Abstract
Background
The purpose of this study was to assess the safety and efficacy of oral glycopyrrolate solution 1 mg/5 mL for 24 weeks in pediatric patients with chronic moderate-to- severe drooling associated with cerebral palsy and other neurologic conditions.
Methods
In this multicenter, open-label, 24-week study, males and females aged 3–18 years weighing at least 27 lb received oral glycopyrrolate solution, starting at 0.02 mg/kg three times daily and titrated in increments of 0.02 mg/kg every 5–7 days for 4 weeks to an optimal maintenance dose or a maximum dose of 0.1 mg/kg, but not exceeding 3 mg three times daily. Safety was assessed by description and tabulation of all adverse events. The primary efficacy endpoint was response, defined as at least a three-point change from baseline to week 24 on the modified Teacher’s Drooling Scale.
Results
Of 137 intent-to-treat participants, 10 (7.3%) received the maximum dose of 0.1 mg/kg three times daily; 122 (89%) had at least one treatment-emergent adverse event, 47% related to oral glycopyrrolate solution, with most being mild-to-moderate in intensity. The most commonly reported treatment-emergent adverse events were constipation (20.4%), vomiting (17.5%), diarrhea (17.5%), pyrexia (14.6%), dry mouth (10.9%), flushing (10.9%), and nasal congestion (10.9%). Nineteen patients (13.9%) discontinued treatment due to an adverse event, but no adverse event was specifically associated with discontinuation. Two patients had clinically significant toxicity grade shifts, one each in platelet count and calcium concentration. No deaths occurred on treatment; deaths of three patients (multisystem organ failure, anoxic encephalopathy, and aspiration pneumonia) within 30 days of their last dose were not considered to be treatment-related. At 24 weeks, 52.3% (95% confidence interval 43.7–60.9) of patients were responders, with at least a three-point decrease in modified Teacher’s Drooling Scale from baseline, with 83.5% of parents/caregivers and 85.8% of investigators rating oral glycopyrrolate solution as being worthwhile.
Conclusion
Oral glycopyrrolate solution 1 mg/5 mL for chronic moderate-to-severe drooling associated with cerebral palsy or other neurologic conditions was well tolerated over 24 weeks by pediatric patients aged 3–18 years.
Introduction
An estimated 10%–37% of developmentally disabled individuals have moderate-to- severe sialorrhea.Citation1–Citation3 In children with cerebral palsy and other neuromuscular conditions, drooling is primarily due to oral motor dysfunction.Citation4,Citation5 Glycopyrrolate (glycopyrronium bromide), a synthetic quaternary ammonium anticholinergic agent,Citation6 was first approved for clinical use in 1961. Indications include adjunctive treatment of peptic ulcer disease in adults, preoperative or intraoperative inhibition of salivation and excessive respiratory tract secretions in adults and children aged ≥2 years, and management of secretions in children with tracheostomies.Citation7,Citation8 Glycopyrrolate penetrates the blood– brain barrier poorly, and therefore has reduced potential to cause central nervous system effects.Citation9,Citation10
Although off-label use of glycopyrrolate tablets has been shown to decrease drooling in children with cerebral palsy,Citation11–Citation14 these tablets require compounding to treat pediatric patients. The highly variable pharmacokinetics of this formulation of glycopyrrolate can result in wide-ranging effective doses and cause adverse events that lead to treatment discontinuation.Citation14 For example, a placebo-controlled trial in 39 children found that the mean highest tolerated dose of glycopyrrolate was 2.49 mg (0.11 mg/kg), ranging from 1.2 mg (0.04 mg/kg) to 3.0 mg (0.2 mg/kg), with most children showing improvement in drooling at doses of 0.1 mg/kg.Citation14 These findings suggest that the initial dose should be 0.04 mg/kg, followed by stepwise increases to an effective dose that can be tolerated without significant adverse events.Citation14 Results from a retrospective trial in 37 patients found that the mean dose of glycopyrrolate was 0.51 mg/kg, with 86% of patients receiving 0.02–0.07 mg/kg doses.Citation15 In an open-label trial, 24 patients were treated with 0.04–0.10 mg/kg glycopyrrolate once daily, with patients started on lower doses and titrated until drooling significantly decreased or stopped.Citation12 In another open-label trial, 40 patients were treated with 0.5 mg glycopyrrolate once or twice daily.Citation11 Effective doses ranged from 0.01–0.82 mg/kg/day (1.0–8.0 mg/ day; median, 0.09 mg/kg/day), with glycopyrrolate administered 1–5 times per day.Citation11
To provide more accurate pediatric dosing and titration, a novel liquid formulation of glycopyrrolate was developed at a concentration of 1.0 mg/5 mL. Glycopyrrolate was started at a dose of 0.2 mg/kg three times daily, with dose increases to 0.04, 0.06, 0.08, and 0.10 mg/kg. This open-label trial assessed the 24-week safety of oral glycopyrrolate solution (1 mg/5 mL) in pediatric patients aged 3–18 years with chronic, moderate-to-severe drooling associated with cerebral palsy and other neurologic conditions. We also assessed the efficacy of maintenance doses of glycopyrrolate oral solution in managing drooling in this patient population.
