Abstract
In January 2012, glucarpidase (Voraxaze®) received approval from the US Food and Drug Administration for intravenous treatment of toxic plasma methotrexate concentrations due to impaired renal clearance. Methotrexate, an antifolate agent, has been used for over 60 years in the treatment of various cancers. High-dose methotrexate has been particularly useful in the treatment of leukemias and lymphomas. However, even with aggressive hydration and urine alkalinization, such regimens can lead to acute renal dysfunction, as indicated by decreases in urine production and concomitant increases in blood urea nitrogen and serum creatinine levels. Because methotrexate is largely excreted by the kidneys, this can greatly potentiate tissue damage. Toxic levels of blood methotrexate can be rapidly and effectively decreased by intravenous administration of glucarpidase. Glucarpidase is a recombinant form of carboxypeptidase G2, a bacterial enzyme that rapidly cleaves methotrexate to form the amino acid glutamate and 2,4-diamino-N10-methylpteroic acid. Catabolites of methotrexate are much less toxic than the parent compound, and are primarily excreted by hepatic mechanisms. Glucarpidase has been available on a compassionate basis since the 1990s, and a variety of case reports and larger clinical trials have demonstrated the safety and efficacy of this drug in patients ranging in age from infants to the elderly and in a variety of races and ethnic groups. Glucarpidase should not be administered within 2 hours of leucovorin, because this agent is a reduced folate which competes with methotrexate for the enzyme and glucarpidase inactivates leucovorin. Side effects of glucarpidase are rare and relatively mild, and include paraesthesia, flushing, nausea, vomiting, pruritus, and headache. Glucarpidase has seen limited use in intrathecal treatment of methotrexate toxicity for which it is also effective. Future applications of this enzyme in chemotherapy continue to be an active area of research.
Brief history of folate, methotrexate, and chemotherapy
An understanding of glucarpidase depends on some knowledge of folic acid and methotrexate. In the earlier half of the 20th century, scientists were working out the dietary requirements for human health. During this time, it was recognized that vitamins were small molecules required in the diet, and that deficiencies resulted in specific syndromes.Citation1,Citation2 Folic acid, or vitamin B9, is a water-soluble vitamin which functions within cells in reduced forms, primarily as tetrahydrofolates subject to polyglutamylation by the enzyme folylpolyglutamate synthetase. Intracellular tetrahydrofolates act as carriers of a wide variety of one-carbon units, and can carry formyl, methenyl, methyl, and methylene groups. Each functions in specific pathways, including biosynthesis of purines, thymidylate, and amino acid metabolism. Adequate folate nutrition is therefore necessary for normal cell growth.
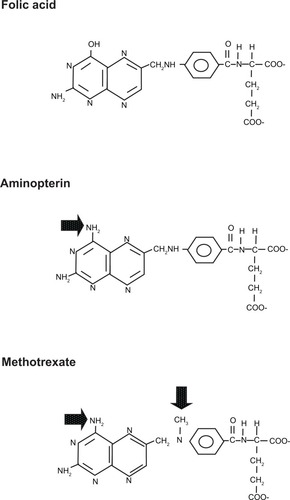
The fundamental role of folates in cell division, especially DNA synthesis, is why folic acid analogs (antifolates) such as methotrexate (amethopterin) and aminopterin, were explored as chemotherapeutic agents.Citation3 In 1948, aminopterin was first successfully used to treat acute lymphocytic leukemia in children.Citation4 Though remissions proved to be temporary, this study provided hope for this line of chemotherapy research. Aminopterin varies from folate by an amino group, while methotrexate also possesses an N-methyl group (). This likely explains why subsequent studies showed methotrexate to have the more favorable therapeutic index. Methotrexate is taken up into cells via the same systems as reduced folates.Citation5 During cellular metabolism, it is acted upon by folylpolyglutamate synthetase, which adds a polyglutamate tail.Citation6 This tail both increases cellular retention and alters bioactivity.Citation3 The classical target of methotrexate is dihydrofolate reductase, but methotrexate and its cellular metabolites accumulate within the cell and interfere with other reactions, including thymidylate synthase, further contributing to its action.Citation6,Citation7 Methotrexate is currently used to treat a variety of cancers, including bladder cancer, breast cancer, head and neck cancers, choriocarcinoma, acute lymphocytic leukemia, non-Hodgkin’s lymphoma, lung cancer, osteosarcoma, meningeal leukemia and carcinomatous meningitis, gestational trophoblastic carcinomas, and colorectal cancer.Citation8,Citation9 Methotrexate is administered via the oral, intravenous, intra-arterial, and intrathecal routes. Side effects include myelosuppression (especially neutropenia), alopecia, nausea, vomiting, mucositis, renal damage, and occasionally portal fibrosis and cirrhosis. While common (and relatively minor) side effects of intrathecal methotrexate include headache, fever, vomiting, meningismus, erythematous rash, and pleocytosis of the cerebral spinal fluid, more severe toxicities can include paralysis, cranial nerve palsies, seizures, coma, demyelinating encephalopathy, and death.Citation8,Citation9
Figure 1 Structures of folic acid, aminopterin and methotrexate.
Because of the variety of pathologies for which methotrexate is prescribed, there is no standard dosing regimen.Citation9 Methotrexate is used as a single low-dose oral medication for treatment of psoriasis and rheumatoid arthritis, though the mechanism of action is not understood and may not be related to inhibition of dihydrofolate reductase.Citation10 Methotrexate is used in higher doses for acute lymphocytic leukemia and for central nervous system lymphomas.Citation9 For other cancers, it is often used in combination with other medications. Intrathecal use of methotrexate may be as a single agent or in combination.
