomi N, Matsushima H, et al. Screening for fabry disease in japanese patients with young-onset stroke by measuring alpha-galactosidase a and globotriaosylsphingosine. J Stroke Cerebrovasc Dis. 2018;27:3563–3569. doi:10.1016/j.jstrokecerebrovasdis.2018.08.025
- Lee TH, Yang JT, Lee JD, et al. Genomic screening of Fabry disease in young stroke patients: the Taiwan experience and a review of the literature. Eur J Neurol. 2019;26:553–555. doi:10.1111/ene.13775
- Sachdev B, Takenaka T, Teraguchi H, et al. Prevalence of anderson-fabry disease in male patients with late onset hypertrophic cardiomyopathy. Circulation. 2002;105:1407–1411. doi:10.1161/01.CIR.0000012626.81324.38
- Nakao S, Takenaka T, Maeda M, et al. An atypical variant of Fabry’s disease in men with left ventricular hypertrophy. N Engl J Med. 1995;333:288–293. doi:10.1056/NEJM199508033330504
- Chimenti C, Pieroni M, Morgante E, et al. Prevalence of Fabry disease in female patients with late-onset hypertrophic cardiomyopathy. Circulation. 2004;110:1047–1053. doi:10.1161/01.CIR.0000139847.74101.03
- Elliott P, Baker R, Pasquale F, et al. Prevalence of anderson-fabry disease in patients with hypertrophic cardiomyopathy: the European anderson-fabry disease survey. Heart. 2011;97:1957–1960. doi:10.1136/heartjnl-2011-300364
- Monserrat L, Gimeno-Blanes JR, Marin F, et al. Prevalence of fabry disease in a cohort of 508 unrelated patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2007;50:2399–2403. doi:10.1016/j.jacc.2007.06.062
- Doheny D, Srinivasan R, Pagant S, Chen B, Yasuda M, Desnick RJ. Fabry disease: prevalence of affected males and heterozygotes with pathogenic GLA mutations identified by screening renal, cardiac and stroke clinics, 1995–2017. J Med Genet. 2018;55:261–268. doi:10.1136/jmedgenet-2017-105080
- Capuano I, Garofalo C, Buonanno P, et al. Identifying Fabry patients in dialysis population: prevalence of GLA mutations by renal clinic screening, 1995–2019. J Nephrol. 2019. doi:10.1007/s40620-019-00663-6.
- Yogasundaram H, Kim D, Oudit O, Thompson RB, Weidemann F, Oudit GY. Clinical features, diagnosis, and management of patients with anderson-fabry cardiomyopathy. Can J Cardiol. 2017;33:883–897. doi:10.1016/j.cjca.2017.04.015
- Favalli V, Disabella E, Molinaro M, et al. Genetic screening of anderson-fabry disease in probands referred from multispecialty clinics. J Am Coll Cardiol. 2016;68:1037–1050. doi:10.1016/j.jacc.2016.05.090
- Laney DA, Peck DS, Atherton AM, et al. Fabry disease in infancy and early childhood: a systematic literature review. Genet Med. 2015;17:323–330. doi:10.1038/gim.2014.120
- Mehta A, Hughes DA. Fabry Disease. In: Adam MP, Ardinger HH, Pagon RA, et al. editors. Genereviews(R) [Internet]. Seattle (WA). August 5, 2002;1993–2019. Available from: http:www.ncbi.nlm.nih.gov/books/NBK1292/. Accessed December 26, 2019.