Materials and methods
Patient selection
We enrolled male and female patients aged 3–18 years and weighing at least 12.3 kg, who had been diagnosed with cerebral palsy, mental retardation, or any other neurologic impairment or condition with chronic drooling, defined as wetness of the chin or clothing on most days. All patients had scores ≥5 on the nine-point modified Teacher’s Drooling Scale (mTDS, ). Both cognitively capable and cognitively impaired patients were eligible.
Table 1 Modified Teacher’s Drooling Scale
All patients lived in a situation where reliable parents/ caregivers were willing and capable of administering medications, as determined by the investigator. Written informed consent, signed by the parent or legally acceptable representative, and written assent, signed by age-appropriate patients if mentally capable, were required for participation. Females of childbearing potential had to have negative pregnancy tests at screening and at week 8. Sexually active females had to agree to use a medically acceptable form of contraception.
The study protocol was approved by the institutional review board of each participating institution, and each patient or the patient’s parent/legal guardian provided written informed consent, in accordance with Good Clinical Practice guidelines, the current version of the Declaration of Helsinki, and the United States Food and Drug Administration Regulations Title 21 code of Federal Regulations Parts 50.20–50.27.
Patients were excluded prior to baseline (day -2) if they had used or received glycopyrrolate (within approximately 24 hours), prohibited medications (within five plasma half-lives of the medication), intrasalivary gland botulinum toxin (within 10 months), intraoral devices or prosthetics for treatment of drooling (within one week), or acupuncture for the treatment of drooling (within 3 months). We also excluded patients with medical conditions contraindicating anticholinergic therapy, known contraindications to the study medication, poorly controlled seizures (defined as daily seizures), a history of intestinal obstruction, or clinically significant hepatic or renal impairment, as well as pregnant or breastfeeding females and any patient who had received any investigational drug within 30 days of study entry. In addition, the investigator could exclude patients and families or parents/caregivers expected to be noncompliant with study procedures, as well as patients unable to meet study requirements for any reason or those with unstable mental disease.
Study design
The study was conducted at 28 sites in the United States. Patients were stratified according to whether they had or had not received glycopyrrolate for drooling within 3 months of initiating the study treatment. The treatment period was 24 weeks, with scheduled visits on day 1 and at weeks 4, 8, 12, 16, 20, and 24.
Treatments
After a washout, screening period, and a 2-day baseline period, oral glycopyrrolate solution 1 mg/5 mL was titrated over 4 weeks in each patient. The starting dose was 0.02 mg/kg three times daily, followed by titration in increments of 0.02 mg/kg every 5–7 days for 4 weeks to an optimal maintenance dose or a maximum dose of 0.1 mg/kg, but not exceeding 3 mg three times daily. The optimal dose was defined as the dose at which a patient received the maximum benefit from the study drug (greatest improvement in drooling) while experiencing minimum side effects, with increases and decreases in doses determined by the investigator in coordination with the parent/caregiver.
Safety
Safety was assessed by description and tabulation of all adverse events. To help identify adverse events, a training manual was provided to the parent/caregiver which included information about the pharmacologic effects and adverse events associated with glycopyrrolate, as well as instructions on how to measure and administer the drug. This manual was provided to the parent/caregiver at visit 1 for mandatory reading and reviewed with qualified study personnel at visit 2 with regard to each parent/caregiver’s understanding of dose measurement, three times daily dosing schedule, dose titration process, and potential adverse events associated with glycopyrrolate. The modified Behavioral and Medical Rating Scale (mBMRS) was completed by the parent/caregiver twice weekly (every 3–4 days) from the first dose of study drug until week 4, then once weekly for the remainder of the study. The mBMRS includes 28 predefined symptoms, each rated on a four-point scale, ie, 1 = not at all, 2 = just a little, 3 = quite a bit, and 4 = very much (). Behavioral, physiological, and total symptom scores were calculated at each visit, with a positive change indicating symptom improvement. Safety was also assessed by physical examination; a 12-lead electrocardiogram at screening and weeks 4, 12 and 24; clinical laboratory evaluation (blood chemistry, hematology, and urinalysis); and measurement of vital signs (heart rate, respiratory rate, systolic and diastolic blood pressure, and temperature). Hematology, gastrointestinal, and renal and electrolyte clinical laboratory results that worsened more than one toxicity grade from baseline were considered clinically significant.
