Abstract
Management of patients with metastatic hormone receptor-positive breast cancer poses a challenge due to the inevitable development of endocrine resistance. Hormone resistance is associated with a complex interaction of the estrogen receptor with growth factors, transmembrane receptors, and intracellular growth cascades. The PI3K/Akt/mTOR pathway plays a major role in hormone resistance and proliferation of breast cancer. Preclinical and clinical data indicate that inhibitors of human epidermal growth factor receptor-2, epidermal growth factor receptor, insulin-like growth factor-1 receptor, and the mammalian target of rapamycin pathway may act synergistically with hormone therapy to circumvent endocrine resistance. Everolimus is currently approved for combination with exemestane in postmenopausal women with advanced hormone receptor-positive breast cancer. However, we still need to unfold the full potential of targeted agents in the hormone-refractory setting and to identify the subsets of patients who will benefit from combination hormonal therapy using targeted agents.
Background
Breast cancer is the most common malignancy among women in the US, accounting for nearly one in three cancers diagnosed.Citation1 It is estimated that 226,870 women will be diagnosed and 39,510 women will die of breast cancer in 2012.Citation2 Approximately two-thirds of breast cancers are estrogen and/or progesterone receptor-positive. Hormone receptor status is determined using immunohistochemistry on paraffin-embedded tissues. The presence of at least 1% staining nuclei is required to define hormone-positive disease and predict clinical response to hormone-directed therapy.Citation3
The natural history of hormone receptor-positive breast cancer tends to be different from hormone receptor-negative disease. The presence of hormone sensitivity is usually associated with a favorable prognosis. Use of adjuvant endocrine therapy has dramatically decreased breast cancer mortality in patients with early-stage disease, and hormone therapy is the cornerstone treatment in advanced stages. However, a subset of hormone receptor-positive breast cancers do not benefit from endocrine therapy (intrinsic resistance), and all hormone receptor-positive metastatic breast cancers ultimately develop resistance to hormonal therapies (acquired resistance). Most patients who have experienced treatment failure after several hormonal agents in the metastatic setting are treated with chemotherapy, which is associated with increased toxicity.Citation4,Citation5
This review focuses on new and emerging treatments for hormone receptor-positive breast cancer and particularly on the role of inhibition of mTOR (mammalian target of rapamycin) in reversing resistance to endocrine agents.
Endocrine therapy
Tamoxifen, a selective estrogen receptor modulator, had been the standard of care for all stages of hormone receptor-positive breast cancer since its initial approval by the US Food and Drug Administration in 1986.Citation6 Aromatase inhibitors, which act by blocking the peripheral conversion of androgens to estrogen and therefore decrease levels of circulating estrogens in postmenopausal women, were approved for the treatment of metastatic breast cancer, and subsequently for early-stage cancer. The currently approved third-generation aromatase inhibitors are divided into steroidal (exemestane) and nonsteroidal (anastrozole and letrozole) agents.Citation7
A study comparing anastrozole and tamoxifen in more than 1000 patients with advanced breast cancer showed that anastrozole was superior to tamoxifen in terms of time to progression, although there was no difference in overall survival.Citation8,Citation9 BIG 1-98 was a Phase III trial of letrozole versus tamoxifen in postmenopausal women with advanced breast cancer, which demonstrated that time to progression was increased from 6 to 9.4 months in the letrozole arm. The response rate and overall clinical benefit were also increased in the letrozole arm when compared with tamoxifen.Citation10–Citation12 Exemestane was also shown to be superior to tamoxifen in terms of clinical benefit in postmenopausal patients with breast cancer.Citation13 The ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial demonstrated that aromatase inhibitors were superior to tamoxifen in the adjuvant setting. Aromatase inhibitors have thus become the preferred regimen in postmenopausal women.Citation14,Citation15
Fulvestrant, an estrogen receptor downregulator with no known agonist activity, was initially found to be equivalent to anastrozole in patients previously treated with tamoxifen.Citation16 Fulvestrant was also compared with tamoxifen in the first-line setting in women with metastatic disease and was found to have similar efficacy in patients with hormone receptor-positive tumors.Citation17 Fulvestrant was initially approved at a dose of 250 mg as a monthly intramuscular injection. Subsequent studies have examined the efficacy of different doses and schedules. CONFIRM (COmparisoN of Faslodex In Recurrent or Metastatic breast cancer) was a Phase III trial examining the difference in progression-free survival between the doses of 250 mg and 500 mg, and demonstrated that the higher dose improved the median progression-free survival, reducing the risk of progression by 20%.Citation18
Combination of hormonal agents
Fulvestrant has been evaluated in combination with anastrozole in two trials with differing results. Mehta et al recently reported the results of a study combining anastrozole and fulvestrant in the metastatic setting.Citation19 The authors hypothesized that the combination would be more effective than anastrozole alone in patients with hormone receptor-positive metastatic breast cancer. The trial randomized postmenopausal women with previously untreated metastatic disease to anastrozole alone or anastrozole plus fulvestrant. Fulvestrant was administered intramuscularly at a dose of 500 mg on day 1, 250 mg on days 14 and 28, and monthly thereafter. The median progression-free survival was 13.5 months in the anastrozole alone arm and 15.0 months in the combination arm (hazards ratio 0.80; P = 0.007). The combination therapy was generally more effective than anastrozole alone in all subgroups, with no significant interactions. Overall survival was also improved in the combination arm compared with anastrozole alone (median 47.7 versus 41.3 months, respectively). In this study, 41% of patients in the anastrozole arm crossed over to fulvestrant after progression. The study concluded that the combination of anastrozole and fulvestrant was more effective and better tolerated than anastrozole alone. It is notable that this study enrolled hormone-naïve patients who, judging from the outcomes seen in the anastrozole alone arm, included a large percentage of hormone-sensitive patients. The results of this study are in contrast with those of FACT (Fulvestrant and Anastrozole in Combination Trial), an open-label, randomized Phase III investigation of fulvestrant plus anastrozole versus anastrozole alone as first-line treatment for patients with receptor-positive postmenopausal breast cancer.Citation20 This trial reported no significant differences in time to progression or median overall survival between the two groups. The different results reported in these two studies may be attributed to the size and choice of patient population. Combination of hormonal therapies may warrant further investigation, but it does not address the issue of hormone resistance, which eventually develops in all patients.
