Abstract
Background
The annual gross domestic expenditure on research and development (GERD) per capita of Indonesia ($24) remains relatively lower than the annual GERD per capita of neighboring countries, such as Vietnam ($36), Singapore ($1804), Malaysia ($361), and Thailand ($111).
Objective
The aim of this study was to conduct a cost-effectiveness analysis of spending on healthcare R&D to address the needs of developing innovative therapeutic products in Indonesia.
Methods
A decision tree model was developed by taking into account four stages of R&D: stage 1 from raw concept to feasibility, stage 2 from feasibility to development, stage 3 from development to early commercialization, and stage 4 from early to full commercialization. Considering a 3-year time horizon, a stage-dependent success rate was applied and analyses were conducted from a business perspective. Two scenarios were compared by assuming the government of Indonesia would increase GERD in health and medical sciences up to 2- and 3-times higher than the baseline (current situation) for the first and second scenario, respectively. Cost per number of innovative products in health and medical sciences was considered as the incremental cost-effectiveness ratio (ICER). Univariate sensitivity analysis was conducted to investigate the effects of different input parameters on the ICER.
Results
There was a statistically significant association (P-value<0.05) between countries’ GERD in medical and health sciences with the number of innovative products. We estimated the ICER would be $8.50 million and $2.04 million per innovative product for the first and second scenario, respectively. The sensitivity analysis showed that the success rates in all stages and total GERD were the most influential parameters impacting the ICER.
Conclusion
The result showed that there was an association between GERD in medical and health sciences with the number of innovative products. In addition, the second scenario would be more cost-effective than the first scenario.
Introduction
Recent concerns about escalating healthcare expenditures and costs have sparked considerable public interest to accelerate the development of innovative therapeutic products as well as enhancing the efficiency of the process. As the results of research and development (R&D) in medical and health sciences, therapeutic products are considered as health products intended for use in humans for therapeutic, preventive, palliative, or diagnostic purposes.Citation1 According to the UNESCO Institute for Statistics, an innovative product can be defined as a good or service that is new or significantly improved with respect to its characteristics or intended uses.Citation2 Currently, the discussions on COVID-19 treatment illustrate the lack of innovative therapeutic products since the death cases of COVID-19 increase significantly, concentrate in the elderly population with chronic diseases, and give an impact on the importance of long-term care cost.Citation3–Citation5
In general, discussion on issues related to growing healthcare expenditure and R&D investments in the field of medical and health sciences have moved from an academic, pharmaceutical industry and government level to the broader stakeholders, including health insurance, which is a very important factor influencing the availability of long-term services in the healthcare system.Citation6 R&D in the field of medical and health sciences plays an important role in the healthcare system to prevent, diagnose, and treat diseases, and to improve patients’ quality-of-life.Citation7 As the consequence of economic growth, increasing environmental problems also have led to serious health problems and have attracted great interest among countries around the world.Citation6,Citation8 Countries’ key contribution to global health and wealth is turning fundamental R&D into innovative treatments.Citation9 Investments in healthcare innovation are leading the way to solve emerging healthcare problems and driving contributions to the global economy. Significant investments over the past 10 years are beginning to pay off since it relates to the treatment of untreatable disorders and chronic diseases, one of the biggest cost drivers in the healthcare system today.
As the fourth most populous country in the world, Indonesia has made significant gains in the economic growth.Citation10 With a GDP per capita of about $4050 in 2019, Indonesia is currently classified as an upper middle-income country with continued economic growth.Citation11 As one of the consequences, Indonesia is facing the challenge of increasing healthcare expenditures, by 222% in the last 8 years, also due to the strive of Indonesia to achieve universal healthcare coverage.Citation12 Compared with other middle-income countries, national healthcare spending in Indonesia (3.1% of GDP) remains below the average.Citation13 The gross domestic expenditure on R&D (GERD) of Indonesia is approximately $6.3 billion in all fields of R&D.Citation2 The annual GERD per capita of Indonesia ($24) remains relatively lower than the annual GERD per capita of neighboring countries, such as Vietnam ($36), Singapore ($1804), Malaysia ($361), and Thailand ($111).Citation2 Obviously, scarce resources should be deployed as efficient as possible by reducing costs and increasing effectiveness.Citation14–Citation16 Therefore, there is a growing need for innovative therapeutic products and its rational use, while reducing costs, specifically in a country with limited healthcare spending. To deal with these challenges, the government of Indonesia has put the healthcare sector as a priority in the 2020–2024 national development plan, with the objective to increase communities’ wellbeing to the highest level possible for Indonesian people to lead healthy and productive lives.Citation17 As a public responsibility, it is necessary to spend increased investments in the healthcare sector as efficient as possible resulting in innovative therapeutic products. For that purpose, we conducted a cost-effectiveness analysis of spending on healthcare R&D to address the needs of innovative therapeutic products in Indonesia, by learning from the experience of other countries.