Table 2 Prespecified behavioral and physiological symptoms in the modified Behavioral and Medical Rating Scale
Efficacy
The primary efficacy endpoint was change from baseline to week 24 on the mTDS, as determined by the parent/ caregiver. Patients showing at least a three-point decrease on the mTDS were defined as responders, whereas those showing a less than three-point decrease were defined as nonresponders. Secondary efficacy endpoints included parent/caregiver assessment of extent of drooling on the day for each applicable visit, as determined on a visual analog scale, and investigator and parent/caregiver global assessment of treatment, as determined by responses to the statement, “This is a worthwhile treatment,” using a five-point scale, ie, strongly agree, agree, neutral, disagree, or strongly disagree.
Statistical methods
Safety analysis included a descriptive tabulation of adverse events, listed in order of decreasing frequency. No statistical hypothesis testing was performed for the safety analysis. Visual analog scores and Global Assessment of Treatment were summarized using descriptive statistics for continuous variables and frequency and percentage for discrete variables. Laboratory values were summarized and change from screening determined. The primary efficacy analysis used the mean of each daily postdose evaluation (ie, calculation of the mean of three postdose evaluations for each day). Patients were classified as responders if they had at least a three-point decrease in mean mTDS from baseline to week 24, otherwise as nonresponders.
Results
This study was performed between April 2007 and May 2008. Of the 160 patients enrolled, 23 were deemed screen failures and did not receive study drug, although one patient who was recorded as a screen failure received study drug and was included in the intent-to-treat population. The baseline characteristics of the 137 who received at least one dose of study drug are summarized in and are included in the analysis. Of these 137 patients, 103 (75.2%) completed the study; of the 34 patients (24.8%) who discontinued early, two did so due to lack of efficacy and 14 due to adverse events. Other reasons for discontinuation included failure to meet entry criteria (n = 2), investigator decision (n = 2), patient noncompliance (n = 2), patient/parent decision (n = 5), loss to follow-up (n = 3), death (n = 3; see Safety section), and other (n = 1).
Table 3 Baseline characteristics of the intent-to-treat population (n = 137)
Overall extent of exposure
The mean daily dose of oral glycopyrrolate solution was 0.15 mg/kg, with 70 of the 137 patients (51.1%) receiving a mean daily dose of ≥0.1 mg/kg to ≤0.2 mg/kg, and 10 (7.3%) receiving the maximum dose of 0.1 mg three times daily. Doses were reduced in about 45% of patients over the 24-week study period. Patients were treated for a mean 139.8 days, with 104 patients (75.9%) treated for >150 days to ≤200 days.
Safety
Most patients (n = 122; 89%) had at least one treatment-emergent adverse event, 47% of which were deemed related to oral glycopyrrolate solution, with most being mild-to-moderate in intensity. The most commonly reported treatment-emergent adverse events are summarized in . Several treatment-emergent adverse events occurred more frequently in the high-dose (>0.2 mg/kg) and middle-dose (≥0.1 to ≤0.2 mg/kg) than in the low-dose (<0.1 mg/kg) group, including vomiting (18.4% versus 18.6% versus 13.8%), dry mouth (15.8% versus 11.4% versus 3.4%), otitis media (10.5% versus 10.0% versus 3.4%), upper respiratory tract infection (7.9% versus 10.0% versus 3.4%), pneumonia (7.9% versus 5.7% versus 0%), streptococcal pharyngitis (7.9% versus 4.3% versus 3.4%), epistaxis (7.9% versus 4.3% versus 3.4%), somnolence (2.6% versus 8.6% versus 0%), pyrexia (18.4% versus 15.7% versus 6.9%), and rash (5.3% versus 11.4% versus 3.4%). Fourteen patients had 20 serious adverse events, eight while taking the study drug and six within 30 days of the last dose. Of these 20 serious adverse events, four were considered treatment-related, ie, nystagmus, esophageal candidiasis, dehydration, and gastrointestinal motility disorder. Nineteen patients discontinued due to an adverse event, including the 14 patients who terminated participation due to an adverse event associated with the study drug, three patients who died of an adverse event not associated with the study drug, and two who withdrew consent to participate. However, there was no obvious trend in type of event that led to discontinuation.