Mechanisms of resistance to endocrine therapy
Estrogen receptor activation leads to phosphorylation, dimerization, and downstream signaling through estrogen response elements which promote cell survival, division, and growth of cancer.Citation21,Citation22 Clinical and preclinical data indicate that hormone receptors interact with growth factor receptors, including human epidermal growth factor receptor (HER2/neu), epidermal growth factor receptor (EGFR), and insulin-like growth factor-1 receptor (IGF1R), which likely play a role in hormone resistance.Citation23,Citation24 Crosstalk between the estrogen receptor and membrane tyrosine kinase receptors (EGFR, HER2, and IGF1R) can lead to gene expression and cell growth independent of hormonal activation, mainly via activation of the mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K) pathways. The estrogen receptor can also be regulated by these membrane receptors, which act as coactivators and lead to estrogen receptor phosphorylation in the absence of estrogen (ligand-independent receptor activation, ). The interaction of the estrogen receptor with growth factor receptors is complex. It is believed that the estrogen receptor can activate membrane growth factors via expression of transforming growth factor-alpha and IGF1. However at the same time, it downregulates EGFR and HER2 while inducing IGF1R. In turn, activation of MAPK and PI3K pathways by growth factor receptors downregulates estrogen receptor signaling.Citation25
Figure 1 Crosstalk between the estrogen receptor and EGFR/HER2/IGF1R membrane tyrosine kinase receptors can lead to gene expression and cell growth independent of hormonal activation, mainly via activation of the MAPK and PI3K pathways.
Abbreviations: EGFR, epidermal growth factor receptor; IGF1R, insulin-like growth factor-1 receptor; mTOR, mammalian target of rapamycin; HER2, human epidermal growth factor receptor-2; ER, estrogen receptor; TSC1/2, tuberous sclerosis complex proteins 1/2; PI3K, phosphatidylinositol 3-kinase; MAPK, mitogen-activated protein kinase; Src, steroid receptor coactivator.
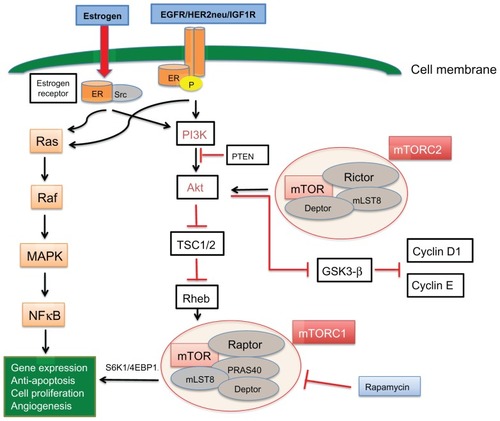
In summary, it appears that membrane growth factor receptors can phosphorylate and activate the estrogen receptor independently of estrogen and they can activate downstream pathways and induce cell growth independently of estrogen receptor activation, but can also downregulate estrogen receptor expression, leading to hormone independence.