Methods
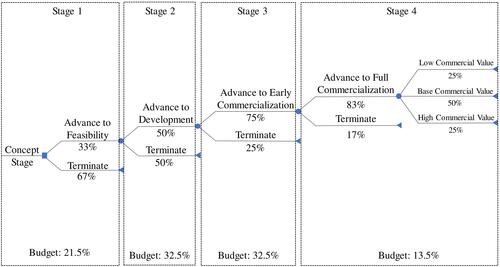
To analyze the cost-effectiveness of healthcare R&D spending on innovation, a decision tree model was developed by taking into account four stages of R&D: stage 1 from raw concept to feasibility, stage 2 from feasibility to development, stage 3 from development to early commercialization, and stage 4 from early to full commercialization (see ). A stage dependent success rate was taken into account. The model used a 3-year time horizon and applied the business perspective. To estimate the success rates of each stage, we applied data from a study by BoerCitation18 that estimated the success rate for R&D stages to become innovations. To conform this study, we applied success rates at 33%, 50%, 75%, and 83% in stages 1, 2, 3 and 4, respectively.Citation18 In stage 4, three options for full commercialization were considered with their respective probabilities of occurrence: low at 25%, base at 50%, and high commercial value (innovative product) at 25%.Citation18
We applied countries’ science, technology, and innovation data from the UNESCO Institute for Statistics, which covered national data on GERD and human resources.Citation2 GERD activities are defined as the total expenditure on R&D performed on the national territory during a given period, including both current costs and capital expenditures.Citation2 In this study, we applied the following inclusion criteria, such as complete national data on GERD, GERD per capita, GERD per researcher, and GERD in medical and health sciences in the last 10 years. Data on GERD with an incomplete percentage of innovative products in medical and health sciences was excluded. A linear regression analysis was applied by considering countries’ GERD in medical and health sciences as the independent variable, and the number of innovative products in medical and health sciences as the dependent variable. Before attempting to fit a linear model to observed data, a significant association between two variables was determined. A linear regression line resulted in an equation of the form Y=a+bX, where X is the explanatory/independent variable, Y is the dependent variable, b is the slope of the line, and a is the intercept (the value of Y when X=0). Applying the current situation as the baseline, we estimated 18.7% of total GERD in Indonesia would be spent in medical and health sciences.Citation19 We assumed the government of Indonesia would increase GERD in health and medical sciences up to 2- and 3-times higher than the baseline for the first and second scenario, respectively. The formula was applied to estimate the increasing number of innovative products in medical and health sciences as a consequence of increasing the number of GERD in medical and health sciences in both scenarios.
To calculate the incremental cost-effectiveness ratio (ICER), we compared each scenario with the baseline. In particular, the number of innovative products in health and medical sciences was considered as the cost-effectiveness measure. This includes significant improvements in technical specifications, components, and materials, incorporated software, user friendliness, or other functional characteristics.Citation2 In addition, to investigate the effects of different input parameters on the ICER, univariate sensitivity analysis was conducted by varying each parameter at a value of ±20% while keeping other parameters constant.Citation20 All parameters that were used in the model can be seen in .
Table 1 Parameters Used in the Model
Results
Applying 164 countries’ data on GERD and innovative products in medical and health sciences, we included only 16 countries’ data that could meet the inclusion and exclusion criteria. These countries represent middle-income (Bulgaria, Ecuador, Kazakhstan, Serbia, and Ukraine) and high-income countries (Croatia, Denmark, Estonia, Hungary, Latvia, Malta, the Netherlands, Poland, Republic of Korea, Romania, and Uruguay). More detailed information about R&D performance of all included countries can be seen in .