Renal toxicity is primarily associated with high-dose therapy, probably because the primary route of excretion for methotrexate and its metabolites is the kidneys. High-dose methotrexate therapy typically involves doses of 1–12 g/m2, with the duration of infusion varying from 0.3 to 24 hours.Citation11 Side effects vary depending on both the dose and schedule. Renal damage is thought to occur either by precipitation of methotrexate and its breakdown products in the renal tubules, or from a direct toxic effect of methotrexate.Citation12,Citation13 The solubility of methotrexate increases with pH, so urine alkalinization and intravenous hydration are necessary treatments accompanying high-dose methotrexate.Citation9 Patients should be screened for adequate hepatic, renal, and bone marrow function; urinary pH should be greater than 7.0, and urinary output should be greater than 100 mL per hour prior to administration of methotrexate. These are typically achieved and maintained with supplemental sodium bicarbonate and 2.5–3.5 L/m2/day of intravenous fluids.Citation11
Some medications may increase the possibility of adverse drug reactions when coadministered with methotrexate, and may precipitate a need for glucarpidase treatment.Citation8,Citation14 The most common pharmacokinetic interaction observed clinically involves coadministration with one of the benzimidazole proton pump inhibitors; these compounds are associated with delayed elimination of methotrexate, and therefore an increased incidence of toxic levels of the antifolate.Citation15
History of carboxypeptidase 2 and related enzymes: purification, characterization, and cloning
A variety of bacterial enzymes have been identified which can remove the carboxyl glutamate from folate and its analogs (). It was observed as early as 1955 that certain bacteria had the capacity to inactivate folate analogs such as methotrexate, presumably via enzymatic cleavage.Citation16 Enzymes with the ability to cleave folate and its derivatives have largely been isolated from soil organisms. In 1967, Levy and Goldman published several studies in which they purified and characterized an enzyme from a Pseudomonas strain which could liberate glutamate from methotrexate (Km 200 μM), aminopterin, and folate.Citation17 At that time, the authors viewed the potential significance of the enzyme as a clinically useful assay for methotrexate levels in clinical samples.Citation17,Citation18 The following year, Pratt et al partially purified and characterized a similar enzyme from a Flavobacterium strain, which they termed folate amidase.Citation19 Their study was motivated by the need to isolate large amounts of pteroic acid, which at the time was a limiting reagent for development of new folate antagonist chemotherapies. In 1971, carboxypeptidase G1 was isolated from Pseudomonas stutzeri,Citation20 and compared with the enzymes of earlier studies, this enzyme was more purified and showed more favorable kinetic properties. Naturally reduced folates (including 5-methyl-tetrahydrofolate and 5-formyl-tetrahydrofolate, also known as leucovorin and citrovorum factor, respectively) were also excellent substrates, but with Km values in the 10–20 μM range. These authors recognized the potential value of the enzyme, not only in preparing pteroic acid, but also as a potential antineoplastic agent, due to its ability to cleave both reduced and oxidized folates. In 1978, Albrecht et al partially characterized a carboxypeptidase from a Flavobacterium species.Citation21
Table 1 Enzymes that cleave glutamate from methotrexate and folate. A low value for Km corresponds to an increased affinity for the substrate. The increasing ratio of (Km CH3THF/Km methotrexate) correlates with the increasing affinity an enzyme shows for methotrexate over CH3THF
In 1983, the gene for carboxypeptidase G2 was cloned from Pseudomonas, first in Escherichia coli and subsequently in Pseudomonas putida;Citation22 expression in the latter was 30-fold higher than in E. coli. This protein possessed properties which were different from the earlier characterized carboxypeptidases, hence the distinct name. Carboxypeptidase G2 requires divalent zinc cations and is composed of two 42 kDa subunits. The complete nucleotide sequence of the gene was subsequently determined.Citation23 Optimized expression enabled enhanced purification yielding homogeneous protein.Citation24 These studies demonstrated that among this family of enzymes, carboxypeptidase G2 displayed the best ability to differentiate between methotrexate and 5-methyl tetrahydrofolate (CH3THF), the circulating form of folate, and thereby paved the way for clinical use of recombinant carboxypeptidase G2. Since carboxypeptidase G2 has found clinical application, the crystal structure of the enzyme has been analyzed as well, enabling the potential for designing enzymes with variable functionality.Citation25
The physiological role of carboxypeptidase enzymes within the bacterial cell remains unknown. They may function in a folate catabolic or a salvage pathway similar to that proposed for p-aminobenzoyl-glutamate hydrolase, a manganese-dependent enzyme isolated from E. coli which hydrolyzes p-aminobenzoyl-glutamate, the folate catabolite, to form p-aminobenzoic acid and glutamate.Citation26–Citation28 However, folate is a very poor substrate for this enzyme.
Mechanism of action of glucarpidase
As an enzyme, glucarpidase rapidly converts methotrexate to the amino acid glutamate and 2,4-diamino-N10-methylpteroic acid (DAMPA, see ).Citation24 Using Rhesus monkeys, Widemann et al studied the pharmacokinetics and metabolism of DAMPA.Citation29 DAMPA was infused over 15 minutes into the monkeys; after reaching a serum concentration of about 50 μM, DAMPA was eliminated rapidly, falling to less than 1 μM within 1–2 hours. About 50% of the DAMPA was excreted in urine and about 50% was metabolized by the liver into hydroxyl-DAMPA, DAMPA-glucuronide, and hydroxy-DAMPA-glucuronide. These latter compounds were identified in both the blood and urine of the monkeys, and also in similar samples from patients treated for methotrexate toxicity with carboxypeptidase G2. Using a human leukemic cell line, they found that DAMPA was not toxic in itself, nor did it alter the cytotoxicity of methotrexate. They concluded that conversion of methotrexate to DAMPA relieves stress on the kidneys for elimination of methotrexate and generates a relatively nontoxic compound.