- Liguori R, Incensi A, de Pasqua S, et al. Skin globotriaosylceramide 3 deposits are specific to Fabry disease with classical mutations and associated with small fibre neuropathy. PLoS One. 2017;12:e0180581. doi:10.1371/journal.pone.0180581
- Wozniak MA, Kittner SJ, Tuhrim S, et al. Frequency of unrecognized Fabry disease among young European-American and African-American men with first ischemic stroke. Stroke. 2010;41:78–81. doi:10.1161/STROKEAHA.109.558320
- van der Tol L, Svarstad E, Ortiz A, et al. Chronic kidney disease and an uncertain diagnosis of Fabry disease: approach to a correct diagnosis. Mol Genet Metab. 2015;114:242–247. doi:10.1016/j.ymgme.2014.08.007
- Nagueh SF. Anderson-Fabry disease and other lysosomal storage disorders. Circulation. 2014;130:1081–1090. doi:10.1161/CIRCULATIONAHA.114.009789
- El-Abassi R, Singhal D, England JD. Fabry’s disease. J Neurol Sci. 2014;344:5–19. doi:10.1016/j.jns.2014.06.029
- Gambarin FI, Disabella E, Narula J, et al. When should cardiologists suspect Anderson-Fabry disease? Am J Cardiol. 2010;106:1492–1499. doi:10.1016/j.amjcard.2010.07.016
- Aerts JM, Groener JE, Kuiper S, et al. Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc Natl Acad Sci U S A. 2008;105:2812–2817. doi:10.1073/pnas.0712309105
- Moon JC, Sachdev B, Elkington AG, et al. Gadolinium enhanced cardiovascular magnetic resonance in Anderson-Fabry disease. Evidence for a disease specific abnormality of the myocardial interstitium. Eur Heart J. 2003;24:2151–2155. doi:10.1016/j.ehj.2003.09.017
- Pastores GM, Hughes DA. To see a world in a grain of sand: elucidating the pathophysiology of Anderson-Fabry disease through investigations of a cellular model. Kidney Int. 2009;75:351–353. doi:10.1038/ki.2008.606
- Germain DP, Elliott PM, Falissard B, et al. The effect of enzyme replacement therapy on clinical outcomes in male patients with Fabry disease: a systematic literature review by a European panel of experts. Mol Genet Metab Rep. 2019;19:100454. doi:10.1016/j.ymgmr.2019.100454
- Schiffmann R, Fuller M, Clarke LA, Aerts JM. Is it Fabry disease? Genet Med. 2016;18:1181–1185. doi:10.1038/gim.2016.55
- Smid BE, van der Tol L, Cecchi F, et al. Uncertain diagnosis of Fabry disease: consensus recommendation on diagnosis in adults with left ventricular hypertrophy and genetic variants of unknown significance. Int J Cardiol. 2014;177:400–408. doi:10.1016/j.ijcard.2014.09.001
- Laney DA, Bennett RL, Clarke V, et al. Fabry disease practice guidelines: recommendations of the national society of genetic counselors. J Genet Couns. 2013;22:555–564. doi:10.1007/s10897-013-9613-3
- Clarke JT. Narrative review: fabry disease. Ann Intern Med. 2007;146:425–433. doi:10.7326/0003-4819-146-6-200703200-00007
- Havndrup O, Christiansen M, Stoevring B, et al. Fabry disease mimicking hypertrophic cardiomyopathy: genetic screening needed for establishing the diagnosis in women. Eur J Heart Fail. 2010;12:535–540. doi:10.1093/eurjhf/hfq073
- Linthorst GE, Bouwman MG, Wijburg FA, Aerts JM, Poorthuis BJ, Hollak CE. Screening for Fabry disease in high-risk populations: a systematic review. J Med Genet. 2010;47:217–222. doi:10.1136/jmg.2009.072116
- Wang RY, Lelis A, Mirocha J, Wilcox WR. Heterozygous Fabry women are not just carriers, but have a significant burden of disease and impaired quality of life. Genet Med. 2007;9:34–45. doi:10.1097/GIM.0b013e31802d8321
- Niemann M, Rolfs A, Stork S, et al. Gene mutations versus clinically relevant phenotypes: lyso-Gb3 defines Fabry disease. Circ Cardiovasc Genet. 2014;7:8–16. doi:10.1161/CIRCGENETICS.113.000249
- Putko BN, Wen K, Thompson RB, et al. Anderson-Fabry cardiomyopathy: prevalence, pathophysiology, diagnosis and treatment. Heart Fail Rev. 2015;20:179–191. doi:10.1007/s10741-014-9452-9
- Benjamin ER, Della Valle MC, Wu X, et al. The validation of pharmacogenetics for the identification of Fabry patients to be treated with migalastat. Genet Med. 2017;19:430–438. doi:10.1038/gim.2016.122
- Schiffmann R, Bichet DG, Benjamin E, Wu X, Giugliani R. The migalastat GLP-HEK assay is the gold standard for determining amenability in patients with Fabry disease. Mol Genet Metab Rep. 2019;20:100494. doi:10.1016/j.ymgmr.2019.100494
- Lukas J, Cimmaruta C, Liguori L, et al. Assessment of gene variant amenability for pharmacological chaperone therapy with 1-deoxygalactonojirimycin in fabry disease. Int J Mol Sci. 2020;21(3):956.