Table 2 R&D Performance of All Included CountriesCitation2
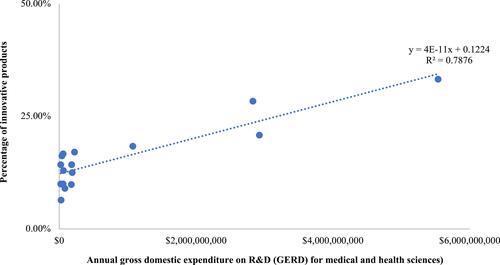
We found that there was a statistically significant association (P-value<0.05) between countries’ GERD in medical and health sciences with the number of innovative products. A linear regression analyses resulted in an equation of Y=0.12+(4.00x10−11)X, with R2=0.79 (see ). Applying GERD in medical and health sciences at $3541 million and GERD per researcher in medical and health sciences at $0.14 million, we estimated the percentage of innovative products would be 16.97%. We also estimated the number of outcomes in stages 1, 2, 3, and 4 would be 5511, 1871, 953, and 537, respectively. In particular, approximately 91 innovative products in medical and health sciences would be resulted in a 3-year time horizon.
Figure 2 Linear regression analysis on countries’ GERD for medical and health sciences with the percentage of innovative products.
Applying the same approach and time horizon, we estimated the percentage of innovative products in the first scenario would be 20.83%. We estimated the number of outcomes in stages 1, 2, 3, and 4 would be 13,531, 5638, 3,5243 and 2437, respectively. As its consequence, approximately 508 innovative products in medical and health sciences would be resulted. For the second scenario, we estimated the percentage of innovative products would be 28.36%. We estimated the number of outcomes in stages 1, 2, 3, and 4 would be 27,627, 15,669, 13,330, and 12,550, respectively. We also estimated the number of innovative products in medical and health sciences would be approximately 3559 in a 3-year time horizon.
Considering the number of innovative products in medical and health sciences as the effectiveness of R&D process, we estimated the ICER would be $8.50 million and $2.04 million per innovative product for the first and second scenario, respectively (see ). Taking several parameters into account (eg, the success rate of stage 1, 2, 3, and 4, shared budget of stage 1, 2, 3, and 4, and total GERD for health and medical sciences), the sensitivity analysis showed that the success rates in all stages and total GERD were the most influential parameters impacting the ICER (see ).
Table 3 Incremental Cost-Effectiveness Ratio (ICER) in a 3-Year Time Horizon Analysis
Table 4 Cost per Innovative Product on the Sensitivity Analysis
Discussion
R&D plays an extremely important role in the healthcare sector, specifically in prevention and treatment of diseases since it would provide greater benefits and yield better care and services for patients. Healthcare innovations can potentially bring savings, higher quality of therapeutic products, and the avoidance of errors to the society. Even though healthcare technology is one of the most innovative industries, its pattern has been the same for decades. This situation brings a potential space for innovations, where healthcare R&D can play an important role as an innovation driver. As a country with emerging middle-class population, demand for innovative therapeutic products in Indonesia is increasing. Nevertheless, innovation rate remains quite modest compared to huge R&D investment, as occurred in other similar countries.Citation21 This situation will continue to experience serious obstacles in the fiscal feasibility of national healthcare system.Citation22 Using essential medicine as a reference case, about 201 essential medicines were considered to be unaffordable in low- and middle-income countries, including in Indonesia.Citation23 The price of the raw medicine substance or active pharmaceutical ingredient is considered to be the most significant component cost of essential medicine production.Citation23–Citation27 An earlier estimation put the number of people lacking regular access to essential medicines at one-third of the global population,Citation28 which highlights that the lack of access to affordable therapeutic products remains a major global health burden.Citation29 Even though there are many pharmaceutical companies in Indonesia, approximately 90% of raw medicine substances are still imported.Citation30 The export value of the Indonesian pharmaceutical industry ($0.14 billion) was reported to be much lower than the import value ($1.5 billion).Citation31 This situation is mainly caused by the lack of R&D innovation in this industry in Indonesia. The results of this study showed that there was a statistically significant association between GERD in medical and health sciences with the number of innovative products. This study also highlighted that increasing GERD in medical and health sciences can encourage a higher number of new products and sales revenue, which is linear with the result of a previous study in Indonesia.Citation32
It is well known that innovations on therapeutic products are challenging, which are characterized by uncertainty, risk, and complexity. As a highly regulated and R&D driven industry, sustainable innovations are required to be developed and implemented according to structured, systematic, and methodologically strict processes. This also includes a practical innovation process according to different phases, which on the one hand leaves enough space for creativity, but also leads to the goal in a focused manner. In general, there are four phases of innovation: idea, concept, solution, and market.Citation33 In this study, we developed a decision tree model by taking into account four stages of R&D and the success rates of each stage. Obviously, each stage has its own characteristics. Typically, the rates in the initial stages are reported to be lower (33–50%) than in the development stages (75–83%). This situation might be caused by the fact that the initial stages usually tend to be less structured and the development stages are very process-oriented and focused.Citation33 In stage 4, three options for full commercialization were considered with their respective probabilities of occurrence: low at 25%, base at 50%, and high commercial value at 25%. In the healthcare sector, new products take the form of raw materials, intermediate and final products with low, base, and high commercial value, respectively.