Figure 2 The reaction catalyzed by glucarpidase (CPG2).
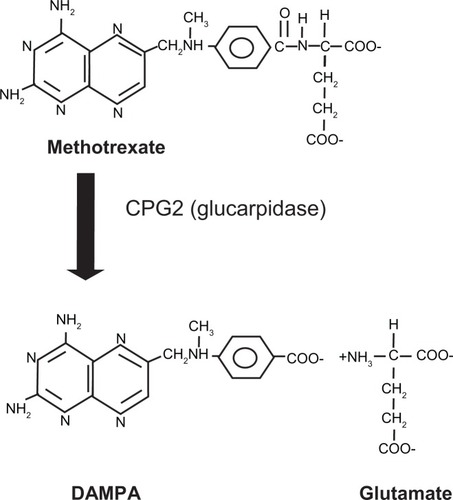
Successful cleavage of methotrexate by glucarpidase results in elevations of glutamate and DAMPA in the bloodstream. Because methotrexate levels are frequently monitored during patient treatment, one should note that DAMPA can interfere with such monitoring. Methotrexate can be measured using a variety of tests, including dihydrofolate reductase assays, an antibody-based immunoassay, and a high-pressure liquid chromatography method.Citation30 However, the antibody-based methods cross-react with the breakdown products of methotrexate, and are not a reliable measure of methotrexate levels in samples from patients after treatment with glucarpidase.
Animal studies of carboxypeptidase G2 for methotrexate toxicity
There are only two animal studies in the literature concerning use of carboxypeptidase G2 for treatment of methotrexate overdose. The first study, in 1991, involved use of adult Rhesus monkeys to investigate intrathecal carboxypeptidase G2 for treatment of intrathecal overdose of methotrexate.Citation31 Using three groups (with three monkeys per group), they first determined the pharmacokinetics in cerebrospinal fluid. One group received methotrexate alone (5 mg, equivalent to 50 mg for humans), another group received methotrexate followed by carboxypeptidase G2 (30 U) five minutes later, and another group received carboxypeptidase G2 alone. In the first group of monkeys, methotrexate reached a concentration of about 3000 μM in the cerebrospinal fluid, with an area under the curve of about 4300 μM × hour. In the group treated with methotrexate and carboxypeptidase G2, cerebrospinal fluid levels of methotrexate decreased by greater than 400-fold within 5 minutes of carboxypeptidase G2 administration; the area under the curve decreased by 80-fold to about 51 μM × hour. Elevations in mononuclear cells were observed in the cerebrospinal fluid of animals receiving carboxypeptidase G2 alone, but no symptoms were apparent. In the second phase of experiments, monkeys were subjected to a toxic intrathecal methotrexate dose and were then rescued using carboxypeptidase G2. Two groups of three monkeys each were either treated with 25 mg or 50 mg of intrathecal methotrexate, followed immediately by 150 U or 300 U, respectively, of intrathecal carboxypeptidase G2. These methotrexate doses were equivalent to 250 mg or 500 mg in humans. While one monkey (receiving the 25 mg dose) experienced a seizure, all six monkeys, regardless of dose, were successfully rescued by carboxypeptidase G2, which reduced the methotrexate levels in all animals by about 1000-fold. However, all animals also demonstrated an asymptomatic elevation of mononucleocytes in cerebrospinal fluid. The data from this study show that carboxypeptidase G2 is promising as a rescue agent for intrathecal methotrexate toxicity.
Follow-up studies in monkeys were performed to investigate the pharmacokinetics of carboxypeptidase G2 as an alternative to leucovorin for “rescue” treatment of toxic overdoses in serum, and as a secondary goal, they investigated the effects of anti-carboxypeptidase G2 antibodies on the effectiveness of carboxypeptidase G2.Citation32 Leucovorin (5-formyltetrahydrofolate) is a form of reduced folate. Like methotrexate (and unlike carboxypeptidase G2) it is taken up and metabolized by cells. While leucovorin administration was commonly used as a rescue agent, it was unclear whether tumor cells were rescued as well as nontumor cells. To investigate the possible superiority of carboxypeptidase G2 as a rescue agent, two groups of monkeys (three in each group) were studied. One group was given a loading dose followed by a continuous 18-hour intravenous infusion of methotrexate designed to maintain a steady-state plasma concentration of approximately 10 μM. At the end of the infusion, carboxypeptidase G2 50 U/kg was administered. Three control animals were treated similarly with methotrexate, but with saline substituted for the carboxypeptidase G2. The results showed that the average concentration of methotrexate during infusion was maintained at 11 μM. Treatment with carboxypeptidase G2 diminished plasma methotrexate levels by over 100-fold within 15 minutes, and nontoxic levels of methotrexate were achieved within 30 minutes of administration of carboxypeptidase G2. The post-infusion area under the curve for the methotrexate-infused animals treated or untreated with carboxypeptidase G2 was 301 μM and 20 μM, respectively. With regard to the secondary goal, monkeys were treated with repeated doses of carboxypeptidase G2 over time to generate antibodies. It was hypothesized that because carboxypeptidase G2 is a protein, it would stimulate synthesis of antibodies, which might bind to and diminish the effectiveness of the enzyme. Using animals confirmed to have high titers of anticarboxypeptidase G2 antibodies, the researchers administered intravenous methotrexate followed by carboxypeptidase G2 rescue. This demonstrated that even in the presence of antibodies, carboxypeptidase G2 still significantly reduced plasma methotrexate concentrations, although the effect was less dramatic than that observed in antibody-negative animals. The authors concluded that carboxypeptidase G2 was a very promising treatment for methotrexate toxicity, with the potential to supplant leucovorin.
