Abstract
Vildagliptin is a selective and potent dipeptidyl peptidase-4 inhibitor that improves glycemic control by inhibiting the degradation of both endogenous glucagon-like peptide-1 and glucose-dependent insulinotropic peptide. This article is a comprehensive review of the safety and efficacy of vildagliptin in patients with type 2 diabetes. Clinical evidence has proven that it effectively decreases hemoglobin A1c with a low risk of hypoglycemia and is weight neutral. The addition of vildagliptin to metformin improves glucose control and significantly reduces gastrointestinal adverse events, particularly in patients inadequately controlled with metformin monotherapy. Its long-term advantages include preservation of β-cell function, reduction in total cholesterol, decrease in fasting lipolysis in adipose tissue, and triglyceride storage in non-fat tissues. Vildagliptin is well tolerated with a low incidence of AEs, and it does not increase the risk of cardiovascular/cerebrovascular (CCV) events. It can be taken before or after meals, and has little drug interaction, thus it will be well accepted.
Introduction
The prevalence of type 2 diabetes mellitus (T2DM) among adults aged 20–79 years in 2010 was approximately 285 million worldwide. It is estimated that this figure will increase to 430 million by the year 2030.Citation1 Many antidiabetic agents are available, including sulfonylureas (SUs), metformin, α-glycosidase inhibitors, thiazolidinediones (TZDs), prandial glucose regulators, insulin, and so on.
Recently, a new therapeutic approach for the treatment of type 2 diabetes that targets the incretin hormones has been developed. These peptide hormones, glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP), are released from the intestine after a meal and stimulate insulin secretion in a glucose-dependent fashion.Citation2 However, their action is limited by rapid inactivation by the enzyme dipeptidyl peptidase (DPP)-4. In addition, patients with T2DM usually do not respond well to GIP and GLP-1.Citation3,Citation4 Inhibition of DPP-4 will increase active incretins; therefore, DPP-4 has become a target in diabetes control.Citation5–Citation7 To date, several DPP-4 inhibitors are available, including sitagliptin, vildagliptin, saxagliptin, and linagliptin. Their pharmacokinetics/pharmacodynamics, efficacy, safety, and tolerability have been assessed in numerous clinical studies.Citation8
Recently, the American Diabetes Association and the European Association for the Study of Diabetes published a joint position statement on the management of hyperglycemia in type 2 diabetes.Citation9
Key points of this statement include:
Glycemic targets and glucose-lowering therapies must be individualized.
Diet, exercise, and education remain the foundation for any type 2 diabetes treatment program.
Unless there are prevalent contraindications, metformin is the optimal first-line drug.
After metformin, there are limited data to guide us. Combination therapy with additional 1–2 oral or injectable agents is reasonable, aiming to minimize side effects where possible.
Ultimately, many patients may require insulin therapy alone or in combination with other agents to maintain glucose control.
All treatment decisions, where possible, should be made in conjunction with the patient, focusing on his/her preferences, needs, and values.
According to this position statement, in most patients, it is important to begin with lifestyle changes; metformin monotherapy is added at, or soon after, diagnosis (unless there are explicit contraindications). If the hemoglobin A1c (HbA1c) target is not achieved after about 3 months, consider one of the five treatment options combined with metformin: a SU, TZD, DPP-4 inhibitor, GLP-1 receptor agonist, or basal insulin. Some studies have shown advantages of adding a third noninsulin agent to a two-drug combination that is not yet or no longer achieving the glycemic target. If combination therapy that includes basal insulin has failed to achieve the HbA1c target after 3–6 months, proceed to a more complex insulin strategy, usually in combination with one or two noninsulin agents. In patients intolerant of, or with contraindications for, metformin, select initial drugs from other classes (such as SUs/glinide, pioglitazone, or a DPP-4 inhibitor; in occasional cases where weight loss is seen as an essential aspect of therapy, initial treatment with a GLP-1 receptor agonist might be helpful), and proceed accordingly.
Vildagliptin is a selective and potent DPP-4 inhibitor that inhibits rapid degradation of endogenous GLP-1 and GIP, and increases α- and β-cell responsiveness to glucose, thereby improving glycemic control in T2DM.Citation10 Having a strong binding ability to DPP-4 and a long half-life, vildagliptin is more potent than other DPP-4 inhibitors such as sitagliptin in suppressing glucagon, and causes less glycemic variation.Citation8
This paper provides the efficacy, safety, and acceptability of vildagliptin in treating patients with type 2 diabetes.
Short-term efficacy
It is well established that hyperglycemia, expressed as elevated HbA1c, is associated with the risk of microvascular and macrovascular complications.Citation11 Reduced rates of microvascular complications in well controlled type 2 diabetic patients were proven by prospective randomized trials.Citation12,Citation13 Thus the American Diabetes Association’s “Standards of Medical Care in Diabetes” recommends lowering HbA1c to 7.0% in most patients.Citation14 Noninsulin agents differ in potency and effective dosages, with a varied expected HbA1c improvement of 0.5% to 1.5%.Citation15 However, some patients failed to reach their HbA1c target due to hypoglycemia. In China, insulin secretagogues are the first choice of oral antidiabetic drugs (OADs) (70.2%), which may account for the high incidence of hypoglycemia (odds ratio 1.76, 95% CI 1.20 to 2.57) and poor glycemic control.Citation16
DPP-4 inhibitors were said to have an intermediate glucose-lowering effect.Citation17 A systematic review of numerous clinical trials had profiled the efficacy and safety of incretin-based therapy. Trials were included in that study if they: (1) were randomized controlled trials of >12 weeks’ duration; (2) had >10 patients with type 2 diabetes per treatment arm; (3) reported changes in hemoglobin HbA1c as the primary endpoint; and (4) studied the effects of adding a single drug (not multiple therapies) in a representative population. That study showed that vildagliptin (50 mg once a day (qd) or twice a day (bid)) is able to reduce HbA1c by 0.98% (−1.46% to −0.52%) after adjusting for differences in baseline HbA1c.Citation18 Plenty of evidence has supported that vildagliptin consistently and effectively improves glucose control in patients with mild to moderate hyperglycemia as a monotherapy or as an addition to other antidiabetic agents.
Monotherapy
The efficacy of vildagliptin has been confirmed in a number of controlled trials.Citation19–Citation29 Most trials were designed to examine the noninferiority of vildagliptin to the comparator.Citation26–Citation29 However, the comparison with other active OADs not only validated its efficacy, but also revealed that it is weight-neutral and has low risk of hypoglycemia.