- Weidemann F, Breunig F, Beer M, et al. The variation of morphological and functional cardiac manifestation in Fabry disease: potential implications for the time course of the disease. Eur Heart J. 2005;26:1221–1227. doi:10.1093/eurheartj/ehi143
- Linhart A, Kampmann C, Zamorano JL, et al. Cardiac manifestations of Anderson-Fabry disease: results from the international Fabry outcome survey. Eur Heart J. 2007;28:1228–1235. doi:10.1093/eurheartj/ehm153
- Weidemann F, Beer M, Kralewski M, Siwy J, Kampmann C. Early detection of organ involvement in Fabry disease by biomarker assessment in conjunction with LGE cardiac MRI: results from the SOPHIA study. Mol Genet Metab. 2019;126:169–182. doi:10.1016/j.ymgme.2018.11.005
- Pieroni M, Chimenti C, Ricci R, Sale P, Russo MA, Frustaci A. Early detection of Fabry cardiomyopathy by tissue Doppler imaging. Circulation. 2003;107:1978–1984. doi:10.1161/01.CIR.0000061952.27445.A0
- Kramer J, Niemann M, Liu D, et al. Two-dimensional speckle tracking as a non-invasive tool for identification of myocardial fibrosis in Fabry disease. Eur Heart J. 2013;34:1587–1596. doi:10.1093/eurheartj/eht098
- Imbriaco M, Nappi C, Ponsiglione A, et al. Hybrid positron emission tomography-magnetic resonance imaging for assessing different stages of cardiac impairment in patients with Anderson-Fabry disease: AFFINITY study group. Eur Heart J Cardiovasc Imaging. 2019;20:1004–1011. doi:10.1093/ehjci/jez039
- Pica S, Sado DM, Maestrini V, et al. Reproducibility of native myocardial T1 mapping in the assessment of Fabry disease and its role in early detection of cardiac involvement by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2014;16:99. doi:10.1186/s12968-014-0099-4
- Sado DM, White SK, Piechnik SK, et al. Identification and assessment of Anderson-Fabry disease by cardiovascular magnetic resonance noncontrast myocardial T1 mapping. Circ Cardiovasc Imaging. 2013;6:392–398. doi:10.1161/CIRCIMAGING.112.000070
- Nappi C, Altiero M, Imbriaco M, et al. First experience of simultaneous PET/MRI for the early detection of cardiac involvement in patients with Anderson-Fabry disease. Eur J Nucl Med Mol Imaging. 2015;42:1025–1031. doi:10.1007/s00259-015-3036-3
- Seydelmann N, Liu D, Kramer J, et al. High-sensitivity troponin: a clinical blood biomarker for staging cardiomyopathy in fabry disease. J Am Heart Assoc. 2016;5:e002839. doi:10.1161/JAHA.115.002839
- Kramer J, Weidemann F. Biomarkers for diagnosing and staging of fabry disease. Curr Med Chem. 2018;25:1530–1537. doi:10.2174/0929867324666170616102112
- Coats CJ, Parisi V, Ramos M, et al. Role of serum N-terminal pro-brain natriuretic peptide measurement in diagnosis of cardiac involvement in patients with anderson-fabry disease. Am J Cardiol. 2013;111:111–117. doi:10.1016/j.amjcard.2012.08.055
- Torralba-Cabeza MA, Olivera S, Hughes DA, Pastores GM, Mateo RN, Perez-Calvo JI. Cystatin C and NT-proBNP as prognostic biomarkers in Fabry disease. Mol Genet Metab. 2011;104:301–307. doi:10.1016/j.ymgme.2011.06.021