While the above two studies are the only ones involving animals and carboxypeptidase G2, there are earlier studies describing successful use of carboxypeptidase G1 to reduce plasma methotrexate levels, which provided the foundation for later work. In 1972, Chabner et al demonstrated that carboxypeptidase G1 successfully reduced plasma methotrexate levels in mice.Citation33 As part of this study, they also performed an experiment in which mice injected with leukemia cells were subsequently treated with methotrexate, methotrexate with leucovorin rescue, or methotrexate with carboxypeptidase G1 rescue. The latter two groups showed very similar protection from methotrexate toxicity. Further studies with carboxypeptidase G1 were not performed owing to loss of the bacterial source.
Clinical studies of glucarpidase for methotrexate overdose
The need to have treatment available for methotrexate overdose results in part from the observation that individuals vary widely in their tolerance to methotrexate,Citation34 with one study of 49 cancer patients observing that the dose causing toxic effects varied between 50 and 900 mg/m2, ie, by a factor of 18.Citation35 The same study investigated the predictive power of various patient characteristics with regard to the potential for a patient to experience an adverse response to methotrexate. They studied a number of factors including history of previous chemotherapy or radiation treatment, weight loss, serum albumin levels, pretreatment blood folate levels, and age. Age was the only factor found to have a predictive value. The authors suggested that this might be due to diminished renal function, which is commonly found in the elderly patient population.
Chabner et al first suggested that enzymatic cleavage of methotrexate might be used clinically in patients suffering from antifolate toxicity back in 1972.Citation33 Because glucarpidase is used to treat dangerously high levels of methotrexate that occur due to impaired metabolism or accidental overdose, there have been no clinical studies involving a control group. However, there are a number of case studies, as well as studies involving small numbers of patients, made possible by the availability of glucarpidase on a compassionate basis for patients experiencing nephrotoxicity following high-dose methotrexate. Carboxypeptidase G2 was used for the first time in 1995 in an 18-year-old woman with osteosarcoma who had been treated with high-dose methotrexate and developed signs of hepatic and renal damage; in this patient, leucovorin rescue was associated with hypercalcemia and ventricular arrhythmia. Administration of carboxypeptidase G2 successfully decreased the patient’s methotrexate levels and she eventually recovered.Citation36 Shortly after this case, and subsequent to their very promising animal studies, Widemann et al carried out a study in 20 patients with a variety of cancers, including osteosarcoma, lymphoid cancer, and gastric cancer. Following treatment with carboxypeptidase G2 and thymidine, all patients experienced a rapid 95%–99% reduction in plasma methotrexate levels, and their serum creatinine levels returned to normal after a median of about 3 weeks.Citation37 In the ensuing decades, a number of reports have been published detailing successful carboxypeptidase G2 treatment in patients with acute renal failure stemming from use of high-dose methotrexate to treat cancer, primarily lymphomaCitation38–Citation40 and osteosarcoma.Citation40–Citation45 These studies have involved both male and female patients, ranging in age from 13 yearsCitation42,Citation46 to 79 years.Citation39 While most of these patients have been from the US or Europe and of nonspecified race or ethnicity, patients known to be Korean,Citation42 Hispanic,Citation44 and Middle EasternCitation45 have been treated successfully.
In the last ten years, several larger studies have further corroborated the promise of carboxypeptidase G2 in the treatment of methotrexate overdose on a background of renal failure (). In 2005, Buchen et al reported a Phase II study in which carboxypeptidase G2 was administered to 82 patients experiencing acute symptoms related to methotrexate overdose and concomitant renal failure.Citation47 This was a prospective, open, nonrandomized multicenter trial in patients with a median age of about 15 (range 0.9–71.8) years. Eligibility criteria included serum methotrexate levels > 10 μM at 36 hours or >5 μM at 42 hours after starting an infusion of methotrexate and documented renal failure. Patients suffered from a range of cancers, including acute lymphocytic leukemia, non-Hodgkin’s lymphoma, osteosarcoma, brain tumors, Hodgkin’s lymphoma, and pleural mesothelioma. The target carboxypeptidase G2 dose was 50 U per kg of body weight. At 15 minutes after administration of carboxypeptidase G2, the methotrexate serum level was reduced by approximately 87% and most patients survived. Despite treatment, four patients died of myelosuppression and septic complications. Because the previous study involved primarily children, Schwartz et al subsequently performed a similar study in Europe involving 43 adult and elderly cancer patients with acute lymphocytic leukemia, lymphoma, germ cell tumor, or osteosarcoma.Citation48 Patients received glucarpidase, followed by leucovorin rescue and standard care. In the 24 patients with samples available, serum methotrexate levels decreased by >97% in a median of 15 minutes after injection of carboxypeptidase G2. Ten of the 43 patients died of complications associated with high-dose methotrexate. Three patients were treated with glucarpidase twice during the same cycle of high-dose methotrexate; in two patients, the glucarpidase response was diminished, while serum methotrexate levels became undetectable in the third patient. The authors speculated that this might be due to inhibition of glucarpidase by DAMPA, or possibly as a result of an immune response to the enzyme.
Table 2 Summary of recent studies using glucarpidase for methotrexate toxicity
Widemann et al performed a retrospective study of 100 cancer patients who received glucarpidase, leucovorin, and in some cases thymidine, for treatment of renal failure induced by high-dose methotrexate ().Citation49 Patients ranged in age from 0.3 to 82 years and suffered from a range of cancers. The plasma methotrexate concentration decreased by 98% within 15 minutes of injection of glucarpidase. However, administration of thymidine did not provide any additional benefit. Of the 12 patients who died, six succumbed to irreversible methotrexate toxicity and organ damage. Multiple-regression analysis identified the presence of grade 4 toxicity before glucarpidase treatment, inadequate leucovorin dosing, and glucarpidase treatment more than 96 hours after methotrexate infusion as risk factors for grade 4 and 5 methotrexate toxicity. Interestingly, 28 patients received two glucarpidase doses 24 hours apart, and seven received three glucarpidase doses every 4 hours, and glucarpidase antibodies were not detected at 3, 7, or 14 days after treatment in any of these patients. The investigators concluded that early intervention with leucovorin and glucarpidase was highly effective in the treatment of renal failure induced by high-dose methotrexate, and that repeated use of glucarpidase was possible.