It has been noticed that the cost of developing a successful therapeutic product is very costly, reflecting the various technical, regulatory, and economic challenges facing R&D pipelines.Citation34 Additionally, R&D in this industry is marked by high failure rates causing many companies to experience lost R&D investments.Citation35 An early-phase compound may have a promising outlook, but only clinical trials will demonstrate its efficacy, quality, and safety. In addition, lost investments may increase when a failure occurs in later R&D phases. A failure in the last stage is significantly more costly than in the initial stage because each phase is associated with a certain amount of required investment. To minimize the risk of failure, the implementation of the Quadruple Helix model should be optimized in Indonesia. In this model of the knowledge-based economy, the main institutions to first invest in R&D have been defined as university, industry, government, and society.Citation36 Learning from the experience of agriculture R&D in Indonesia, public engagement on the R&D process can promote higher productivity by improving the interaction between physical and human capital production inputs among all stakeholders.Citation37
In a comparison with the average GERD per capita of 16 countries in this study ($405), GERD per capita in Indonesia ($24) remains relatively low.Citation2 In this study, we compared two scenarios that were based on international benchmarking data from other countries by analyzing their R&D performance in medical and health sciences and using the number of innovative products as the effectiveness of R&D process. Assuming the government of Indonesia would increase GERD in health and medical sciences up to 2- and 3-times higher than the baseline for the first and second scenario, respectively, the result showed that the second scenario would be more cost-effective than the first scenario in the context of cost per innovative product that can be produced. To enhance the cost-effectiveness value, there is a strong relationship between industry capabilities and innovation incentives, implying that an optimal outcome can only be achieved through the rigorous implementation of approaches.Citation38 Another critical issue is the importance of the Indonesian government’s role in prioritizing innovation incentives to encourage R&D in medical and health sciences, which consistently presents the strongest causal relationship in the current situation and in the future.Citation38 Furthermore, the sensitivity analysis in this study showed that the success rate on each stage and total GERD were the most influential parameters impacting the cost-effectiveness value. Given the limited budget of R&D, these results are not to diminish the innovative drive of the healthcare industry in Indonesia but rather to encourage adoption of a new model of innovation. In Indonesia, the government provides the major share in total healthcare R&D spending, which is different with the situation in HICs where the private sector contributions make up 60% of R&D investment.Citation39 Open collaboration by facilitating partnerships involving academia and the public and private sectors are ways to increase the effectiveness of the R&D process in the healthcare sector.Citation35 These collaborations facilitate the sharing of expertiseand technologies such as compound libraries in order to accelerate the invention of therapeutic products in Indonesia. In the context of sustainability, WHO’s recommendations to strengthen R&D capacity can be implemented by the government of Indonesia, such as capacity building and technology transfer, promotion of partnerships and collaborations based on joint agendas and priority setting, development and retention of human resources and expertise, institutional and infrastructure development, and sustainable medium- and long-term collaborations.Citation40
This is the first study to analyze the cost-effectiveness of healthcare R&D spending to address the challenges of innovative therapeutic products in Indonesia. Some limitations apply to our study. We developed a decision tree model by taking into account four stages of R&D innovations. Despite the majority of parameters being derived from country-specific data, we applied international data on the success rates and distribution of the R&D budget of each stage due to the lack of local data. To deal with the uncertainty, we took these issues into account in the sensitivity analysis. Despite the inherent limitations discussed, the current work represents a valid initial overview to evaluate substantial R&D investment in the healthcare sector to systematically address the challenges of innovative therapeutic products’ development in Indonesia by drawing on experiences of other countries.