HER2/EGFR
Breast cancers with high levels of HER2 expression are more likely to be resistant to hormonal therapy. Transfection of HER2 in estrogen receptor-positive breast cancer cells renders them resistant to tamoxifen.Citation26,Citation27 Further, it has been shown that selective estrogen receptor modulator-resistant breast cancer cells have increased expression of HER2 compared with selective estrogen receptor modulator-sensitive breast cancer cells.Citation28,Citation29 A meta-analysis by De Laurentiis et al reported that HER2-positive patients with metastatic receptor-positive breast cancer treated with tamoxifen had a shorter time to treatment failure when compared with patients having HER2-negative disease.Citation30 These findings suggest that HER2 plays a significant role both in intrinsic and acquired hormone resistance. Preclinical evidence supports that crosstalk between HER2 and the estrogen receptor leads to tamoxifen resistance, and disruption of this crosstalk can restore tamoxifen sensitivity.Citation31,Citation32
A randomized Phase III, double-blind, multicenter study by Johnston et al enrolled 1286 postmenopausal patients with advanced or metastatic estrogen receptor-positive and/or progesterone receptor-positive breast cancer. No prior treatment was allowed, except for neoadjuvant/adjuvant hormonal or anti-HER2 therapy. Patients were randomly assigned to receive letrozole and lapatinib or letrozole and placebo. Median progression-free survival was significantly improved in patients with HER2-positive disease who received lapatinib plus letrozole, compared with letrozole alone (3 months in the placebo arm and 8.2 months in the combination arm, hazards ratio 0.71, P = 0.019). Among the HER2-negative patients, there was no significant improvement in progression-free survival or clinical benefit. However, a subset of patients with HER2-negative disease and estrogen receptor expression in the lowest quartile appeared to benefit from adding lapatinib to letrozole (progression-free survival 13.6 versus 6.6 months, hazards ratio 0.65, P < 0.005).Citation33,Citation34
Similarly, expression of EGFR in vivo and in vitro has been shown to be associated with endocrine resistance, and in preclinical models EGFR inhibition can restore sensitivity to hormone treatment.Citation35–Citation38 A Phase II, randomized, double-blind, placebo-controlled study by Cristofanilli et al evaluated the combination of anastrozole and gefitinib (a selective EGFR tyrosine kinase inhibitor) versus anastrozole and placebo in postmenopausal patients with hormone receptor-positive metastatic breast cancer. The study population consisted of patients who had not received prior endocrine therapy for this stage or had developed metastatic disease during/after adjuvant tamoxifen. Although the study was closed early due to slow accrual, the combination arm showed improvement in progression-free survival versus placebo (median progression-free survival 14.7 versus 8.4 months, respectively). The treatment was tolerated very well.Citation39 Osborne et al reported a randomized Phase II trial of tamoxifen with or without gefitinib in patients with metastatic disease who had experienced treatment failure while on tamoxifen or aromatase inhibitors. The combination arm showed improved progression-free survival in patients who had relapsed after adjuvant tamoxifen. However, no clinical benefit was seen in patients who had been previously treated with aromatase inhibitors.Citation40
IGF1R
The IGF1R pathway plays a significant role in tumor growth and inhibition of apoptosis. IGF1R can activate the estrogen receptor pathway in the absence of estrogen and thus lead to tumor growth. It appears that there is crosstalk between IGF1R and the estrogen receptor, which possibly contributes to hormone resistance. In vivo and in vitro models show that IGF1R inhibition can act synergistically with hormone therapy.Citation41–Citation44 However, a clinical study of AMG 479 (a human anti-IGF1R monoclonal antibody) in combination with exemestane or fulvestrant in postmenopausal women failed to show a clinical benefit or difference in progression-free survival with IGF1R inhibition.Citation45
Steroid receptor coactivator
The steroid receptor coactivator (Src) is a nonreceptor tyrosine kinase, which plays an essential role in the life cycle of the cell.Citation46 Breast cancer tissue has higher expression of Src than normal breast tissue. In hormone receptor-positive breast cancer cells, Src binds and phosphorylates the estrogen receptor and activates downstream signaling pathways (). Src is thought to play a pivotal yet complex role in endocrine resistance. Elevated levels of cytoplasmic Src have been linked with an attenuated response to hormone therapy in vitro, and high expression of Src has been associated with increased metastatic potential and poor survival in the clinical setting.Citation47,Citation48 Preclinical studies indicate that treatment of resistant cells with Src inhibitors restores sensitivity to tamoxifen.Citation49 However, a Phase II study of dasatinib (an oral multi-BCR/ABL and Src family tyrosine kinase inhibitor) as a single agent showed very limited activity in women with advanced HER2-positive or estrogen receptor-positive metastatic breast cancer, probably due to the complexity of the cellular circuits which ultimately lead to hormone resistance.Citation50
PI3K, Akt, and mTOR
The PI3K/Akt/mTOR pathway is a major intracellular cascade, which can be regulated by nutrient availability and growth factor receptors, including EGFR, HER2, IGF1R, and the estrogen receptor. When activated, this pathway induces tumor growth, proliferation, and resistance to targeted agents and chemotherapy.Citation51,Citation52 The PI3K/Akt pathway can activate both estrogen-dependent and estrogen-independent estrogen receptor alpha.Citation53
In this pathway, a central role is played by PI3K heterodimer, which consists of a p85 regulatory and p110 catalytic subunit. Activation of PI3K will phosphorylate Akt. Akt is a serine/threonine kinase, which activates major downstream intracellular effectors. Akt can directly activate the estrogen receptor by phosphorylation in the absence of estrogen, thus promoting estrogen-independent growth and resistance to hormone therapy.Citation54–Citation58 The PI3K/Akt pathway is often aberrantly regulated in cancer, and the PIK3CA mutation is the most common point mutation seen in breast cancer.Citation59 Akt can be also activated by loss of PTEN, a mechanism that has been associated with a poor prognosis and increased risk of relapse after treatment with tamoxifen.Citation60
PI3K/Akt mutations, loss of PTEN, and constitutive activation of the PI3K/Akt pathway have been associated with hormone resistance. Activation of the PI3K pathway has been associated with intrinsic and acquired hormone resistance, and preclinical data indicate that PI3K inhibitors are active when combined with endocrine therapy.Citation56,Citation61 Multiple clinical studies are currently evaluating PI3K inhibitors in hormone receptor-positive tumors.