In a 2-year study of 546 patients, Foley and SreenanCitation26 compared vildagliptin (50 mg bid) with a SU (gliclazide up to 320 mg/day). Similar reductions in HbA1c were found (−0.5% versus −0.6%), but vildagliptin was weight-neutral and posed little risk of hypoglycemia to patients.
Schweizer et alCitation29 reported a study, in which 780 patients (mean baseline HbA1c, 8.7%) were randomized to accept vildagliptin (50 mg bid) or metformin (2 g daily). After 2 years of treatment, a significant HbA1c reduction was noticed both in the vildagliptin (−1.0%) and metformin (−1.4%) groups. However, the metformin group had a twofold higher incidence of gastrointestinal adverse events (AEs).
In another 24-week randomized trial of 786 patients (baseline HbA1c 8.7%), vildagliptin (50 mg bid) was compared to rosiglitazone (8 mg qd). Vildagliptin was noninferior to rosiglitazone in reducing HbA1c (−1.1% versus −1.3%).Citation28 It is worth noting that vildagliptin induced a weight loss of 0.3 kg, whereas rosiglitazone caused a weight gain of 1.6 kg (P < 0.001). In addition, patients treated with vildagliptin had a lower risk of peripheral edema and a better lipid profile.
We had conducted a head-to-head study in which 661 Chinese patients (HbA1c 7.5%–11.0%, mean 8.6%) were randomized to vildagliptin 50 mg bid (n = 441) or acarbose up to 100 mg three times a day (n = 220) for 24 weeks.Citation27 The adjusted mean HbA1c change (from baseline to the end-point) was −1.4% and −1.3% in the vildagliptin group and acarbose group, respectively, meeting the statistical criterion for noninferiority. Body weight did not change in the vildagliptin group, but it did decrease in the acarbose group.
According to a pooled analysis of data from five clinical trials with 1569 patients, 24 weeks of vildagliptin monotherapy induced a mean change in HbA1c of −1.0%. According to baseline HbA1c 8.0%, 8.0%–9.0%, 9.0%–10.0% and 10.0%, HbA1c decreased 0.6%, 0.9%, 1.6%, and 1.9% from baseline respectively.Citation30 Similarly, HbA1c reduction was more substantial in the more obese patients. In addition, the response to vildagliptin in older patients was similar to younger ones (−1.2% versus −1.0%, respectively, P = 0.092). Moreover, the rate of hypoglycemia was low (0.8%).
Combination therapy
Vildagliptin has been assessed in randomized, double-blind trials as an add-on therapy to metformin, SU, thiazolidinedione, and insulin treatment.
Vildagliptin as an add-on therapy to metformin
Vildagliptin can work well with metformin. First, vildagliptin improves islet function by increasing the sensitivity of the α- and β-cells to glucose,Citation25 whereas metformin reduces hepatic glucose and improves insulin resistance.Citation31 It is rational to combine an agent primarily targeting insulin sensitivity within the pancreas, like vildagliptin, with an agent primarily targeting insulin resistance, like metformin. Metformin is the optimal first-line drug for patients who failed to achieve their target with lifestyle interventions alone. Secondly, metformin also has a positive effect on promoting endogenous intact GLP-1 levels,Citation32 presumably by increasing its synthesis but not inhibiting degradation.Citation33,Citation34 Subjects taking vildagliptin with metformin caused about two times elevations in fasting plasma GLP-1 than in taking vildagliptin alone,Citation35,Citation36 The following section presents some clinical evidence supporting vildagliptin as an add-on therapy to metformin in patients inadequately controlled with metformin monotherapy.
Vildagliptin versus placebo as an add-on therapy to metformin
A 24-week study was performed in 544 patients who failed to achieve glucose control (HbA1c 7.5%–11%) with metformin (≥1500 mg/day) alone. The patients were randomized to receive an addition of vildagliptin 50 mg/day (n = 177), or an addition of vildagliptin 100 mg/day (n = 185), or metformin/placebo (n = 182). Compared with the metformin/placebo arm, a change in HbA1c from baseline to the study endpoint was −0.7% and −1.1% in patients receiving 50 mg or 100 mg of vildagliptin daily, respectively; likewise, the between-treatment difference (vildagliptin – placebo) in fasting plasma glucose was −0.8 mmol/L and −1.7 mmol/L in the vildagliptin 50 mg or 100 mg groups, respectively. Body weight did not change significantly from baseline after 24 weeks of treatment with vildagliptin 50 mg/day (−0.4 kg) or vildagliptin 100 mg/day (+0.2 kg), while in patients receiving metformin/placebo, body weight decreased by −1.0 kg (P < 0.001). There were no significant differences between the three groups in terms of AEs, except for the experience of gastrointestinal AEs. Gastrointestinal AEs were reported by 9.6%, 14.8%, and 18.2% of patients receiving 50 mg vildagliptin daily, 100 mg vildagliptin daily, or placebo, respectively (P = 0.022 versus placebo). One patient in each treatment group experienced one mild hypoglycemic event.Citation37
In another randomized control trial, T2DM patients inadequately controlled with metformin 1500 mg/day (7% ≤ HbA1c ≤ 7% to 11%, mean 8.14% to 8.16%) were assigned to the 1500 mg of metformin and 50 mg of vildagliptin bid group (n = 132), and the metformin 2500 mg group (n = 125). After 24 weeks of treatment, HbA1c decreased by 1.25% in the vildagliptin/metformin group and 0.9% in the metformin group (P < 0.0001). AEs and gastrointestinal AEs were significantly less in the vildagliptin/metformin group (3.8%, 3.0%) than those in metformin arm (10.7%, 9.2%).Citation38
Vildagliptin versus SUs as an add-on therapy to metformin
A total of 3118 T2DM patients who failed to achieve their HbA1c target (HbA1c 6.5% to 8.5%, mean 7.3%) after metformin monotherapy were randomized into either metformin/vildagliptin (n = 1562) or metformin/glimepiride treatment groups (n = 1556). The dosage of vildagliptin was 50 mg bid, while up to 6 mg/day of glimepiride was prescribed in the control arm. After 2 years of treatment, the adjusted mean change in HbA1c was similar between the two groups: −0.1% (0.0%) and −0.1% (0.0%), respectively. Although the initial response rate was similar between the two groups, more patients with vildagliptin reached their target HbA1c without hypoglycemia (36.0% versus 28.8%, P = 0.004). Hypoglycemic events that occurred in the metformin/glimepiride group were 14-fold as high as those in metformin/vildagliptin group (838 versus 59). Body weight change was −0.3 kg in the vildagliptin group versus 1.2 kg in glimepiride group (P < 0.001). Both treatments were well tolerated and displayed similar safety profiles.Citation39
Vildagliptin versus TZDs as an add-on therapy to metformin