In countries with complex healthcare problems, setting healthcare R&D priorities appears to be important since it represents the most urgent needs to address health risks of the population.Citation41–Citation44 In the context of Indonesia, the current major challenge in healthcare R&D is associated with the rising prevalence of NCDs and their associated risk factors since these diseases have long duration and generally slow progression. NCDs are closely related to the increased longevity of most contemporary societies, which pose substantial challenges to the health financing sustainability.Citation45 To deal with this issue, countries’ capability and willingness to invest resources could strengthen efforts for eradicating NCDs.Citation46 Public and private donors have marshaled resources and created organizational structures to accelerate the development of new therapeutic products.Citation47 Major challenges continue to be population aging, the rising incidence of lifestyle diseases, universal health coverage, and inequities to healthcare access.Citation48 These challenges can be attributed to inefficient resource allocation strategies in the healthcare system and unsatisfactory funding strategies.Citation48 The last few years have been marked by a bold increase in countries’ healthcare spending.Citation49 In Indonesia, increasing healthcare spending is associated with relying heavily on the development assistance, specifically for the pursuit of universal health coverage. Learning from the experience of Brazil, Russia, India, China, and South Africa, successful health reforms in leading emerging markets require an effective healthcare system management, which will significantly assist countries to achieve common health goals (eg, decreasing NCDs and increasing life expectancy) and to catch up with innovative therapeutic products.Citation50
Conclusion
The result of this study showed that there was a statistically significant association between GERD in medical and health sciences with the number of innovative products. Assuming the government of Indonesia would increase GERD in health and medical sciences up to 2- and 3-times higher than the baseline (current situation) for the first and second scenario, respectively, the result showed that the second scenario would be more cost-effective than the first scenario in the context of cost per innovative product that can be produced. The sensitivity analysis showed that the success rates in all stages and total GERD were the most influential parameters impacting the ICER.
Acknowledgment
This research was partially funded by the Indonesian Ministry of Research and Technology/National Agency for Research and Innovation, and Indonesian Ministry of Education and Culture, under the World Class University Program managed by Institut Teknologi Bandung for Rizky Abdulah.
Disclosure
Professor Maarten Jacobus Postma reports Health-Ecore and PAG BV stocks, grants, and personal fees from Astra Zeneca, GSK, Boehringer Ingelheim, Janssen, MSD, Sanofi, and Sequiris, grants from BMS, WHO, and BioMerieux, and personal fees from Merck, Alexion, Asc Academics, Parexel, Pfizer, IQVIA, Novo Nordisk, and Sanofi, outside the submitted work. The authors report no other potential conflicts of interest for this work.
References
- Health Sciences Authority. Regulatory overview of therapeutic products. Available from: https://www.hsa.gov.sg/therapeutic-products/overview. Accessed April 10, 2020.
- UNESCO Institute for Statistics. Science, Technology and Innovation. Available from: http://data.uis.unesco.org/#. Accessed June 10, 2020.
- Xu X, Zhang L, Chen L, Wei F. Does COVID-2019 have an impact on the purchase intention of commercial long-term care insurance among the elderly in China? Healthcare. 2020;8(2):126.
- Xu X, Chen L. Projection of long-term care costs in china, 2020–2050: based on the bayesian quantile regression method. Sustainability. 2019;11:3530. doi:10.3390/su11133530
- Xu X, Huang X, Zhang X, Chen L. Family economic burden of elderly chronic diseases: evidence from China. Healthcare. 2019;7:99. doi:10.3390/healthcare7030099
- Chen L, Zhang X, Xu X. Health insurance and long-term care services for the disabled elderly in china: based on charls data. Risk Manag Healthc Policy. 2020;13:155–162. doi:10.2147/RMHP.S233949
- DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development cost. J Health Econ. 2003;22:151–185. doi:10.1016/S0167-6296(02)00126-1
- Xu X, Xu Z, Chen L, Li C. How does industrial waste gas emission affect health care expenditure in different regions of china: an application of bayesian quantile regression. Int J Environ Res Public Health. 2019;16(15):2748. doi:10.3390/ijerph16152748
- Daemmrich A, Mohanty A. Healthcare reform in the United States and China: pharmaceutical market implications. J Pharm Policy Pract. 2014;7(1):1–13. doi:10.1186/2052-3211-7-9
- World Bank. World development indicators. Available from: http://wdi.worldbank.org/tables. Accessed April 10, 2020.
- World Bank. Indonesia – Supporting Primary Healthcare Reform Project: Technical Assessment (English). Washington DC: World Bank Group; 2018.