Downstream of PI3K and Akt, mTOR is a serine/threonine protein kinase, which is activated by inhibition of the tuberous sclerosis complex proteins 1/2.Citation62 mTOR exerts its effects via two very different protein complexes. The mTORC1 complex includes the regulatory-associated protein of mTOR (Raptor), mLST8, and proline-rich Akt substrate 40.Citation63 It is irreversibly inhibited by rapamycin and exerts its action by activating S6K1 (40S ribosomal protein S6 kinase 1) and eukaryotic initiation factor 4E-binding protein, thus leading to protein production, cell activation, division, and tumor growth.Citation64,Citation65 mTORC2 has been traditionally thought to be insensitive to rapamycin, but there is evidence that prolonged exposure to rapamycin can induce sufficient inhibition of mTORC2.Citation66 Although its role in the cell cycle remains largely unknown, mTORC2 is believed to modulate cell lipid metabolism and cell growth via Akt, by inhibition of glycogen synthase kinase-3β and cyclin D1/E proteolysis.Citation63 Studies suggest that targeted inhibition of TORC2 inhibits breast cancer cells in vitro and in vivo.Citation67
Several preclinical studies provide evidence that mTOR inhibition can restore hormone sensitivity and induce apoptosis in breast cancer cells. The mTOR inhibitor, rapamycin, can reverse resistance to endocrine therapy when combined with tamoxifen or fulvestrant.Citation68,Citation69 Interestingly, restoration of sensitivity to endocrine therapy can be associated with increased estrogen receptor-α protein expression levels and alteration of the phospho-ser167 estrogen receptor-α to total estrogen receptor-α ratio.Citation69 Treatment of letrozole-resistant or fulvestrant-resistant breast cancer cells with low concentrations of the mTOR inhibitor, everolimus, reverses Akt-mediated resistance and restores responsiveness to antiestrogen treatment.Citation70 When combined with letrozole, everolimus acts synergistically to promote cell cycle arrest and induce apoptosis.Citation71 In summary, these preclinical data strongly support that mTOR inhibition could play a significant role in the treatment of hormone receptor-positive breast cancer, especially in resistant tumors.
Clinical studies with mTOR inhibitors
Rapamycin (sirolimus) was the first identified mTOR inhibitor, and was initially used as an immunosuppressant to prevent organ transplant rejection. The novel inhibitors, everolimus, temsirolimus, and ridaforolimus, are rapamycin analogs with improved pharmacological properties.
Temsirolimus
In a randomized, Phase II three-arm study of temsirolimus in combination with letrozole in postmenopausal women with hormone receptor-positive metastatic breast cancer, combination treatment with an intermittent schedule of temsirolimus was tolerable and showed clinical activity, with preliminary results indicating improvement in progression-free survival.Citation72,Citation73 A subsequent Phase III study by Chow et al who enrolled patients with metastatic breast cancer randomly assigned patients to letrozole or combination of letrozole with temsirolimus.Citation74 The study had to be closed prematurely because there was no clinical benefit from the combination. An unplanned subset analysis suggested that patients who had been previously treated with chemotherapy might benefit from addition of the mTOR inhibitor to hormonal therapy.Citation75 It is possible that this study failed to reach its endpoint due to suboptimal dosing and inappropriate selection of the study population.
Everolimus
BOLERO-2 (Breast Cancer Trials of Oral Everolimus) is a randomized Phase III investigation by Baselga et al which evaluated a combination of everolimus with the steroidal aromatase inhibitor, exemestane, in postmenopausal patients with advanced estrogen receptor-positive breast cancer who had recurrence or progression while receiving a nonsteroidal aromatase inhibitor.Citation76 In total, 724 patients were randomized to receive exemestane 25 mg daily plus everolimus 10 mg daily or exemestane 25 mg daily plus placebo. At the interim analysis, with a median follow-up of 12.5 months, the patients treated with everolimus had a significant improvement in progression-free survival compared with the placebo arm (by local assessment, 6.9 months versus 2.8 months respectively, hazards ratio 0.43, P < 0.001; by central assessment 10.6 versus 4.1 months respectively, hazards ratio 0.36, P < 0.001). More serious adverse events were reported in the combination group, and a higher percentage of patients discontinued everolimus (19% versus 4%). The most common grade 3 or 4 adverse events were stomatitis, anemia, dyspnea, hyperglycemia, fatigue, and pneumonitis.
TAMRAD (tamoxifen and RAD001) was an open-label Phase II study that randomized patients to tamoxifen alone or tamoxifen in combination with everolimus 10 mg daily.Citation77 The clinical benefit rate, which was the primary endpoint, was significantly improved in patients receiving tamoxifen plus everolimus versus tamoxifen alone (61% versus 42%, respectively, P = 0.045). Time to progression was also significantly improved in patients treated with tamoxifen and everolimus compared with tamoxifen alone (8.6 versus 4.5 months, respectively). Preliminary analysis demonstrated that the risk of death was also reduced by 55% with everolimus. Patients with secondary resistance seemed to benefit more from the addition of everolimus to tamoxifen than patients with primary resistance. The main toxicities seen in the everolimus arm were fatigue, stomatitis, rash, anorexia, and diarrhea.