A 52-week, randomized, active-controlled study compared vildagliptin (50 mg bid, n = 295) with pioglitazone (30 mg qd, n = 281) in patients inadequately controlled (HbA1c 7.5% to 11%) with a stable dose of metformin (≥1500 mg). Vildagliptin was found to be noninferior to pioglitazone in lowering HbA1c throughout the trial period. In addition, vildagliptin did not significantly increase body weight (+0.2 kg), whereas pioglitazone induced a weight gain of 2.6 kg. Hypoglycemia occurred rarely in both groups. There were no significant differences in the overall AE rates between the two groups; however, serious AEs occurred more frequently in the pioglitazone group.Citation40,Citation41
Another 12-week randomized open-label study compared vildagliptin (100 mg, n = 1653) with TZD (agent and dose at the investigators’ discretion, n = 825) as an add-on therapy in patients inadequately controlled (HbA1c 7% to 10%) on a stable dose of metformin (≥1000 mg/day). The mean change in HbA1c from baseline to the study endpoint was −0.68% in the vildagliptin group and −0.57% in the TZD group, meeting the noninferiority criteria. Body weight increased in the TZD group (0.33 kg) and decreased in the vildagliptin group (−0.58 kg, P < 0.001). Adverse events occurred in similar proportions of patients in both groups (vildagliptin 39.5% and TZD 36.3%), and hypoglycemia was rare.Citation42
Vildagliptin as an add-on therapy to SU
A 24-week, multicenter, randomized, double-blinded, placebo-controlled study assessed the effect of vildagliptin (50 mg qd or bid) versus placebo added to glimepiride (4 mg qd) in 515 T2DM patients. Compared with placebo, a change in HbA1c from baseline to the study’s endpoint was −0.6% in patients receiving vildagliptin 50 mg/day, and −0.7% in those receiving vildagliptin 100 mg/day. Greater efficacy was seen in patients ≥ 65 years of age (−0.7% for 50 mg/day, −0.8% for 100 mg/day), and in patients with HbA1c levels > 9.0% (−1.0% for 50 mg/day, −0.9% for 100 mg/day). The incidences of AEs (67.1%, 66.3%, and 64.2%) and serious AEs (2.9%, 2.4%, and 5.1%) were similar in the three groups. The incidence was low but slightly higher in the vildagliptin 100 mg group (3.6%) than in both the vildagliptin 50 mg group (1.2%) and the control arm (0.6%). The results showed that in T2DM patients inadequately controlled with SU monotherapy, the addition of vildagliptin to glimepiride improves glycemic control and is well tolerated. The addition of vildagliptin 50 mg/day to a SU may be a particularly attractive therapy in elderly patients.Citation43
Vildagliptin as an add-on therapy to other antidiabetic drugs
Vildagliptin had been added to other antidiabetic drugs, such as pioglitazone,Citation44 and insulin in patients inadequately controlled with monotherapy.Citation45–Citation47 The reduction in HbA1c varied from 0.5% to 1.0% with different dosages of vildagliptin. Vildagliptin posed a low risk of hypoglycemia when combined with pioglitazone or insulin,Citation44,Citation46,Citation47 and the weight gain was more apparent in the vildagliptin/insulin group.Citation46
Long-term benefit of vildagliptin
Vildagliptin showed long-term benefit for type 2 diabetes by preserving β-cell function and normalizing the lipid profile.
Effect of vildagliptin on β-cell function
In animal studies, vildagliptin was observed to preserve β-cell function by increasing β-cell mass,Citation48 stimulating β-cell replication,Citation49 or reducing apoptosis.Citation50 The β-cell mass increase may partly be attributed to the developmental regulation and suppression of oxidation and endoplasmic reticulum stress.Citation51
Studies of vildagliptin in patients with type 2 diabetes demonstrated improvements in meal-test derived markers of β-cell function.Citation21,Citation25,Citation28,Citation37,Citation44,Citation52–Citation54 The homeostatic model assessment-β was increased while the proinsulin to insulin ratio was decreased by vildagliptin.Citation53 Insulin secretion [defined as the ratio of the incremental area under the curve (ΔAUC) for C-peptide to the ΔAUC for glucose during standard meal tests] increased by >30% from 12 weeks throughout 1 year of treatment.Citation54 Some trials calculated the insulin secretory rate (ISR) by deconvolution of C-peptide levels, and used the ΔAUC for ISR/ΔAUC for glucose (ISR/G) as a β-cell function index. This index was consistently found to be increased in patients receiving vildagliptin in monotherapy,Citation53 or as an add-on to glimepiride,Citation43 a thiazolidinedione,Citation44 or metformin.Citation55 Further, given a single dose of vildagliptin before the evening meal, insulin secretion relative to glucose (ISR/G) increased significantly overnight.Citation52 There is evidence that has shown that vildagliptin increased β-cell function (assessed as ISR/G) in different populations, including those with IFG,Citation57 impaired glucose tolerance,Citation56 or T2DM, and mild hyperglycemia.Citation20 Clinical trials have also demonstrated that the insulin demand was brought down by adding vildagliptin, which achieved a negative caloric balance.Citation45,Citation46
Effect of vildagliptin on plasma lipids
The lipid profile is an important determinant of cardiovascular risk in type 2 diabetes.Citation58,Citation59 It is conceivable that glucose-lowering agents have a positive impact on the lipid profile due to the close relationship between glucose and lipid metabolism. A meta-analysis including five trials showed that vildagliptin significantly reduced the total cholesterol level (the difference in means, −0.42 mmol/L, 95% CI −0.59 to −0.25 mmol/L, P < 0.001),Citation60 but the results of other lipid parameters are inconsistent across trials (see ).Citation19,Citation28,Citation29,Citation41,Citation61
Table 1 Comparison of effect of vildagliptin with other antidiabetic agents on blood lipids
Some researchers have addressed the underlying mechanism of the change in lipid profiles after vildagliptin therapy. In animal studies, both GLP-1 and GIP inhibit lipolysis, indicating that the effect of vildagliptin is incretin hormone-mediated. In humans, vildagliptin was found to reduce fasting lipolysis, and increase postprandial lipolysis in adipose tissue as well as postprandial fat oxidation in muscle. These results indicated that the fat accumulated in adipocytes during fasting, and became mobilized and burned in muscle after meals.Citation62 Further, decreasing fasting lipolysis over 6 weeks is predicted to decrease stored triglycerides in nonfat tissues.Citation63 In summary, in addition to preserving β-cell function, vildagliptin improves insulin resistance by reducing glucose-toxicity, decreasing fasting lipolysis in fat cells, and reducing stored triglycerides in muscle, the liver, and pancreas.