- Kramer J, Niemann M, Stork S, et al. Relation of burden of myocardial fibrosis to malignant ventricular arrhythmias and outcomes in Fabry disease. Am J Cardiol. 2014;114:895–900. doi:10.1016/j.amjcard.2014.06.019
- Eng CM, Germain DP, Banikazemi M, et al. Fabry disease: guidelines for the evaluation and management of multi-organ system involvement. Genet Med. 2006;8:539–548. doi:10.1097/01.gim.0000237866.70357.c6
- Schiffmann R, Waldek S, Benigni A, Auray-Blais C. Biomarkers of Fabry disease nephropathy. Clin J Am Soc Nephrol. 2010;5:360–364. doi:10.2215/CJN.06090809
- Riccio E, Sabbatini M, Capuano I, Pisani A. Early biomarkers of fabry nephropathy: a review of the literature. Nephron. 2019;143:274–281. doi:10.1159/000502907
- Wanner C, Oliveira JP, Ortiz A, et al. Prognostic indicators of renal disease progression in adults with Fabry disease: natural history data from the Fabry Registry. Clin J Am Soc Nephrol. 2010;5:2220–2228. doi:10.2215/CJN.04340510
- Glass RB, Astrin KH, Norton KI, et al. Fabry disease: renal sonographic and magnetic resonance imaging findings in affected males and carrier females with the classic and cardiac variant phenotypes. J Comput Assist Tomogr. 2004;28:158–168. doi:10.1097/00004728-200403000-00002
- Tondel C, Bostad L, Hirth A, Svarstad E. Renal biopsy findings in children and adolescents with Fabry disease and minimal albuminuria. Am J Kidney Dis. 2008;51:767–776. doi:10.1053/j.ajkd.2007.12.032
- Burlina AP, Sims KB, Politei JM, et al. Early diagnosis of peripheral nervous system involvement in Fabry disease and treatment of neuropathic pain: the report of an expert panel. BMC Neurol. 2011;11:61.
- Moller AT, Jensen TS. Neurological manifestations in Fabry’s disease. Nat Clin Pract Neurol. 2007;3:95–106. doi:10.1038/ncpneuro0407
- Low M, Nicholls K, Tubridy N, et al. Neurology of Fabry disease. Intern Med J. 2007;37:436–447. doi:10.1111/j.1445-5994.2007.01366.x
- Reisin RC, Romero C, Marchesoni C, et al. Brain MRI findings in patients with Fabry disease. J Neurol Sci. 2011;305:41–44. doi:10.1016/j.jns.2011.03.020
- Lee HJ, Hung SC, Hsu TR, et al. Brain MR imaging findings of cardiac-type fabry disease with an IVS4+919G>A mutation. AJNR Am J Neuroradiol. 2016;37:1044–1049. doi:10.3174/ajnr.A4677
- Morrison AS. Screening. In: Rothman KJ, Greenland S, editors. Modern Epidemiology. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 1998:499–518.
- Hagege A, Reant P, Habib G, et al. Fabry disease in cardiology practice: literature review and expert point of view. Arch Cardiovasc Dis. 2019;112:278–287. doi:10.1016/j.acvd.2019.01.002
- Auray-Blais C, Lavoie P, Abaoui M, et al. High-risk screening for Fabry disease in a Canadian cohort of chronic kidney disease patients. Clin Chim Acta. 2019;501:234–240.
- Sodi A, Nicolosi C, Vicini G, Lenzetti C, Virgili G, Rizzo S. Computer-assisted retinal vessel diameter evaluation in Fabry disease. Eur J Ophthalmol. 2019;1120672119886985.
- Schiffmann R, Hughes DA, Linthorst GE, et al. Screening, diagnosis, and management of patients with Fabry disease: conclusions from a “Kidney Disease: improving Global Outcomes” (KDIGO) controversies conference. Kidney Int. 2017;91:284–293.