Baselga et al reported a neoadjuvant study of everolimus plus letrozole versus placebo plus letrozole in estrogen receptor-positive disease.Citation78 Two hundred and seventy postmenopausal women with operable estrogen receptor-positive breast cancer were randomly assigned to receive 4 months of neoadjuvant treatment with letrozole 2.5 mg/day and either everolimus 10 mg/day or placebo. The primary endpoint was clinical response by palpation. The response rate was higher in the everolimus arm (68% versus 59%). Biopsies were obtained at baseline and after 2 weeks of treatment. Progesterone receptor and cyclin D1 expression were decreased in both treatment arms, but phospho-S6 was downregulated significantly in the everolimus arm. Ki67 expression also decreased more dramatically in the everolimus arm compared with placebo. This study showed that everolimus significantly increased the efficacy of letrozole in the neoadjuvant setting.
These clinical studies () continue to support that everolimus has synergistic anticancer activity and improves outcomes when combined with hormonal therapy in hormone receptor-positive breast cancer.
Table 1 Phase II and III trials of everolimus in patients with hormone receptor-positive, HER2-negative breast cancer
Sirolimus
Bhattacharyya et al have reported a Phase I/II trial that evaluated the combination of tamoxifen and sirolimus 2 mg daily in hormone receptor-positive and HER2-negative breast cancer.Citation79 The study was divided into two groups and included a total of 400 patients. The first group included patients who could not afford aromatase inhibitors and thus were hormone-naïve. The second group included patients who had experienced treatment failure on aromatase inhibitors or tamoxifen. Response rates and time to progression were the primary endpoints. The Phase II study showed a response rate of 4% versus 40% and time to progression of 3 versus 11 months in the tamoxifen alone versus the tamoxifen plus sirolimus arm, respectively. The patients who had progression of disease within 6 months had less benefit (2.2 versus 7.4 months), and both hormone-naïve and hormone-resistant patients seemed to benefit from the combination therapy. This study concluded that the combination of sirolimus and tamoxifen was effective and well tolerated.Citation80
Resistance to mTOR inhibitors
Drug resistance is a potential challenge that may arise with the use of mTOR inhibitors, and can be mediated by dysregulation of p27, feedback activation of PI3K/Akt by S6K, activation of the ERK, PIM, and PDK1 pathways, alterations in protein synthesis, and increased bcl-2.Citation81,Citation82 Resistance can potentially be overcome by a combination of agents that target the mTOR pathway and PI3K/Akt, EGFR, or mTORC2 inhibitors. There are ongoing Phase I and II studies which are investigating the combination of these agents.Citation83 BELLE-3 (clinicaltrials.gov, NCT01633060) is an ongoing Phase III study evaluating the combination of BKM120 and fulvestrant in previously treated, hormone receptor-positive, HER2-negative patients who have progressed on or after an mTOR inhibitor. BKM120 is an oral pan-class PI3K inhibitor, which can potentially overcome mTOR resistance by targeting upstream PI3K signaling.Citation61
Conclusion and future directions
The development of resistance to hormonal agents represents a significant challenge in the management of advanced hormone receptor-positive breast cancer. Several cellular pathways have been investigated as potential targets in an effort to bypass the estrogen receptor and block tumor growth. Preclinical data suggest that there is crosstalk between the estrogen receptor and membrane growth factors, which can stimulate cell growth independent of hormonal activation. HER2, EGFR and mTOR inhibitors appear to have activity and act synergistically with hormonal therapy. On the other hand, a recent review reports that it may be possible to identify a subset of patients who are HER2-positive and estrogen receptor-positive who will benefit from the combination of HER2 and hormone inhibition, while patients with low hormone expression may not benefit from hormonal therapy and should be treated with chemotherapy and HER2-directed therapy instead.Citation84
Recent studies have shown that everolimus is active in combination with hormonal therapy in the metastatic and neoadjuvant setting, with an acceptable side effect profile. Based on the progression-free survival data in the control arm of the BOLERO-2 trial, as well as the data from the TAMRAD trial, it is possible that mTOR inhibitors may be more effective in tumors that have developed secondary resistance to endocrine agents.
It is unclear why the temsirolimus trials did not produce the clinical benefit that was seen with everolimus, but it may be attributed to the selection of hormone-naïve patients.
Ridaforolimus is another rapamycin analog, which is currently being studied in multiple tumors. In hormone receptor-positive and HER2-negative breast cancer, ridaforolimus is currently being evaluated in combination with dalotozumab (an IGF1R inhibitor) in one study that has completed recruitment (clinicaltrials.gov, NCT01234857). Another study, which is comparing the combination of ridaforolimus plus dalotozumab plus exemestane versus ridaforolimus plus exemestane, is currently recruiting patients (clinicaltrials.gov, NCT01605396). Another study which is currently recruiting patients is comparing trastuzumab or everolimus in combination with endocrine therapy in patients with hormone-refractory, HER2-negative metastatic breast cancer (clinical trials.gov, NCT00912340).