Effects of vildagliptin on surrogate markers of atherosclerosis
In an animal study, 17-week-old ApoE (−/−) mice fed an atherogenic diet were administered a DPP-4 inhibitor, vildagliptin analogue (PKF275-055 [PKF], 100 μg/kg/day), over a period of 4 weeks. Aortic atherosclerosis and oxidized low-density lipoprotein-induced foam cell formation were determined. PKF increased the plasma levels of active glucagon-like peptide-1 by 3.5-fold, increased the total glucose-dependent insulinotropic polypeptide levels by twofold, reduced body weight by 13%, and reduced plasma cholesterol levels by 30%. Compared with drinking water controls, PKF significantly suppressed total aortic atherosclerotic lesions, atheromatous plaques in the aortic root, and macrophage accumulation in the aortic wall by 30% to 40% (P < 0.001). Foam cell formation was suppressed by 40%.Citation64
In another animal study, 14 pigs were randomized to receive either a DPP-4 inhibitor (vildagliptin; 50 mg) or normal saline intravenously prior to a 90-minute left anterior descending artery occlusion, followed by a 120-minute reperfusion period. The hemodynamic, cardiac, electrophysiological, and arrhythmic parameters, as well as the infarct size were determined before and during interventional radiology. During interventional radiology, the DPP-4 inhibitor stabilized the cardiac electrophysiology by preventing the event-related potential shortening, decreasing the number of preventricular contractions, increasing the ventricular fibrillation threshold, and decreasing the infarct size.Citation65
In a 12-week animal study, male Wistar rats were fed with either a normal diet or a high-fat diet (n = 24 in each group). Rats in each group were divided into three subgroups to receive the vehicle, metformin (30 mg/kg, bid), or vildagliptin (3 mg/kg, qd) for another 21 days. Heart rate variability, cardiac function, and cardiac mitochondrial function were determined and compared among these treatment groups. Rats exposed to a high-fat diet developed increased body weight, visceral fat, plasma insulin, cholesterol, oxidative stress, depressed heart rate variability, and cardiac mitochondrial dysfunction. It was found that vildagliptin was more effective in preventing cardiac sympathovagal imbalance and cardiac dysfunction, as well as cardiac mitochondrial dysfunction, than metformin in rats with insulin resistance induced by a high-fat diet.Citation66
Matsui et alCitation67 investigated whether vildagliptin inhibits vascular damage in the thoracic aorta of Otsuka Long–Evans Tokushima Fatty rats (OLETF rats), an animal model of type 2 diabetes with obesity, by blocking the advanced glycation end product–receptor for advanced glycation end product (AGE–RAGE) axis. OLETF and control Long–Evans Tokushima Otsuka (LETO) rats at 22 weeks old were given a vehicle or 3 mg/kg of vildagliptin for another 12 weeks. Vildagliptin treatment significantly inhibited levels of AGE, RAGE, mRNA, protein, an oxidative stress marker, 8-hydroxydeoxyguanosine, two membrane components of nicotinamide adenine dinucleotide phosphate oxidase, p22, and gp91phox mRNAs, as well as phospho-NF-κB p65. Vildagliptin also significantly reduced both mRNA and protein levels of monocyte chemoattractant protein-1, vascular cell adhesion molecule-1, and plasminogen activator inhibitor-1. All these data suggest that vildagliptin could play a protective role against vascular injury in diabetes partly by attenuating the deleterious effects of the AGE–RAGE-oxidative stress axis.
Safety
The most frequent AEs of vildagliptin were gastrointestinal AEs, hypoglycemia, headache, nasopharyngitis, influenza, cough, edema, and dizziness. Although the absolute occurrence was low, the incidence of AEs while taking vildagliptin varied in different trials when compared with placebo.Citation20,Citation21,Citation68–Citation70 However, the incidence of gastrointestinal AEs, as well as the total AEs were significantly lower with vildagliptin compared with metformin,Citation55,Citation71 and α-glycosidase inhibitors.Citation27,Citation72 Dizziness and upper respiratory infections were more common in the vildagliptin group compared with TZDs.Citation28,Citation61 A meta-analysis summarized the AEs of vildagliptin.Citation73 In that analysis, vildagliptin was not related to an increase in overall risk for any AEs when compared with placebo (relative risk (RR), 0.97; 95% CI 0.94 to 0.99); the incidence of hypoglycemia was low with vildagliptin, and the risk was similar to the comparators (RR 0.85, 95% CI 0.49 to 1.47). The use of vildagliptin did not pose any additional risks of infection for nasopharyngitis (RR 1.03, 95% CI 0.94 to 1.13) or for upper respiratory tract infections (RR 1.07, 95% CI 0.90 to 1.27).
Risk of hypoglycemia
There is emerging concern about the increased risk of brain dysfunction in patients with repeated episodes of hypoglycemia, which may also lead to dysrhythmias, dizziness, confusion, or infection, which can be especially dangerous in the elderly.Citation74 Further, the frequency of both minor and major hypoglycemia was threefold higher in intensively managed patients than the frequency associated with conventional therapy in the ACCORD trial,Citation75 indicating a tight control target is more likely to cause hypoglycemia events. Accordingly, in at-risk individuals, drug selection should favor agents that do not precipitate such events and, in general, blood glucose targets may need to be moderated.Citation9
Clinical evidence has shown that vildagliptin caused fewer hypoglycemic events than insulin secretagogues, no matter of whether it was used as a monotherapy or combined with other agents. Twenty-nine studies with almost 15,000 participants showed that the risk of hypoglycemia occurs at the placebo level for all gliptins in the absence of insulin or SU cotherapy.Citation88 Moreover, ten trials with over 4700 participants indicated that as part of a combination therapy with an SU or insulin, the risk of hypoglycemia is elevated with sitagliptin and or linagliptin, but vildagliptin and saxagliptin appear to be safer agents to use in this context.Citation76 Of course, further studies are needed to clarify these differing hypoglycemia potentials.