- WHO SEARO. The Republic of Indonesia Health System Review. New Delhi: WHO; 2017.
- Institute for Scientific Information. The Annual G20 Scorecard research Performance 2019. Available from: https://clarivate.com/webofsciencegroup/campaigns/the-annual-g20-scorecard-research-performance-2019/. Accessed April 5, 2020.
- Han E, Chae SM, Kim NS, Park S. Effects of pharmaceutical cost containment policies on doctors’ prescribing behavior: focus on antibiotics. Health Policy (New York). 2015;119(9):1245–1254. doi:10.1016/j.healthpol.2015.05.005
- Bae G, Park C, Lee H, Han E, Kim DS, Jang S. Effective policy initiatives to constrain lipid-lowering drug expenditure growth in South Korea. BMC Health Serv Res. 2014;14:100. doi:10.1186/1472-6963-14-100
- Cho MH, Yoo KB, Lee HY, et al. The effect of new drug pricing systems and new reimbursement guidelines on pharmaceutical expenditures and prescribing behavior among hypertensive patients in Korea. Health Policy (New York). 2015;119(5):604–611. doi:10.1016/j.healthpol.2015.01.002
- Ministry of Health, Republic of Indonesia. Strategic plan 20202024. Available from: https://www.kemkes.go.id/resources/download/info-terkini/Rakerkesnas-2020/. Accessed March 30, 2020.
- Boer FP. The Valuation of Technology: Business and Financial Issues in R&D, Operations Management Series for Professionals. New York: John Wiley; 1999.
- CNN Indonesia. Indonesia Research Budget; 2020. Available from: https://www.cnnindonesia.com/teknologi/20200128122325-199-469363/anggaran-riset-dan-pengabdian-masyarakat-2020-capai-rp146-t. Accessed April 10, 2020.
- Jayasundara K, Hollis A, Krahn M, et al. Estimating the clinical cost of drug development for orphan versus non-orphan drugs. Orphanet J Rare Dis. 2019;14(1):12. doi:10.1186/s13023-018-0990-4
- Jakovljevic MB. BRIC’s growing share of global health spending and their diverging pathways. Front Public Health. 2015;3:135. doi:10.3389/fpubh.2015.00135
- Jakovljevic M, Getzen TE. Growth of global health spending share in low and middle income countries. Front Pharmacol. 2016;7:21. doi:10.3389/fphar.2016.00021
- Wirtz VJ, Hogerzeil HV, Gray AL, et al. Essential medicines for universal health coverage. Lancet. 2017;389:403–476. doi:10.1016/S0140-6736(16)31599-9
- Wagner AK, Graves AJ, Reiss SK, et al. Access to care and medicines, burden of health care expenditures, and risk protection: results from the world health survey. Health Policy (New York). 2011;100:151–158. doi:10.1016/j.healthpol.2010.08.004
- Hill AM, Barber MJ, Gotham D. Estimated costs of production and potential prices for the WHO essential medicines list. BMJ Glob Health. 2018;3:e000571. doi:10.1136/bmjgh-2017-000571
- Fortunak JM, de Souza RO, Kulkarni AA, et al. Active pharmaceutical ingredients for antiretroviral treatment in low- and middle-income countries: a survey. Antivir Ther. 2014;19(Suppl 3):15–29. doi:10.3851/IMP2897
- Basu P, Joglekar G, Rai S, et al. Analysis of manufacturing costs in pharmaceutical companies. J Pharm Innov. 2008;3:30–40. doi:10.1007/s12247-008-9024-4
- World Health Organization. The world medicines situation. Available from: http://apps.who.int/medicinedocs/pdf/s6160e/s6160e.pdf. Accessed March 2, 2020.
- UN Secretary-General. The United Nations secretary-general’s high-level panel on access to medicines report: promoting innovation and access to health technologies; [cited 2020 April 1]. Available from: http://www. unsgaccessmeds.org/s/UNSG- HLP- Report- FINAL- 12- Sept- 2016. Pdf. Accessed April 2, 2020.
- The Jakarta Post. Pharma companies still dependent on imported APIs, says ministry. Available from: https://www.thejakartapost.com/news/2018/10/18/pharma-companies-still-dependent-on-imported-apis-says-ministry.html. Accessed April 2, 2020.