The most common side effects of mTOR inhibitors include stomatitis, rash, pneumonitis, hyperglycemia, and hyperlipidemia. Pneumonitis may warrant interruption of treatment and dose reduction if moderate or severe. The clinician should be aware that use of mTOR inhibitors is associated with an increased cost and side effects, especially in elderly patients with multiple comorbidities. In future research, we need to define biomarkers to help us identify better those patients who will benefit from addition of mTOR inhibitors, such as those with known mutations of the PI3K pathway. Use of gene expression profiling might also identify subsets of patients who will benefit from a combination of targeting therapies, such as patients with luminal B tumors, which are associated with high recurrence rates and poor survival.Citation85,Citation86
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRNaishadhamDAhmedin JemalACancer Statistics, 2012CA Cancer J Clin201262102922237781
- National Cancer InstituteSEER statistic fact sheet: breast cancer Available from: http://seer.cancer.gov/statfacts/html/breast.htmlAccessed August 6, 2012
- HarveyJMClarkGMOsborneCKAlfredDCEstrogen receptor status by immunohistochemistry is superior to ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancerJ Clin Oncol1999171474148110334533
- ColleoniMGelberSCoatesASInfluence of endocrine-related factors on response to perioperative chemotherapy for patients with node-negative breast cancerJ Clin Oncol2001194141414911689582
- WinerEPOptimizing endocrine therapy for breast cancerJ Clin Oncol2005231609161015755964
- JaiyesimiIABuzedarAUDeckerDAHortobagyiGNUse of tamoxifen for breast cancer: twenty-eight years laterJ Clin Oncol1995135135297844613
- CardosoFBischoffJBrainEA review of the treatment of endocrine responsive metastatic breast cancer in postmenopausal womenCancer Treat Rev7252012 [Epub ahead of print.]
- NabholtzJMBonneterreJBuzdarARobertsonJFThurlimannBAnastrozole (Arimidex) versus tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: survival analysis and updated safety resultsEur J Cancer2003391684168912888362
- BonneterreJBuzdarANabholtzJMAnastrozole is superior to tamoxifen as first-line therapy in hormone receptor positive advanced breast carcinomaCancer2001922247225811745278
- MouridsenHGershanovichMSunYSuperior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a Phase III study of the International Letrozole Breast Cancer GroupJ Clin Oncol2001192596260611352951
- MouridsenHGershanovichMSunYPhase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer GroupJ Clin Oncol2003212101210912775735
- MouridsenHGiobbie-HurderAGoldhirschALetrozole therapy alone or in sequence with tamoxifen in women with breast cancer. BIG 1-98 Collaborative GroupN Engl J Med200936176677619692688
- ParidaensRDirixLLohrischCMature results of a randomized phase II multicenter study of exemestane versus tamoxifen as first-line hormone therapy for postmenopausal women with metastatic breast cancerAnn Oncol2003141391139812954578
- ForbesJFCuzickJBuzdarAHowellATobiasJSBaumMEffect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trialLancet Oncol20089455318083636
- CoatesASKeshaviahAThurlimannBFive years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98J Clin Oncol20072548649217200148
- RobertsonJFOsborneCKHowellAFulvestrant versus anastrozole for the treatment of advanced breast carcinoma in postmenopausal women: a prospective combined analysis of two multicenter trialsCancer20039822923812872340
- HowellARobertsonJFAbramPComparison of fulvestrant versus tamoxifen for the treatment of advanced breast cancer in postmenopausal women previously untreated with endocrine therapy: a multinational, double-blind, randomized trialJ Clin Oncol2004221605161315117982
- Di LeoAJerusalemGPetruzelkaLResults of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancerJ Clin Oncol201028304594460020855825
- MehtaRSBarlowWEAlbainKSCombination anastrozole and fulvestrant in metastatic breast cancerN Engl J Med201236743544422853014
- BerghJJönssonPELidbrinkEKFACT: an open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancerJ Clin Oncol2012301919192522370325
- ShangYHuXDiRenzoJLazarMABrownMCofactor dynamics and sufficiency in estrogen receptor-regulated transcriptionCell2000103684385211136970
- OsborneCKSchiffRFuquaSAShouJEstrogen receptor: current understanding of its activation and modulationClin Cancer Res200174338s4342s11916222
- MassarwehSSchiffRUnraveling the mechanism of endocrine resistance in breast cancer: new therapeutic opportunitiesClin Cancer Res2007131950195417404074
- PietrasRJArboledaJReeseDMHER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cellsOncogene199510243524467784095
- OsborneCKSchiffRMechanisms of endocrine resistance in breast cancerAnnu Rev Med20116223324720887199
- BenzCCScottGKSarupJCEstrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neuBreast Cancer Res Treat19922485958095168
- MackeyJRKaufmanBClemensMTrastuzumab prolongs progression free survival in hormone-dependent and HER2-positive metastatic breast cancerBreast Cancer Res Treat2006100Suppl 1 Abstract 3