The distinctive pharmacological characteristics of vildagliptin could explain its advantage. The mechanism of action of vildagliptin is quite different from that of SUs, which work by closing adenosine triphosphate-sensitive potassium channels on β-cells and stimulates insulin release.Citation77 SUs increase insulin secretion independent of plasma glucose levels over time,Citation78 decrease the sensitivity of the α- and β-cells to glucose,Citation79 and exacerbate islet dysfunction after long-term use.Citation80 On the contrary, active endogenous GLP-1 and GIP concentrations are increased two- to threefold by vildagliptin, lowering blood sugar in a glucose-dependent way after a meal, which is anticipated to have a low risk of hypoglycemia. Furthermore, vildagliptin not only suppresses inappropriate glucagon secretion in response to glucose or mixed meals, but also enhances α-cell responsiveness to the stimulatory effect of hypoglycemia.Citation25 When hypoglycemia occurs, glucagon levels were significantly higher in the vildagliptin group than in the placebo group (see ),Citation25 possibly due to the attenuation or abstentions of inhibition of glucagon secretion by GLP-1, and maximized stimulation of glucagon secretion by GIP.Citation81 Vildagliptin also extends the surge in GLP-1 and GIP after meals to intermeal and overnight periods,Citation52,Citation82 which may protect the body from hypoglycemia (see ). Taken together, the bifunctional effect on glucagon by vildagliptin can well explain the low risk of hypoglycemia caused by vildagliptin when glycemic control is achieved.
Figure 1 Effect of vildagliptin on α-cell function in hyperglycemia (A) and hypoglycemia (B).
Adapted with permission from Ahrén B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab. 2004;89(5):2078–2084.24 ©2009, The Endocrine Society.
Abbreviation: AUC, area under the curve.
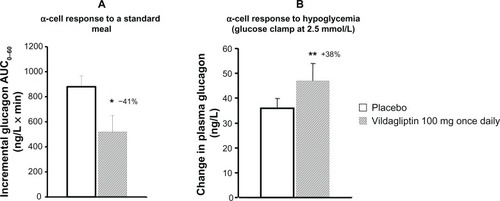
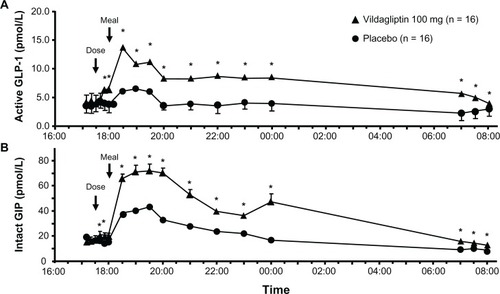
Figure 2 Instant effect of vildagliptin 100 mg/day versus placebo on glucose fluctuations.
Adapted with permission from Balas B, Baig MR, Watson C, et al. The dipeptidyl peptidase IV inhibitor vildagliptin suppresses endogenous glucose production and enhances islet function after single-dose administration in type 2 diabetic patients. J Clin Endocrinol Metab. 2007;92(4):1249–1255.Citation52 © 2007, The Endocrine Society.
Abbreviations: GLP-1, glucagon-like peptide-1; GIP, gastric inhibitory polypeptide.
Effect of vildagliptin on body weight
In overweight or obese patients with type 2 diabetes, successful weight loss can improve glycemic control and reduce concomitant cardiovascular risk factors, such as hypertension and dyslipidemia.Citation83 The effect of antidiabetic drugs on weight is considered to be an important issue in the selection of therapy. GLP-1 receptor agonists and metformin were associated with weight loss, whereas insulin, insulin secretagogues (eg, SUs and TZDs) were all associated with weight gain.Citation14
Clinical trials have demonstrated that vildagliptin has a neutral effect on weight, which is partly explained by its low risk for hypoglycemia. Hypoglycemia is more common in patients treated with weight-gain insulin or SUs. In addition, the experience of hypoglycemia was often followed by “defensive eating,” leading to an excess of caloric intake.Citation12 Vildagliptin can prevent defensive eating by decreasing the risk of hypoglycemia.
It has also been found that vildagliptin significantly lowered chylomicron lipid and apolipoprotein levels, suggesting that it may inhibit intestinal fat extraction.Citation63 Another trial demonstrated that vildagliptin can increase norepinephrine levels and promote lipolysis through sympathetic stimulation, in conjunction with the postprandial fatty acid mobilization and oxidation.Citation62 These new potential roles of vildagliptin may also contribute to its weight-neutral effect.
Cardiovascular and cerebrovascular safety
Cardiovascular and cerebrovascular (CCV) events are comorbidities of T2DM, and the major cause of death in such settings.Citation84,Citation85 To reduce CCV incidence is always the treatment target of type 2 diabetes. Unexpectedly, however, some antidiabetic agents are associated with increased risk of CV AEs.Citation86,Citation87 Thus, the evaluation of CCV risk of therapy is very important. A systematic review pooled studies of patients treated with vildagliptin and analyzed its CCV safety.Citation88 A total of 25 Phase III studies of vildagliptin (n = 7509), used either as monotherapy or as part of combination therapy (placebo or comparators, n = 6061), with durations of 12 weeks to ≥2 years were included. Outcomes included the composite endpoints of acute coronary syndrome, transient ischemic attack (with imaging evidence of infarction), stroke, and CCV death. Compared with all comparators, the RRs for the composite endpoint were <1 for both vildagliptin 50 mg qd (RR 0.88, 95% CI 0.37 to 2.11) and vildagliptin 50 mg bid (RR 0.84, 95% CI 0.62 to 1.14), which were also consistent across subgroups defined by age, gender, and CV risk status. As a conclusion, vildagliptin did not increase the risk of adjudicated CCV events relative to all comparators in the broad population of type 2 diabetics, including those at increased risk of CCV events.
Safety of vildagliptin in patients with chronic kidney disease
In a 24-week study of 515 patients with T2DM and moderate or severe renal impairment (RI), vildagliptin was added to ongoing antidiabetic therapy. After 24 weeks, the between-treatment difference in the adjusted mean change in HbA1c was −0.5% ± 0.1% (P < 0.0001) in moderate RI (baseline HbA1c = 7.9%), and −0.6% ± 0.1% (P < 0.0001) in severe RI (baseline HbA1c = 7.7%). In patients with moderate RI, similar proportions of those receiving vildagliptin or placebo experienced any AE (68% versus 73%, respectively), any serious AE (9% versus 9%, respectively), any AE leading to discontinuation (3% versus 5%, respectively), or death (1% versus 1%, respectively). This was also true for patients with severe RI: AEs (73% versus 74%, respectively), serious AEs (19% versus 21%, respectively), AEs leading to discontinuation (9% versus 6%, respectively), and death (2% versus 4%, respectively). Vildagliptin added to ongoing antidiabetic therapy had a safety profile similar to placebo. Furthermore, relative to placebo, vildagliptin elicited a satisfactory and clinically significant decrease in HbA1c in patients with moderate or severe RI.Citation89
Patients’ acceptability
Diabetes, including T2DM is usually a lifelong (chronic) disease. One study has shown that patients’ poor adherence to therapy is a great obstacle in achieving their glycemic control target.Citation90 Therefore, the patients’ acceptability of antidiabetic agents is as important as their efficacy and safety. Unlike some SUs, meglitinides (which should be given before a meal), and alpha-glucosidase inhibitors (which should be taken just prior to ingesting the first portion of each meal), vildagliptin can be ingested at any time of the day. Unlike insulin, which requires an injection, vildagliptin is an oral glucose-lowering agent. In addition, it has little drug interaction and it can be combined with other OADs. Vildagliptin is currently approved in the European Union for use in combination with metformin and TZD at 50 mg bid, with SU at 50 mg qd, or prepared in a fixed-dose and combined with metformin (50 mg/500 mg, 50 mg/850 mg, and 50 mg/1000 mg). All of these advantages of vildagliptin promote patients’ adherence for long-term treatment.