In 2011, researchers at St Jude Children’s Research Hospital undertook a retrospective study of pediatric cancer patients treated with high-dose methotrexate between 1998 and 2010, some of whom had received glucarpidase for nephrotoxicity. The goal of this study was to determine the safety of resuming treatment with methotrexate following such treatment.Citation50 Of 20 children who received glucarpidase, 13 were later treated again with methotrexate. Although one patient suffered neurotoxicity with further high-dose methotrexate, renal function returned to baseline in all patients, and all survived treatment. This study demonstrates that high-dose methotrexate treatment can be resumed after treatment with glucarpidase for methotrexate nephrotoxicity.
Widemann et al analyzed data from 492 cancer patients in the US and the European Union who were treated with glucarpidase for methotrexate toxicity between 1993 and 2009; while not yet published, this work was presented at the American Society of Clinical Oncology annual meeting in 2012.Citation51 Most (94%) of the patients had non-Hodgkin’s lymphoma, osteogenic sarcoma, or acute lymphocytic leukemia, and ranged in age from 5 weeks to 85 years, with a median age of 18 years. While 76% of these patients had one dose of glucarpidase, 22% had two doses, and 2% had three doses. Of the 156 patients for whom reliable data were available, blood methotrexate levels decreased by a median of 99% relative to the preglucarpidase baseline at 15 minutes after administration of glucarpidase. Adverse reactions included paresthesia (2%), flushing (1.8%), and headache (1%). The patients who died within 30 days of glucarpidase treatment (8%) succumbed for reasons unrelated to glucarpidase.
Pharmacokinetics of glucarpidase
In 2008, Phillips et al performed an open-label, single-site pharmacokinetic study in which 12 adult volunteers without cancer received glucarpidase.Citation52 Eight subjects had normal renal function and four had impaired renal function. Each subject received a single intravenous dose of glucarpidase 50 U/kg. Subjects with normal renal function had a mean maximum serum glucarpidase concentration of 31 μg/mL, with a mean half-life of 9.0 hours and a mean area under the serum concentration-time curve of 23.4 μg × h/mL. The data for subjects with renal impairment were similar, indicating that renal impairment has a minimal effect on the pharmacokinetics of glucarpidase in the bloodstream.
Drug interactions between glucarpidase and leucovorin
Glucarpidase should not be administered simultaneously with leucovorin, because leucovorin serves as an alternative substrate and therefore can compete with methotrexate for the enzyme. Also, leucovorin is inactivated by glucarpidase.Citation49,Citation53 While glucarpidase is effective for reducing methotrexate levels in the bloodstream, it has no effect on intracellular methotrexate; because leucovorin enters cells, it combats intracellular methotrexate. Therefore, coadministration of leucovorin and carboxypeptidase G2 diminishes the effectiveness of both medications. Leucovorin rescue is often used in chemotherapy regimens in which the antifolate is administered first, and after some period of delay, leucovorin is then given.Citation54–Citation56 The goal is to kill tumor cells, which typically grow rapidly and therefore take up more folate (and therefore also antifolate), and then “rescue” the normal host cells. This approach was first successfully demonstrated as early as 1954 in a mouse model of leukemia, in which Goldin et al showed that aminopterin followed by delayed administration of citrovorum factor (leucovorin) was more effective than aminopterin used alone.Citation57 Since that time, administration of methotrexate partnered with leucovorin rescue has become an established therapy for a variety of cancers.Citation9 It is interesting that early studies in the area of treatment for methotrexate overdose also investigated the use of carboxypeptidase G2, not as a rescue agent but as a method whereby folate levels in the bloodstream might be reduced in order to enhance the biologic effectiveness of the antifolate.Citation58
Side effects of glucarpidase
Adverse reactions to glucarpidase are rare, but include paresthesia, flushing, nausea and/or vomiting, hypotension, pruritus, fever, and headache.Citation37,Citation47,Citation59
Dosing
Glucarpidase (Voraxaze®) is administered as a single intravenous injection of 50 U/kg body weight.Citation60 It is supplied as a lyophilized powder, with 1000 U per vial. Treatment with glucarpidase is warranted in patients with plasma methotrexate concentrations greater than 1 μM and concomitant impaired renal function. Leucovorin should not be administered within 2 hours before or after a dose of glucarpidase.
Intrathecal use
Methotrexate is used intrathecally for the treatment of cancer, usually leukemia, or to prevent metastasis via the cerebrospinal fluid. However, intrathecal overdose of methotrexate can be fatal. Given the very promising data obtained in the animal studies in which monkeys administered lethal doses of methotrexate were rescued with carboxypeptidase G2,Citation31 it is perhaps not surprising that accidental intrathecal methotrexate overdose has been successfully treated with carboxypeptidase G2. The first case involved a 6-year-old boy with acute lymphocytic leukemia who received 600 mg of methotrexate rather than the intended 12 mg; despite acute neurotoxic symptoms he recovered fully after receiving 2000 U of intrathecal carboxypeptidase G2, which decreased his cerebrospinal fluid methotrexate by 150-fold.Citation61 Similarly, other patients with accidental intrathecal methotrexate overdose have been treated successfully with carboxypeptidase G2, although in several instances the patients also underwent cerebrospinal fluid exchange.Citation62 These data, although limited, suggest that carboxypeptidase G2 is a safe and effective treatment for accidental intrathecal methotrexate overdose.