Table 4 Most frequently observed (>5%) treatment-emergent adverse events (n = 137)
Generally, adverse event rates were slightly higher in the 84 patients naïve to treatment with oral glycopyrrolate solution than in the 53 patients previously treated with this drug. These included rates of treatment-emergent adverse events (90.5% versus 86.8%), drug-related treatment-emergent adverse events (50.0% versus 41.5%), severe treatment-emergent adverse events (9.5% versus 5.7%), serious adverse events (11.9% versus 7.5%), deaths (2.4% versus 1.9%) and adverse events resulting in discontinuation of study (14.3% versus 3.8%). In contrast, naïve patients had lower rates of serious treatment-emergent adverse events than non-naïve patients (4.8% versus 7.5%).
The mBMRS, intended to help the parent/caregiver identify possible adverse event-related behaviors and physiological effects in patients taking oral glycopyrrolate solution, enabled the identification of 24.7% of adverse events by parents/caregivers and 1.8% by investigators. Clinically significant toxicity grade shifts, from grade 0 at baseline to grade 2 at week 24/exit visit, were observed in two patients, one in platelet count (from 3.9 × 105/μL at baseline to 6.7 × 104/μL at week 24/exit visit) and one in calcium concentration (from 9.2 mg/dL at baseline to 7.2 mg/dL at week 24/exit visit). No deaths were reported while patients were on oral glycopyrrolate solution. Three deaths occurred within 30 days of the last dose, one each due to multisystem organ failure, anoxic encephalopathy, and aspiration pneumonia, but none was considered treatment-related by the investigators.
There were no notable changes in mean hematology or chemistry parameters. Hematology parameters were measured in 65% and 79% of patients at baseline and week 24, respectively. Decreases >10% from the normal reference range at baseline to below that range at week 24 were observed for absolute neutrophil (11.2%) and red blood cell (11.1%) count, and increases >10% from the normal reference range at baseline to above that at week 24 were observed for monocyte (16.7%) and absolute monocyte (11.2%) counts. Chemistry parameters were measured in 68% and 82% of patients at baseline and the final visit, respectively, except for bicarbonate, which was measured in only 15 patients at both time points. Decreases >10% from the normal reference range at baseline to below that range at week 24 were observed for carbon dioxide (15.1%), bicarbonate (13.3%), and creatinine (10.7%) concentrations, but there were no increases >10% from the normal reference range at baseline to above that range at week 24.
Although there were minor fluctuations in vital signs, including mean systolic and diastolic blood pressure, pulse rate, respiration rate, temperature, and weight, over the course of the study, none of these changes was clinically notable. Shifts from normal at baseline to abnormal and clinically significant at week 24 in >1% of patients who underwent physical examinations at both visits were observed in the extremities (3.2%), head, ears, eyes, nose, and throat (1.6%), and in psychology (1.6%).
Electrocardiographic interval durations were examined across six dose levels at weeks 4, 12, and 24 by both time averaged and per time point techniques. No abnormal or clinically significant shifts in electrocardiographic findings were observed in patients with electrocardiographic results at baseline and week 24. The electrocardiographic interval data showed that oral glycopyrrolate solution had no obvious effect on heart rate or atrioventricular conduction, as measured by PR interval, or depolarization, as measured by QRS duration. There was no evidence of any clinically relevant changes in QTc duration, or imbalances in specific and nonspecific outliers. Morphological changes were noted in T wave changes, which were likely spurious (not dose-related and of very low frequency). One patient had an abnormal, clinically significant electrocardiographic tracing during the study, indicating sinus tachycardia, incomplete right bundle branch block, and left atrial enlargement; this patient died of multiorgan failure before completing the study, but the death was deemed unrelated to the study drug by the investigator.
Efficacy
At the week 24/exit visit, 52.3% (95% confidence interval [CI] 43.7–60.9) of patients had an at least three-point decrease in mTDS from baseline and were classified as responders to treatment with oral glycopyrrolate solution. The proportion of responders from week 4 (visit 1) to week 24 (exit visit) is shown in . The percentage of the 53 non-naïve patients (ie, patients previously treated with glycopyrrolate) classified as responders (58.5%; 95% CI 45.2–71.8) was higher than that of the 84 naïve patients (48.1%; 95% CI 36.9–59.2). The percentages of patients with profuse, severe, and moderate drooling decreased substantially after 24 weeks of treatment, from 31.6% to 2.3%, from 36.6% to 8.3%, and from 32.1% to 25.6%, respectively. At the end of the study, 15% of patients no longer drooled. The percentages of responders by dose group are summarized in .