Conclusion
Vildagliptin is a potent DPP-4 inhibitor. When used alone or added to other OADs, it effectively improves glycemic control, preserves both the α- and β-cell function, and reduces lipotoxicity and insulin resistance. This drug is well tolerated and is weight-neutral. It poses a low risk of AEs to patients, including hypoglycemia, as well as CCV events. Vildagliptin can be taken before or after a meal, and has little drug interaction; thus, it will be well accepted.
Disclosure
The authors report no conflicts of interest in this work.
References
- ShawJESicreeRAZimmetPZGlobal estimates of the prevalence of diabetes for 2010 and 2030Diabetes Res Clin Pract201087141419896746
- HolzGG4thKühtreiberWMHabenerJFPancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1 (7–37)Nature199336164103623658381211
- FreemanJSThe pathophysiologic role of incretinsJ Am Osteopath Assoc2007107SupplS6S917724014
- NauckMABallerBMeierJJGastric inhibitory polypeptide and glucagon-like peptide-1 in the pathogenesis of type 2 diabetesDiabetes200453Suppl 3S190S19615561910
- AhrénBFoleyJEThe islet enhancer vildagliptin: mechanisms of improved glucose metabolismInt J Clin Pract Suppl200815981418269436
- BanerjeeMYounisNSoranHVildagliptin in clinical practice: a review of literatureExpert Opin Pharmacother200910162745275719874253
- PalalauAITahraniAAPiyaMKBarnettAHDPP-4 inhibitors in clinical practicePostgrad Med200912167010019940419
- ThornberryNAGallwitzBMechanism of action of inhibitors of dipeptidyl-peptidase-4 (DPP-4)Best Pract Res Clin Endocrinol Metab200923447948619748065
- InzucchiSEBergenstalRMBuseJBDiamantMfor American Diabetes Association (ADA)European Association for the Study of Diabetes (EASD)Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD)Diabetes Care20123561364137922517736
- KeatingGMVildagliptin: a review of its use in type 2 diabetes mellitusDrugs201070162089211220964454
- StrattonIMAdlerAINeilHAAssociation of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational studyBMJ2000321725840541210938048
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) GroupLancet199835291318378539742976
- UK Prospective Diabetes Study (UKPDS)VIII. Study design, progress and performanceDiabetologia199134128778901778353
- American Diabetes AssociationStandards of medical care in diabetes – 2011Diabetes Care201134Suppl 1S11S6121193625
- BennettWLMaruthurNMSinghSComparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinationsAnn Intern Med2011154960261321403054
- JiLLJWengJChina Type 2 Diabetes Treatment Status Survey of Treatment Pattern of Oral Drugs Users (China DiaSTAGE)BerlinThe 48th European Association for the Study of Diabetes2012
- PetersAIncretin-based therapies: review of current clinical trial dataAm J Med2010123Suppl 3S28S3720206729
- ArodaVRHenryRRHanJHuangWEfficacy of GLP-1 Receptor Agonists and DPP-4 Inhibitors: Meta-Analysis and Systematic ReviewClin Ther201263461247125822608780
- DejagerSRazacSFoleyJESchweizerAVildagliptin in drug-naïve patients with type 2 diabetes: a 24-week, double-blind, randomized, placebo-controlled, multiple-dose studyHorm Metab Res200739321822317373638
- ScherbaumWASchweizerAMariAEvidence that vildagliptin attenuates deterioration of glycaemic control during 2-year treatment of patients with type 2 diabetes and mild hyperglycaemiaDiabetes Obes Metab200810111114112418355325
- ScherbaumWASchweizerAMariAEfficacy and tolerability of vildagliptin in drug-naïve patients with type 2 diabetes and mild hyperglycaemia*Diabetes Obes Metab200810867568218248490
- FoleyJEBunckMCMöller-GoedeDLBeta cell function following 1 year vildagliptin or placebo treatment and after 12 week washout in drug-naive patients with type 2 diabetes and mild hyperglycaemia: a randomised controlled trialDiabetologia20115481985199121547496
- Pi-SunyerFXSchweizerAMillsDDejagerSEfficacy and tolerability of vildagliptin monotherapy in drug-naïve patients with type 2 diabetesDiabetes Res Clin Pract200776113213817223217
- AhrénBLandin-OlssonMJanssonPASvenssonMHolmesDSchweizerAInhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetesJ Clin Endocrinol Metab20048952078208415126524
- AhrénBSchweizerADejagerSVildagliptin enhances islet responsiveness to both hyper- and hypoglycemia in patients with type 2 diabetesJ Clin Endocrinol Metab20099441236124319174497
- FoleyJESreenanSEfficacy and safety comparison between the DPP-4 inhibitor vildagliptin and the sulfonylurea gliclazide after two years of monotherapy in drug-naïve patients with type 2 diabetesHorm Metab Res2009411290590919705345
- PanCYangWBaronaJPComparison of vildagliptin and acarbose monotherapy in patients with Type 2 diabetes: a 24-week, double-blind, randomized trialDiabet Med200825443544118341596
- RosenstockJBaronMADejagerSMillsDSchweizerAComparison of vildagliptin and rosiglitazone monotherapy in patients with type 2 diabetes: a 24-week, double-blind, randomized trialDiabetes Care200730221722317259484
- SchweizerACouturierAFoleyJEDejagerSComparison between vildagliptin and metformin to sustain reductions in HbA(1c) over 1 year in drug-naive patients with Type 2 diabetesDiabet Med200724995596117509069
- PratleyRERosenstockJPi-SunyerFXManagement of type 2 diabetes in treatment-naive elderly patients: benefits and risks of vildagliptin monotherapyDiabetes Care200730123017302217878242
- KirpichnikovDMcFarlaneSISowersJRMetformin: an updateAnn Intern Med20021371253312093242
- MigoyaEMMJLarsonPJTanenMRSitagliptin, a selective DPP-4 inhibitor, and metformin have complementary effects to increase43rd EASD Annual MeetingAmsterdam2007
- HinkeSAKühn-WacheKHoffmannTPedersonRAMcIntoshCHDemuthHUMetformin effects on dipeptidylpeptidase IV degradation of glucagon-like peptide-1Biochem Biophys Res Commun200229151302130811883961
- YasudaNInoueTNagakuraTEnhanced secretion of glucagon-like peptide 1 by biguanide compoundsBiochem Biophys Res Commun2002298577978412419322
- D’AlessioDADenneyAMHermillerLMTreatment with the dipeptidyl peptidase-4 inhibitor vildagliptin improves fasting islet-cell function in subjects with type 2 diabetesJ Clin Endocrinol Metab2009941818818957505