- Indonesia Investments. Pharmaceutical Exports Indonesia Remain Under Pressure in 2017. Available from: https://www.indonesia-investments.com/business/business-columns/pharmaceutical-exports-indonesia-remain-under-pressure-in-2017/item7867. Accessed April 2, 2020.
- Simanjuntak DG, Tjandrawinata RR. A study on influencers of total sales revenue of generic pharmaceutical companies in Indonesia. MPRA. 2011. 31628:111. Available from: https://mpra.ub.uni-muenchen.de/31628/. Accessed September 4, 2020.
- LEAD Innovation Management. The 4 phases of innovation. Available from: https://www.lead-innovation.com/english-blog/the-4-phases-of-innovation. Accessed April 2, 2020.
- DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: new estimates of R&D costs. J Health Econ. 2016;47:20–33. doi:10.1016/j.jhealeco.2016.01.012
- International Federation of Pharmaceutical Manufacturers & Associations. The pharmaceutical industry and global health, facts and figures 2017. Available from: https://www.ifpma.org/wp-content/uploads/2017/02/IFPMA-Facts-And-Figures-2017.pdf. Accessed April 5, 2020.
- Carayannis EG, Campbell DFJ. ‘Mode 3ʹ and ‘quadruple helix’: toward a 21st century fractal innovation ecosystem. Int J Technol Manage. 2009;46(3/4):201–234. doi:10.1504/IJTM.2009.023374
- Armas EB, Osorio CG, Moreno-Dodson B, Abriningrum DE. Agriculture public spending and growth in indonesia. Policy research working paper; no. WPS 5977. World Bank. 2012.Available from: https://openknowledge.worldbank.org/handle/10986/3263. Accessed September 4, 2020.
- Siagian RC, Ayuningtyas D. Gap analysis for drug development policy-making: an attempt to close the gap between policy and its implementation. PLoS One. 2019;14(8):e0220605. doi:10.1371/journal.pone.0220605
- Rottingen JA, Regmi S, Eide M, et al. Mapping of available health research and development data: what’s there, what’s missing, and what role is there for a global observatory? Lancet. 2013;382:1286–1307. doi:10.1016/S0140-6736(13)61046-6
- WHO. Research and development to meet health needs in developing countries: strengthening global financing and coordination. Available from: https://apps.who.int/iris/bitstream/handle/10665/254706/9789241503457-eng.pdf;jsessionid=D7D1D78319B6D54CD9AFAE78FA5DCECD?sequence=1. Accessed September 4, 2020.
- Cole CB, Trolle S, Edwards DJ. Developing the latest framework to measure and incentivise pharmaceutical industry contributions to health research and development. Health Res Policy Syst. 2018;16(1):73. doi:10.1186/s12961-018-0332-y
- Leydesdorff L The knowledge-based economy and the triple helix model. Available from: https://www.leydesdorff.net/arist09/arist09.pdf. Accessed April 2, 2020.
- WHO. Country Pharmaceutical Situations, Fact Book on WHO Level 1 Indicators 2007. Geneve: WHO; 2009.
- Suwantika AA, Postma MJ. Effect of breastfeeding promotion interventions on cost-effectiveness of rotavirus immunization in Indonesia. BMC Public Health. 2013;13(1):1106. doi:10.1186/1471-2458-13-1106
- Jakovljevic MB, Milovanovic O. Growing burden of non-communicable diseases in the emerging health markets: the case of BRICS. Front Public Health. 2015;3:65. doi:10.3389/fpubh.2015.00065
- Jakovljevic M, Jakab M, Gerdtham U, et al. Comparative financing analysis and political economy of noncommunicable diseases. J Med Econ. 2019;22(8):722–727. doi:10.1080/13696998.2019.1600523
- Morel CM, Acharya T, Broun D, et al. Health innovation networks to help developing countries address neglected diseases. Science. 2005;309(5733):401–404. doi:10.1126/science.1115538
- Jakovljevic M, Groot M, Souliotis K. Editorial: health care financing and affordability in the emerging global markets. Front Public Health. 2016;4:2.
- Jakovljevic MM. Comparison of historical medical spending patterns among the BRICS and G7. J Med Econ. 2016;19(1):70–76. doi:10.3111/13696998.2015.1093493
- Jakovljevic M, Timofeyev Y, Ekkert NV, et al. The impact of health expenditures on public health in BRICS nations. J Sport Health Sci. 2019;8(6):516–519. doi:10.1016/j.jshs.2019.09.002