Impact of pharmacogenomics
Recent advances in the burgeoning field of pharmacogenomics have resulted in greatly increased knowledge of the individual genetic alterations affecting drug metabolism, including methotrexate. Altered genes impacting methotrexate metabolism could encode for proteins involved in transport, metabolism, or function of folates and antifolates. Such differences could explain both lack of efficacy and increased toxicity. Several papers have described studies in which gene polymorphisms have been identified that impact methotrexate uptake and metabolism.Citation63,Citation64 While at this time one cannot order a battery of genetic tests to determine methotrexate dosing better, this sort of testing and personal dosing is on the horizon, and will possibly decrease the occurrence of toxic side effects (and the need for glucarpidase).
Other potential clinical applications for carboxypeptidase G2
A variety of alternative applications have been explored for bacterial carboxypeptidases. In 1972, carboxypeptidase G1 was used to deplete blood folate in a mouse leukemia model, which demonstrated that depletion of folate both successfully decreased blood folate and prolonged survival in mice.Citation65 Later studies demonstrated that simultaneous administration of carboxypeptidase G1 and nonclassical folate antagonists (which the enzyme did not cleave) enhanced the antitumor effects of the drugs.Citation66,Citation67 In 1987, carboxypeptidase G2 was covalently linked to soluble dextran and injected into mice,Citation68 and this dextran-carboxypeptidase G2 conjugate had a prolonged lifetime in the circulation. The authors proposed using such systems to target therapeutic enzymes specifically to the liver. Similarly, carboxypeptidase G2 has been used in a two-step treatment mouse model of human choriocarcinoma.Citation69–Citation72 First, carboxypeptidase G2 was conjugated with a fragment of a monoclonal antibody attached to a subunit of human chorionic gonadotrophin. This was then used in conjunction with a prodrug consisting of a toxin attached to a substrate of carboxypeptidase G2. After the modified antibody-carboxypeptidase G2 conjugate was injected and allowed to localize to the tumor, the modified toxin-prodrug was injected, and the carboxypeptidase G2 released the active drug only at the tumor sites. Similarly, carboxypeptidase G2 has been used to activate phenol mustard prodrugs.Citation73,Citation74 More recently, carboxypeptidase G2 has been used in gene-directed enzyme prodrug therapy; this two-step approach involves first introducing the gene for carboxypeptidase G2 into the tumor via a virus or liposome, then allowing sufficient time for protein expression. Subsequently, the nontoxic prodrug is administered, and is activated specifically at the tumor site by carboxypeptidase G2.Citation75,Citation76 This strategy will be used in a Phase I clinical study involving patients with head and neck cancer.Citation77 It is clear that glucarpidase or perhaps a similarly modified form of carboxypeptidase G2 may find new roles in the fight against cancer.
Acknowledgements
The expert assistance of Catherine Lencioni with internet research and reference management is gratefully acknowledged. This work was supported by funds from Midwestern University and by award number R15 GM085760 from the National Institute of General Medical Sciences.
Disclosure
The content of this paper is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
References
- HitchingsGHBurchallJJInhibition of folate biosynthesis and function as a basis for chemotherapyAdv Enzymol1965274174684387360
- CarmelRFolic acidShikeMRossACModern Nutrition in Health and Disease10th edPhiladelphia, PALippincott Williams & Wilkins2006
- BertinoJRKarnofsky memorial lecture. Ode to methotrexateJ Clin Oncol1993115148418242
- HeinleRWWelchADExperiments with pteroylglutamic acid and pteroylglutamic acid deficiency in human leukemiaJ Clin Invest19482753918935160
- MatherlyLHHouZDengYHuman reduced folate carrier: translation of basic biology to cancer etiology and therapyCancer Metastasis Rev20072611112817334909
- ChabnerBAAllegraCJCurtGAPolyglutamation of methotrexate. Is methotrexate a prodrug?J Clin Invest1985769079122413074
- RosenfeltFMethotrexate and the need for continued researchYale J Biol Med197548971031154813
- SkeelRTAntineoplastic drugs and biologic response modifiers: classification, use, and toxicity of clinically useful agentsSkeelRTHandbook of Cancer Chemotherapy7th edPhiladelphia, PALippincott Williams & Wilkins2003
- PerryMCThe Chemotherapy Source Book4th edPhiladelphia, PALippincott Williams & Wilkins2008
- KemperARVan MaterHACoeytauxRRWilliamsJWJrSandersGDSystematic review of disease-modifying antirheumatic drugs for juvenile idiopathic arthritisBMC Pediatr2012122922420649
- ChabnerBAAllegraCJAntifolatesChabnerBALongoDLCancer Chemotherapy and Biotherapy: Principles and Practice5th edPhiladelphia, PALippincott, Williams & Wilkins2010
- TreonSPChabnerBAConcepts in use of high-dose methotrexate therapyClin Chem199642132213298697606
- WidemannBCAdamsonPCUnderstanding and managing methotrexate nephrotoxicityOncologist20061169470316794248
- LevequeDSantucciRGourieuxBHerbrechtRPharmacokinetic drug-drug interactions with methotrexate in oncologyExpert Rev Clin Pharmacol2011474375022111860