- DunningBELigueros-SaylanMD’AlessioDADifferential effects of DPP-4 inhibition on incretin hormone levels in drug-naïve and metformin-treated patients with type 2 diabetesDiabetologia200649S1S110
- BosiECamisascaRPColloberCRochotteEGarberAJEffects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metforminDiabetes Care200730489089517277036
- KimNHSSungYAAhnWCEfficacy and safety of add-on vildagliptin to metformin in comparison to uptitrating metformin therapyDiabetes201261S1A297
- MatthewsDRDejagerSAhrenBVildagliptin add-on to metformin produces similar efficacy and reduced hypoglycaemic risk compared with glimepiride, with no weight gain: results from a 2-year studyDiabetes Obes Metab201012978078920649630
- BolliGDottaFRochotteECohenSEEfficacy and tolerability of vildagliptin vs pioglitazone when added to metformin: a 24-week, randomized, double-blind studyDiabetes Obes Metab2008101829018034842
- BolliGDottaFColinLMinicBGoodmanMComparison of vildagliptin and pioglitazone in patients with type 2 diabetes inadequately controlled with metforminDiabetes Obes Metab200911658959519515179
- BlondeLDagogo-JackSBanerjiMAComparison of vildagliptin and thiazolidinedione as add-on therapy in patients inadequately controlled with metformin: results of the GALIANT trial – a primary care, type 2 diabetes studyDiabetes Obes Metab2009111097898619614942
- GarberAJSchweizerABaronMARochotteEDejagerSVildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled studyDiabetes Obes Metab20079216617417300592
- GarberAJFoleyJEBanerjiMAEffects of vildagliptin on glucose control in patients with type 2 diabetes inadequately controlled with a sulphonylureaDiabetes Obes Metab200810111047105618284434
- FonsecaVBaronMShaoQDejagerSSustained efficacy and reduced hypoglycemia during one year of treatment with vildagliptin added to insulin in patients with type 2 diabetes mellitusHorm Metab Res200840642743018401832
- FonsecaVSchweizerAAlbrechtDBaronMAChangIDejagerSAddition of vildagliptin to insulin improves glycaemic control in type 2 diabetesDiabetologia20075061148115517387446
- LukashevichVKozlovskiPFoleyJVildagliptin combined with insulin reduces HbA1c without increasing risk of hypoglycemia and weight gain in patients with type 2 diabetes mellitusProceedings from the 72nd ADA Scientific SessionsPhiladelphia, PA, USA2012
- DuttaroyAVoelkerFMerriamKThe DPP-4 inhibitor vildagliptin increases pancreatic beta cell mass in neonatal ratsEur J Pharmacol20116502–370370721070766
- AkarteASSrinivasanBPGandhiSVildagliptin selectively ameliorates GLP-1, GLUT4, SREBP-1c mRNA levels and stimulates β-Cell proliferation resulting in improved glucose homeostasis in rats with streptozotocin-induced diabetesJ Diabetes Complications201226426627422626875
- SatoKNakamuraAShirakawaJImpact of the dipeptidyl peptidase-4 inhibitor vildagliptin on glucose tolerance and β-cell function and mass in insulin receptor substrate-2-knockout mice fed a high-fat dietEndocrinology201215331093110222315446
- HamamotoSKandaYShimodaMVildagliptin preserves the mass and function of pancreatic β cells via the developmental regulation and suppression of oxidative and endoplasmic reticulum stress in a mouse model of diabetesDiabetes Obes Metab201215215316322950702
- BalasBBaigMRWatsonCThe dipeptidyl peptidase IV inhibitor vildagliptin suppresses endogenous glucose production and enhances islet function after single-dose administration in type 2 diabetic patientsJ Clin Endocrinol Metab20079241249125517244786
- PratleyRESchweizerARosenstockJRobust improvements in fasting and prandial measures of beta-cell function with vildagliptin in drug-naïve patients: analysis of pooled vildagliptin monotherapy databaseDiabetes Obes Metab2008101093193818093207
- AhrénBPaciniGFoleyJESchweizerAImproved meal-related beta-cell function and insulin sensitivity by the dipeptidyl peptidase-IV inhibitor vildagliptin in metformin-treated patients with type 2 diabetes over 1 yearDiabetes Care20052881936194016043735
- BosiEDottaFJiaYGoodmanMVildagliptin plus metformin combination therapy provides superior glycaemic control to individual monotherapy in treatment-naive patients with type 2 diabetes mellitusDiabetes Obes Metab200911550651519320662
- UtzschneiderKMTongJMontgomeryBThe dipeptidyl peptidase-4 inhibitor vildagliptin improves beta-cell function and insulin sensitivity in subjects with impaired fasting glucoseDiabetes Care200831110811317909087
- RosenstockJFoleyJERendellMEffects of the dipeptidyl peptidase-IV inhibitor vildagliptin on incretin hormones, islet function, and postprandial glycemia in subjects with impaired glucose toleranceDiabetes Care2008311303517947341
- AvogaroAGiordaCMagginiMfor Diabetes and Informatics Study GroupAssociation of Clinical DiabetologistsIstituto Superiore di SanitàIncidence of coronary heart disease in type 2 diabetic men and women: impact of microvascular complications, treatment, and geographic locationDiabetes Care20073051241124717290034
- GaedePLund-AndersenHParvingHHPedersenOEffect of a multifactorial intervention on mortality in type 2 diabetesN Engl J Med2008358658059118256393
- MonamiMLamannaCDesideriCMMannucciEDPP-4 inhibitors and lipids: systematic review and meta-analysisAdv Ther2012291142522215383
- RosenstockJKimSWBaronMAEfficacy and tolerability of initial combination therapy with vildagliptin and pioglitazone compared with component monotherapy in patients with type 2 diabetesDiabetes Obes Metab20079217518517300593
- BoschmannMEngeliSDobbersteinKDipeptidyl-peptidase-IV inhibition augments postprandial lipid mobilization and oxidation in type 2 diabetic patientsJ Clin Endocrinol Metab200994384685219088168
- AhrénBSchweizerADejagerSVillhauerEBDunningBEFoleyJEMechanisms of action of the dipeptidyl peptidase-4 inhibitor vildagliptin in humansDiabetes Obes Metab201113977578321507182
- TerasakiMNagashimaMWatanabeTEffects of PKF275-055, a dipeptidyl peptidase-4 inhibitor, on the development of atherosclerotic lesions in apolipoprotein E-null miceMetabolism201261797497722225957
- ChindaKPaleeSSurinkaewSPhornphutkulMChattipakornSChattipakornNCardioprotective effect of dipeptidyl peptidase-4 inhibitor during ischemia-reperfusion injuryInt J Cardiol Epub January 26, 2012.