- SchaferJMBentremDJTakeiHA mechanism of drug resistance to tamoxifen in breast cancerJ Steroid Biochem Mol Biol200283758312650703
- KaklamaniVGCicconiJGradisharWIncreased HER2 expression in women with recurrent ER-positive breast cancerProc Am Soc Clin Oncol200725569s
- De LaurentiisMArpinoGMassarelliEA meta-analysis on the interaction between HER2 expression and response to endocrine therapy in advanced breast cancerClin Cancer Res2005114741474816000569
- ShouJMassarwehSOsborneCMechanisms of tamoxifen resistance: increased estrogen receptor- HER2/neu cross-talk in ER/HER2-positive breast cancerJ Natl Cancer Inst20049692693515199112
- LearyAFDrurySDetreSLapatinib restores hormone sensitivity with differential effects on ER signaling in cell models of HER2-negative breast cancer with acquired endocrine resistanceClin Cancer Res2010161486149720179226
- JohnstonSPippenJJrPivotXLapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancerJ Clin Oncol2009275538554619786658
- FinnRSPressMDeringJProgression-free survival (PFS) of patients with HER2-negative, estrogen receptor (ER)-low metastatic breast cancer (MBC) with the addition of lapatinib to letrozole: biomarker results of EGF30008Proc Am Soc Clin Oncol20092745s
- NicholsonRIMcClellandRAFinlayPRelationship between EGF-R, c-erb B-2 protein expression and Ki67 immunostaining in breast cancer and hormone sensitivityEur J Cancer19937101810238098946
- GeeJMHarperMEHutchesonIRThe antiepidermal growth factor receptor agent gefitinib (ZD1839/Iressa) improves antihormone response and prevents development of resistance in breast cancer in vitroEndocrinology20031445105511712960029
- MassarwehSShouJDiPietroMTargeting the epidermal growth factor receptor pathway improves the anti-tumor effect of tamoxifen and delays acquired resistance in a xenograft model of breast cancerBreast Cancer Res Treat200276S33 Abstract 18
- McClellandRABarrowDMaddenTAEnhanced epidermal growth factor receptor signaling in MCF7 breast cancer cells after long-term culture in the presence of the pure antiestrogen ICI 182,780 (Faslodex)Endocrinology20011422776278811415996
- CristofanilliMValeroVMangalikAPhase II, randomized trial to compare anastrozole combined with gefitinib or placebo in postmenopausal women with hormone receptor-positive metastatic breast cancerClin Cancer Res2010161904191420215537
- OsborneCKNevenPDirixLYGefitinib or placebo in combination with tamoxifen in patients with hormone receptor-positive metastatic breast cancer: a randomized phase II studyClin Cancer Res2011171147115921220480
- StoicaASacedaMFakhroAJoynerMMartinMBRole of insulin-like growth factor-I in regulating estrogen receptor-a gene expressionJ Cell Biochem20007660561410653980
- HouXHuangFMacedoLFDual IGF-1R/InsR inhibitor BMS-754807 synergizes with hormonal agents in treatment of estrogen-dependent breast cancerCancer Res2011717597760722042792
- FoxEMMillerTWBalkoJMA kinome-wide screen identifies the insulin/IGF-I receptor pathway as a mechanism of escape from hormone dependence in breast cancerCancer Res2011716773678421908557
- ZhangYMoerkensMRamaiahgariSElevated insulin-like growth factor 1 receptor signaling induces antiestrogen resistance through the MAPK/ERK and PI3K/Akt signaling routesBreast Cancer Res201113R5221595894
- KaufmanPAFerreroJMBourgeoisHA randomized, double-blind, placebo-controlled, phase 2 study of AMG 479 with exemestane (E) or fulvestrant (F) in postmenopausal women with hormone-receptor positive (HR+) metastatic (M) or locally advanced (LA) breast cancer (BC)Cancer Res201070Suppl 2 Abstract S1–S4
- FrameMCSrc in cancer: deregulation and consequences for cell behaviorBiochim Biophys Acta2002160211413012020799
- MorganLGeeJPumfordSElevated Src kinase activity attenuates tamoxifen response in vitro and is associated with poor prognosis clinicallyCancer Biol Ther200981550155819830888
- McBryanJTheissenSMByrneCMetastatic progression with resistance to aromatase inhibitors is driven by steroid receptor coactivator SRC-1Cancer Res20127254855922108824
- YueWFanPWangJMechanisms of acquired resistance to endocrine therapy in hormone-dependent breast cancer cellsJ Steroid Biochem Mol Biol200710610211017616457
- MayerELBaurainJFSparanoJA phase 2 trial of dasatinib in patients with advanced HER2-positive and/or hormone receptor-positive breast cancerClin Cancer Res2011176897690421903773
- FedelePCalvaniNMarinoATargeted agents to reverse resistance to endocrine therapy in metastatic breast cancer: where are we now and where are we going?Crit Rev Oncol Hematol20128424325122494933
- CarrawayHHidalgoMNew targets for therapy in breast cancer: mammalian target of rapamycin (mTOR) antagonistsBreast Cancer Res2004621922415318929
- SunMPacigaJFeldmanRPhosphatidylinositol-3-OH kinase (PI3K)/AKT2, activated in breast cancer, regulates and is induced by estrogen receptor α (ERα) via interaction between ERα and PI3KCancer Res2001615985599111507039