- JoergerMHuitemaADvan den BongardHJDeterminants of the elimination of methotrexate and 7-hydroxy-methotrexate following high-dose infusional therapy to cancer patientsBr J Clin Pharmacol200662718016842380
- WebbMInactivation of analogues of folic acid by certain non-exacting bacteriaBiochim Biophys Acta19551721222513239661
- LevyCCGoldmanPThe enzymatic hydrolysis of methotrexate and folic acidJ Biol Chem1967242293329386027254
- GoldmanPLevyCCCarboxypeptidase G: purification and propertiesProc Natl Acad Sci U S A196758129913065237864
- PrattAGCrawfordEJFriedkinMThe hydrolysis of mono-, di-, and triglutamate derivatives of folic acid with bacterial enzymesJ Biol Chem1968243636763725726892
- McCulloughJLChabnerBABertinoJRPurification and properties of carboxypeptidase G1J Biol Chem1971246720772135129727
- AlbrechtAMBoldizsarEHutchisonDJCarboxypeptidase displaying differential velocity in hydrolysis of methotrexate, 5-methyltetrahydrofolic acid, and leucovorinJ Bacteriol197813450651326657
- MintonNPAtkinsonTSherwoodRFMolecular cloning of the Pseudomonas carboxypeptidase G2 gene and its expression in Escherichia coli and Pseudomonas putidaJ Bacteriol1983156122212276358192
- MintonNPAtkinsonTBrutonCJSherwoodRFThe complete nucleotide sequence of the Pseudomonas gene coding for carboxypeptidase G2Gene19843131386396165
- SherwoodRFMeltonRGAlwanSMHughesPPurification and properties of carboxypeptidase G2 from Pseudomonas sp. strain RS-16. Use of a novel triazine dye affinity methodEur J Biochem19851484474533838935
- RowsellSPauptitRATuckerADMeltonRGBlowOMBrickPCrystal structure of carboxypeptidase G2, a bacterial enzyme with applications in cancer therapyStructure199753373479083113
- CarterELJagerLGardnerLHallCCWillisSGreenJMEscherichia coli abg genes enable uptake and cleavage of the folate catabolite p-aminobenzoyl-glutamateJ Bacteriol20071893329333417307853
- GreenJMHollandsworthRPitstickLCarterELPurification and characterization of the folate catabolic enzyme p-aminobenzoyl-glutamate hydrolase from Escherichia coliJ Bacteriol20101922407241320190044
- HusseinMJGreenJMNicholsBPCharacterization of mutations that allow p-aminobenzoyl-glutamate utilization by Escherichia coliJ Bacteriol1998180626062689829935
- WidemannBCSungEAndersonLPharmacokinetics and metabolism of the methotrexate metabolite 2, 4-diamino-N(10)-methylpteroic acidJ Pharmacol Exp Ther200029489490110945838
- MonahanBPAllegraCJAntifolatesChabnerBALongoDLCancer Chemotherapy and Biotherapy: Principles and Practice4th edPhiladelphia, PALippincott, Williams & Wilkins2006
- AdamsonPCBalisFMMcCullyCLRescue of experimental intrathecal methotrexate overdose with carboxypeptidase-G2J Clin Oncol199196706742066764
- AdamsonPCBalisFMMcCullyCLGodwinKSPoplackDGMethotrexate pharmacokinetics following administration of recombinant carboxypeptidase-G2 in rhesus monkeysJ Clin Oncol199210135913641634927
- ChabnerBAJohnsDGBertinoJREnzymatic cleavage of methotrexate provides a method for prevention of drug toxicityNature197223939539712635300
- StollerRGHandeKRJacobsSARosenbergSAChabnerBAUse of plasma pharmacokinetics to predict and prevent methotrexate toxicityN Engl J Med1977297630634302412
- HansenHHSelawryOSHollandJFMcCallCBThe variability of individual tolerance to methotrexate in cancer patientsBr J Cancer1971252983054256007
- ZoubekAZaunschirmHALionTSuccessful carboxypeptidase G(2) rescue in delayed methotrexate elimination due to renal failurePediatr Hematol Oncol1995124714778519632
- WidemannBCBalisFMMurphyRFCarboxypeptidase-G2, thymidine, and leucovorin rescue in cancer patients with methotrexate-induced renal dysfunctionJ Clin Oncol199715212521349164227
- DeAngelisLMTongWPLinSFleisherMBertinoJRCarboxypeptidase G2 rescue after high-dose methotrexateJ Clin Oncol199614214521498683248
- KrackhardtASchwartzSKorfelAThielECarboxypeptidase G2 rescue in a 79 year-old patient with cranial lymphoma after high-dose methotrexate induced acute renal failureLeuk Lymphoma19993563163510609804
- KrauseASWeihrauchMRBodeUCarboxypeptidase-G2 rescue in cancer patients with delayed methotrexate elimination after high-dose methotrexate therapyLeuk Lymphoma2002432139214312533039
- WidemannBCHetheringtonMLMurphyRFBalisFMAdamsonPCCarboxypeptidase-G2 rescue in a patient with high dose methotrexate-induced nephrotoxicityCancer1995765215268625136
- ParkESHanKHChoiHSShinHYAhnHSCarboxypeptidase-G2 rescue in a patient with high dose methotrexate-induced nephrotoxicityCancer Res Treat20053713313519956493
- EsteveMADevictor-PierreBGalyGSevere acute toxicity associated with high-dose methotrexate (MTX) therapy: use of therapeutic drug monitoring and test-dose to guide carboxypeptidase G2 rescue and MTX continuationEur J Clin Pharmacol200763394217115148
- PeyriereHCociglioMMargueritteGVallatCBlayacJPHillaire-BuysDOptimal management of methotrexate intoxication in a child with osteosarcomaAnn Pharmacother20043842242714970366
- QudsiRAbdulhadiOSultanILow-dose carboxypeptidase-G2 for methotrexate toxicity in a childPediatr Blood Cancer2010551439144020981700
- VilayAMMuellerBAHainesHAltenJAAskenaziDJTreatment of methotrexate intoxication with various modalities of continuous extracorporeal therapy and glucarpidasePharmacotherapy20103011120030480