Figure 1 Proportion of responders after 4–24 weeks of treatment with oral glycopyrrolate solution 1 mg/5 mL. Response was defined as at least a three-point decrease on the Modified Teachers’ Drooling Scale. The percentage of responders at each time point from week 4 to week 20 was assessed relative to the number of patients remaining on study at that time point. The percentage of responders at week 24 was assessed relative to all intent-to-treat patients (n = 137), except for seven patients with missing values. Patients who discontinued treatment due to lack of efficacy had their worst observation carried forward, whereas patients who discontinued due to any other reason had their last observation carried forward.
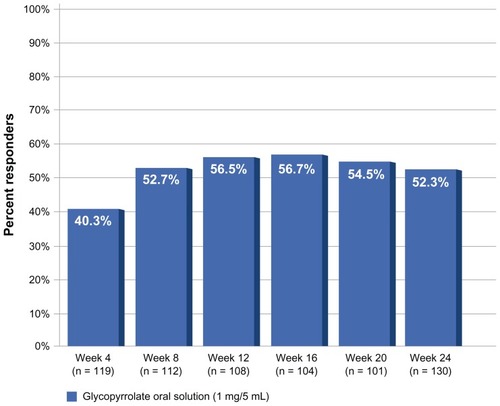
Table 5 Responders by dose group
Improvements in the extent of drooling were also observed using the visual analog scale assessment. The mean visual analog score was reduced from 6.56 at baseline to 3.21 at 24 weeks. These scores were similar in glycopyrrolate-naïve and glycopyrrolate-non-naïve patients. At the end of the study, 83.5% of parents/caregivers and 85.8% of investigators rated oral glycopyrrolate solution as a worthwhile treatment.
Discussion
This study demonstrated that 24 weeks of treatment with oral glycopyrrolate solution 1 mg/5 mL for chronic, moderate- to-severe drooling associated with cerebral palsy and other neurologic conditions was well tolerated by children 3–18 years of age. More than 80% of patients had cerebral palsy classified as spastic or quadriplegic; >90% were developmentally disabled and had impaired speech, approximately 75% resided at home with a parent or a foster parent or guardian, and >60% were glycopyrrolate-naïve.
The treatment-emergent adverse events observed in these patients were similar to those in patients treated with glycopyrrolate tablets.Citation11–Citation14 Clinical trials of glycopyrrolate tablets to manage drooling in patients with cerebral palsy and other neurodevelopmental conditions have reported behavioral changes (including hyperactivity and irritability), constipation, diarrhea, excessive oral dryness, urinary retention, headache, drowsiness, dilated pupils, blurred vision, facial flushing, nasal congestion, vomiting, dizziness, dehydration, fever, and rash.Citation11–Citation14 No unexpected safety issues were identified in 24 weeks of treatment with oral glycopyrrolate solution.
The primary adverse event observed with oral glycopyrrolate solution was constipation; other adverse events were related to its mechanism of action as an anticholinergic. The increase in adverse events observed with higher doses of oral glycopyrrolate solution was similar to that in a study of glycopyrrolate tablets in developmentally disabled children with sialorrhea, which attempted to identify the dose at which the curve of reduced drooling crossed the curve of increased adverse effects.Citation14 Adverse events increased as the dose increased; 20% of children stopped taking glycopyrrolate due to adverse events, the most frequent being behavioral problems, constipation, excessive oral dryness, and urinary retention.Citation14
Although the overall incidence of abnormal laboratory test results in this study was low, two patients had clinically significant toxicity grade shifts in laboratory values. No clinically notable changes in vital signs were observed. Although the low frequency of electrocardiograms, the lack of a placebo control, and the small overall sample size limit the interpretability of our electrocardiographic results, we did not observe any notable electrocardiographic changes associated with treatment with oral glycopyrrolate solution. Moreover, to our knowledge, no association has been reported between glycopyrrolate treatment and torsade de pointes or QT prolongation.
We found that the percentage of patients with profuse, severe, or moderate drooling was markedly reduced after 24 weeks of treatment with oral glycopyrrolate solution. Improvements in the extent of drooling were also observed using the visual analog scale assessment. Most parents/ caregivers and investigators rated oral glycopyrrolate solution as a worthwhile treatment.