- Hernandez-AyaLFGonzalez-AnguloAMTargeting the phosphatidylinositol 3-kinase signaling pathway in breast cancerOncologist20111640441421406469
- BaselgaJTargeting the phosphoinositide-3 (PI3) kinase pathway in breast cancerOncologist201116Suppl 1121921278436
- CampbellRABhat-NakshatriPPatelNMPhosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for antiestrogen resistanceJ Biol Chem20012769817982411139588
- SheriAMartinLAJohnstonSTargeting endocrine resistance: is there a role for mTOR inhibition?Clin Breast Cancer201010Suppl 3S79S8521115426
- SimonciniTHafezi-MoghadamABrazilDPInteraction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinaseNature200028:40753854111029009
- BachmanKEArganiPSamuelsYThe PIK3CA gene is mutated with high frequency in human breast cancersCancer Biol Ther2004377277515254419
- ShomanNKlassenSMcFaddenABickisMGTorlakovicEChibbarRReduced PTEN expression predicts relapse in patients with breast carcinoma treated by tamoxifenMod Pathol20051825025915475931
- MillerTWBalkoJMFoxEMERα-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancerCancer Discov2011133835122049316
- KenersonHLAicherLDTrueLDActivated mammalian target of rapamycin pathway in the pathogenesis of tuberous sclerosis complex renal tumorsCancer Res2002625645565012384518
- WanderSAHennessyBTSlingerlandJMNext-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategyJ Clin Invest20111211231124121490404
- DowlingRJTopisirovicIFonsecaBDDissecting the role of mTOR: lessons from mTOR inhibitorsBiochim Biophys Acta201018044349
- HoltzMKThe role of S6K1 in ER-positive breast cancerCell Cycle2012113159316522895181
- SarbassovDDAliSMSenguptaSProlonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKBMol Cell20062215916816603397
- LiHLinJWangXTargeting of mTORC2 prevents cell migration and promotes apoptosis in breast cancerBreast Cancer Res Treat20121341057106622476852
- ChangSMironPMironARapamycin inhibits proliferation of estrogen-receptor-positive breast cancer cellsJ Surg Res2007138374417109887
- GhayadSEBiecheIVendrellJAmTOR inhibition reverses acquired endocrine therapy resistance of breast cancer cells at the cell proliferation and gene-expression levelsCancer Sci2008991992200319016759
- BeeramMTanQTekmalRRAkt-induced endocrine therapy resistance is reversed by inhibition of mTOR signalingAnn Oncol2007181323132817693645
- BoulayARudloffJYeJDual inhibition of mTOR and estrogen receptor signaling in vitro induces cell death in models of breast cancerClin Cancer Res2005115319532816033851
- BaselgaJFumoleauPGilMPhase II, 3 arm study of CCI-779 in combination with letrozole in postmenopausal women with locally advanced or metastatic breast cancer. Preliminary resultsJ Clin Oncol200422Suppl 14544
- CarpenterJTRochéHCamponeMRandomized 3-arm, phase 2 study of temsirolimus (CCI-779) in combination with letrozole in postmenopausal women with locally advanced or metastatic breast cancerJ Clin Oncol200523:16S564
- ChowLWCSunYJassemJPhase 3 study of temsirolimus with letrozole or letrozole alone in postmenopausal women with locally advanced or metastatic breast cancerBreast Cancer Res Treat2006100Suppl 1 Abstract 6091
- RugoHSKeckSReversing hormone resistance: have we found the golden key?J Clin Oncol2012302707270922753913
- BaselgaJCamponeMPiccartMEverolimus in postmenopausal hormone-receptor-positive advanced breast cancerN Engl J Med201236652052922149876
- BachelotTBourgierCCropetCRandomized phase II trial of everolimus in combination with tamoxifen versus tamoxifen alone in patients with hormone-receptor positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO studyJ Clin Oncol2012302718272422565002
- BaselgaJSemiglazovVvan DamPPhase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancerJ Clin Oncol2009272630263719380449
- BhattacharyyaGSBiswasJSinghJKReversal of tamoxifen resistance (hormone resistance) by addition of sirolimus (mTOR inhibitor) in metastatic breast cancer2011 European Multidisciplinary Cancer CongressEdition Stockholm, Sweden2011
- Villarreal-GarzaCCortesJAndreFVermaSmTOR inhibitors in the management of hormone receptor-positive breast cancer: the latest evidence and future directionsAnn Oncol2012232526253522553196
- CarewJSKellyKRNawrockiSTMechanisms of mTOR inhibitor resistance in cancer therapyTarget Oncol20116172721547705
- O’ReillyKERojoFSheQBmTOR inhibition induces upstream receptor tyrosine kinase signaling and activates AktCancer Res2006661500150816452206
- MargaritiNFoxSBBottiniAGeneraliDOvercoming breast cancer drug resistance with mTOR inhibitors. Could it be a myth or a real possibility in the short-term future?Breast Cancer Res Treat201112859960620945086
- NahtaRO’ReganRMTherapeutic implications of estrogen receptor signaling in HER2-positive breast cancersBreast Cancer Res Treat2012135394822527112
- SorlieTPerouCMTibshiraniRGene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implicationsProc Natl Acad Sci U S A200198108691087411553815
- Harichand-HerdtSZelnakAO’ReganREndocrine therapy for the treatment of postmenopausal women with breast cancerExpert Rev Anticancer Ther2009918719819192957