- BuchenSNgampoloDMeltonRGCarboxypeptidase G2 rescue in patients with methotrexate intoxication and renal failureBr J Cancer20059248048715668713
- SchwartzSBornerKMullerKGlucarpidase (carboxypeptidase g2) intervention in adult and elderly cancer patients with renal dysfunction and delayed methotrexate elimination after high-dose methotrexate therapyOncologist2007121299130818055849
- WidemannBCBalisFMKimAGlucarpidase, leucovorin, and thymidine for high-dose methotrexate-induced renal dysfunction: clinical and pharmacologic factors affecting outcomeJ Clin Oncol2010283979398620679598
- ChristensenAMPauleyJLMolinelliARResumption of high-dose methotrexate after acute kidney injury and glucarpidase use in pediatric oncology patientsCancer20121184321438022252903
- WidemannBCJayaprakashNHowardSCDaughertyCChauhanRKRushJClinical trial and compassionate use experience with glucarpidase for methotrexate toxicityJ Clin Oncol2012Suppl 306530
- PhillipsMSmithWBalanGWardSPharmacokinetics of glucarpidase in subjects with normal and impaired renal functionJ Clin Pharmacol20084827928418192538
- HempelGLinggRBoosJInteractions of carboxypeptidase G(2) with 6S-leucovorin and 6R-leucovorin in vitro: implications for the application in case of methotrexate intoxicationsCancer Chemother Pharmacol20055534735315723260
- LevittMMosherMBDeContiRCImproved therapeutic index of methotrexate with “leucovorin rescue”Cancer Res197333172917344541737
- BernardSEtienneMCFischelJLFormentoPMilanoGCritical factors for the reversal of methotrexate cytotoxicity by folinic acidBr J Cancer1991633033071997110
- CapizziRLDeContiRCMarshJCBertinoJRMethotrexate therapy of head and neck cancer: improvement in therapeutic index by the use of leucovorin “rescue”Cancer Res197030178217884248129
- GoldinAMantelNGreenhouseSWVendittiJMHumphreysSREffect of delayed administration of citrovorum factor on the antileukemic effectiveness of aminopterin in miceCancer Res195414434813126932
- BertinoJRLevittMMcCulloughJLChabnerBNew approaches to chemotherapy with folate antagonists: use of leucovorin “rescue” and enzymic folate depletionAnn N Y Acad Sci19711864864954943812
- BinscheckTAmbachLGroboschTSchwartzSGlucarpidase – a fast and efficient antidote in methotrexate poisoningClin Toxicol201048299
- Voraxaze® (glucarpidase, full prescribing information)West Conshohocken, PABTG International Inc2012
- O’MarcaighASJohnsonCMSmithsonWASuccessful treatment of intrathecal methotrexate overdose by using ventriculolumbar perfusion and intrathecal instillation of carboxypeptidase G2Mayo Clin Proc1996711611658577190
- WidemannBCBalisFMShalabiATreatment of accidental intrathecal methotrexate overdose with intrathecal carboxypeptidase G2J Natl Cancer Inst2004961557155915494606
- GervasiniGPolymorphisms in methotrexate pathways: what is clinically relevant, what is not, and what is promisingCurr Drug Metab20091054756619702537
- CastaldoPMagiSNastiAAClinical pharmacogenetics of methotrexateCurr Drug Metab20111227828621470106
- ChabnerBAChelloPLBertinoJRAntitumor activity of a folate-cleaving enzyme, carboxypeptidase G1Cancer Res197232211421195080761
- KalghatgiKKMorosonBAHorvathCBertinoJREnhancement of antitumor activity of 2,4-diamino-5-(3′,4′-dichlorophenyl)-6-methylpyrimidine and Baker’s antifol (triazinate) with carboxypeptidase G1Cancer Res19793934413445476673
- RomaniniASobreroAFChouTCSherwoodRFBertinoJREnhancement of trimetrexate cytotoxicity in vitro and in vivo by carboxypeptidase G2Cancer Res198949601960232529027
- MeltonRGWiblinCNBaskervilleAFosterRLSherwoodRFCovalent linkage of carboxypeptidase G2 to soluble dextrans-II. In vivo distribution and fate of conjugatesBiochem Pharmacol1987361131212432899
- BagshaweKDSpringerCJSearleFA cytotoxic agent can be generated selectively at cancer sitesBr J Cancer1988587007033265633
- SearleFBierCBuckleyRGThe potential of carboxypeptidase G2-antibody conjugates as anti-tumour agents. I. Preparation of antihuman chorionic gonadotrophin-carboxypeptidase G2 and cytotoxicity of the conjugate against JAR choriocarcinoma cells in vitroBr J Cancer1986533773843964540
- MeltonRGSearleFSherwoodRFBagshaweKDBodenJAThe potential of carboxypeptidase G2: antibody conjugates as anti-tumour agents. II. In vivo localising and clearance properties in a choriocarcinoma modelBr J Cancer1990614204242328209
- SenterPDActivation of prodrugs by antibody-enzyme conjugates: a new approach to cancer therapyFASEB J199041881932404820
- BlakeyDCDaviesDHDowellRIAnti-tumour effects of an antibody-carboxypeptidase G2 conjugate in combination with phenol mustard prodrugsBr J Cancer199572108310887577451
- PedleyRBSharmaSKHawkinsREChesterKAAntibody-directed enzyme-prodrug therapyMethods Mol Med20049049151414657581
- DaviesLCFriedlosFHedleyDNovel fluorinated prodrugs for activation by carboxypeptidase G2 showing good in vivo antitumor activity in gene-directed enzyme prodrug therapyJ Med Chem2005485321538816078849
- HedleyDOgilvieLSpringerCCarboxypeptidase-G2-based gene-directed enzyme-prodrug therapy: a new weapon in the GDEPT armouryNat Rev Cancer2007787087917943135
- HunterPThe fourth front against cancer. The first clinical trials to test engineered viruses that attack tumour cells have yielded promising results for future cancer therapiesEMBO Rep20111276977121799527