Results of a randomized, placebo-controlled efficacy trialCitation16 and this study led to approval by the US Food and Drug Administration in July 2010 of oral glycopyrrolate solution 1 mg/5 mL to reduce chronic severe drooling in patients aged 3–16 years with neurologic conditions associated with problem drooling (eg, cerebral palsy).Citation17 The liquid formulation provides for consistent and reliable dose adjustments based on individual patient response to treatment and permits more precise weight-based dosing than is possible with oral tablets. Once an initial dose has been selected, clinical signs can be used to titrate the dose of oral glycopyrrolate solution for each child over several weeks until control of drooling is satisfactory, with maintenance dose individualized. Oral glycopyrrolate solution is contraindicated in patients with conditions that preclude anticholinergic therapy (eg, glaucoma, paralytic ileus, unstable cardiovascular status in acute hemorrhage, severe ulcerative colitis, toxic megacolon complicating ulcerative colitis, myasthenia gravis), in those taking solid oral forms of potassium chloride, and in those with constipation or intestinal pseudo-obstruction.Citation17
Conclusion
Treatment for 24 weeks with oral glycopyrrolate solution 1 mg/5 mL for the management of pathologic drooling associated with neurologic conditions was well tolerated by children aged 3–18 years. Oral glycopyrrolate solution substantially reduced the percentage of patients with profuse, severe, or moderate drooling.
Disclosure
RSZ has served as an advisor to Shionogi Inc. H-ML, PC, and JD are employees of Shionogi Inc. The authors thank BelMed Professional Resources, New Rochelle, NY, for editorial support, with funding provided by Shionogi Inc. The study was sponsored by Shionogi Inc and ResearchPoint, a Shionogi company.
References
- EkedahlCSurgical treatment of droolingActa Otolaryngol19747732152204819034
- Van De HeyningPHMarquetJFCretenWLDrooling in children with cerebral palsyActa Otorhinolaryngol Belg19803466917057223419
- SyedaFAhsanFNunezDAQuality of life outcome analysis in patients undergoing submandibular duct repositioning surgery for sialorrhoeaJ Laryngol Otol2007121655555817078897
- HarrisSRPurdyAHDrooling and its management in cerebral palsyDev Med Child Neurol19872968078113319742
- HusseinIKershawAETahmassebiJFFayleSAThe management of drooling in children and patients with mental and physical disabilities: a literature reviewInt J Paediatr Dent1998813119558540
- ReddihoughDSReidSMPloverCEvaluation of glycopyrrolate in the treatment of chronic droolingDegenerative Neurological and Neuromuscular Disease20111137
- Robinul Injectable [package insert]Deerfield, ILBaxter Healthcare Corporation31999
- American Society of Health-System PharmacistsGlycopyrrolateMcEvoyGKAFHS Drug Information 2006Bethesda, MDAmerican Society of Health-System Pharmacists200612721273
- OlsenAKSjøgrenPOral glycopyrrolate alleviates drooling in a patient with tongue cancerJ Pain Symptom Manage199918430030210534970
- RautakorpiPMannerTAli-MelkkilaTKailaTOlkkolaKKantoJPharmacokinetics and oral bioavailability of glycopyrrolate in childrenPharmacol Toxicol19988331321349783332
- BlascoPAStansburyJCGlycopyrrolate treatment of chronic droolingArch Pediatr Adolesc Med199615099329358790123
- SternLMPreliminary study of glycopyrrolate in the management of droolingJ Paediatr Child Health199733152549069045
- BachrachSJWalterRSTrzcinskiKUse of glycopyrrolate and other anticholinergic medications for sialorrhea in children with cerebral palsyClin Pediatr (Phila)19983784854909729704
- MierRJBachrachSJLakinRCBarkerTChildsJMoranMTreatment of sialorrhea with glycopyrrolate: a double-blind, dose- ranging studyArch Pediatr Adolesc Med2000154121214121811115305
- RautakorpiPAli-MelkkilaTKailaTPharmacokinetics of glycopyrrolate in childrenJ Clin Anesth1994632172208060629
- ZellerRSLeeHMCavanaughPDavidsonJRandomized phase III evaluation of the efficacy and safety of a novel glycopyrrolate oral solution for the management of chronic severe drooling in children with cerebral palsy or other neurologic conditionsTher Clin Risk Manage2011in press
- Cuvposa™ [package insert]Atlanta, GAShionogi Pharma Inc2010