- ApaijaiNPintanaHChattipakornSCChattipakornNCardioprotective effects of metformin and vildagliptin in adult rats with insulin resistance induced by a high-fat dietEndocrinology201215383878388522621958
- MatsuiTNishinoYTakeuchiMYamagishiSVildagliptin blocks vascular injury in thoracic aorta of diabetic rats by suppressing advanced glycation end product-receptor axisPharmacol Res201163538338821320599
- PratleyREJauffret-KamelSGalbreathEHolmesDTwelve-week monotherapy with the DPP-4 inhibitor vildagliptin improves glycemic control in subjects with type 2 diabetesHorm Metab Res200638642342816823726
- KikuchiMAbeNKatoMTeraoSMimoriNTachibanaHVildagliptin dose-dependently improves glycemic control in Japanese patients with type 2 diabetes mellitusDiabetes Res Clin Pract200983223324019118913
- MonamiMCremascoFLamannaCMarchionniNMannucciEPredictors of response to dipeptidyl peptidase-4 inhibitors: evidence from randomized clinical trialsDiabetes Metab Res Rev201127436237221309062
- GökeBHershonKKerrDEfficacy and safety of vildagliptin monotherapy during 2-year treatment of drug-naïve patients with type 2 diabetes: comparison with metforminHorm Metab Res2008401289289518726829
- IwamotoYKashiwagiAYamadaNEfficacy and safety of vildagliptin and voglibose in Japanese patients with type 2 diabetes: a 12-week, randomized, double-blind, active-controlled studyDiabetes Obes Metab201012870070820590747
- CaiLCaiYLuZJZhangYLiuPThe efficacy and safety of vildagliptin in patients with type 2 diabetes: a meta-analysis of randomized clinical trialsJ Clin Pharm Ther201237438639822191695
- BerlieHDGarwoodCLDiabetes medications related to an increased risk of falls and fall-related morbidity in the elderlyAnn Pharmacother201044471271720215495
- GersteinHCMillerMEGenuthSfor ACCORD Study GroupLong-term effects of intensive glucose lowering on cardiovascular outcomesN Engl J Med2011364981882821366473
- GooßenKGräberSLonger term safety of dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes mellitus: systematic review and meta-analysisDiabetes, Obesity and Metabolism2012141210611072
- BryanJCraneAVila-CarrilesWHBabenkoAPAguilar-BryanLInsulin secretagogues, sulfonylurea receptors and K(ATP) channelsCurr Pharm Des200511212699271616101450
- WardWKBeardJCHalterJBPfeiferMAPorteDJrPathophysiology of insulin secretion in non-insulin-dependent diabetes mellitusDiabetes Care1984754915026094129
- TanMHBaksiAKrahulecBfor GLAL Study GroupComparison of pioglitazone and gliclazide in sustaining glycemic control over 2 years in patients with type 2 diabetesDiabetes Care200528354455015735185
- KahnSEHaffnerSMHeiseMAfor ADOPT Study GroupGlycemic durability of rosiglitazone, metformin, or glyburide monotherapyN Engl J Med2006355232427244317145742
- ChristensenMVedtofteLHolstJJVilsbøllTKnopFKGlucose-dependent insulinotropic polypeptide: a bifunctional glucose-dependent regulator of glucagon and insulin secretion in humansDiabetes201160123103310921984584
- MarfellaRBarbieriMGrellaRRizzoMRNicolettiGFPaolissoGEffects of vildagliptin twice daily vs sitagliptin once daily on 24-hour acute glucose fluctuationsJ Diabetes Complications2010242798319261490
- NathanDMBuseJBDavidsonMBfor American Diabetes AssociationEuropean Association for Study of DiabetesMedical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of DiabetesDiabetes Care200932119320318945920
- BeckerABosGde VegtFCardiovascular events in type 2 diabetes: comparison with nondiabetic individuals without and with prior cardiovascular disease. 10-year follow-up of the Hoorn StudyEur Heart J200324151406141312909069
- LeeCDFolsomARPankowJSBrancatiFLfor Atherosclerosis Risk in Communities (ARIC) Study InvestigatorsCardiovascular events in diabetic and nondiabetic adults with or without history of myocardial infarctionCirculation2004109785586014757692
- NissenSEWolskiKEffect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causesN Engl J Med2007356242457247117517853
- KendallDMRubinCJMohideenPImprovement of glycemic control, triglycerides, and HDL cholesterol levels with muraglitazar, a dual (alpha/gamma) peroxisome proliferator-activated receptor activator, in patients with type 2 diabetes inadequately controlled with metformin monotherapy: A double-blind, randomized, pioglitazone-comparative studyDiabetes Care20062951016102316644631
- SchweizerADejagerSFoleyJECouturierALigueros-SaylanMKothnyWAssessing the cardio-cerebrovascular safety of vildagliptin: meta-analysis of adjudicated events from a large Phase III type 2 diabetes populationDiabetes Obes Metab201012648549420518804
- LukashevichVSchweizerAShaoQGroopPHKothnyWSafety and efficacy of vildagliptin versus placebo in patients with type 2 diabetes and moderate or severe renal impairment: a prospective 24-week randomized placebo-controlled trialDiabetes Obes Metab2011131094795121733061
- SchillingerDGrumbachKPietteJAssociation of health literacy with diabetes outcomesJAMA2002288447548212132978