Abstract
Introduction
Elevated heart rate is linked with poor prognosis and has been shown to accelerate the progress of atherosclerosis. However, the association between heart rate and new-onset PAD is unknown.
Methods
A total of 3463 participants without PAD at baseline from a community-based cohort in Beijing were included and followed up for 2.3 years. PAD was defined as ankle–brachial index (ABI) ≤0.9. We used multivariate logistic regression models to investigate the association of heart rate and the risk of new-onset PAD.
Results
Participants were 56.67 ± 8.54 years old, and 36.12% were men. The baseline ABI was 1.11 ± 0.08, and the incidence of new-onset PAD was 2.97%. Multivariate regression models, adjusted for sex, age, risk factor of atherosclerosis, medications, and baseline ABI, showed that heart rate was significantly associated with incidence of PAD (odds ratio [OR] = 1.22, 95% confidence interval [CI]: 1.03–1.43, P = 0.020); every increase of 10 heart beats per minute (bpm) was associated with a 22% increase in the odds of developing new-onset PAD. Respondents in the higher-heart rate group (≥80 bpm) had an increased risk of new-onset PAD, compared with those in the lower-heart rate group (<80 bpm) (OR = 1.73, 95% CI: 1.14–2.63, P = 0.010). Subgroup analyses revealed no significant heterogeneity among the analyzed subgroups.
Conclusion
Elevated heart rate was independently associated with the risk of new-onset PAD in a community-based population in Beijing. Heart rate management should be considered for the purpose of PAD prevention.
Introduction
Peripheral arterial disease (PAD) is defined as ankle–brachial index (ABI) ≤0.9 based on the 2016 American Heart Association/American College of Cardiology guideline concerning the management of patients with lower-extremity PAD. PAD leads to claudication or critical limb ischemia, significantly reducing quality of lifeCitation1 and increasing the possibility of cardiovascular events.Citation2,Citation3 Moreover, patients with PAD have a higher prevalence of systemic atherosclerosis, including in the coronary arteries,Citation4 carotid arteries,Citation5 and renal arteries.Citation6 In 1993, the Framingham Study first reported the association between heart rate and mortality,Citation7 and many epidemiological studies have demonstrated that elevated heart rate is associated with poor prognosis among patients with hypertension,Citation8–Citation10 coronary artery disease,Citation11,Citation12 and heart failure.Citation13,Citation14 Studies have revealed that elevated heart rate could accelerate the progress of atherosclerosis.Citation15–Citation17 Moreover, several cross-sectional studies have previously revealed an inverse correlation between heart rate and the ABI.Citation18–Citation21 However, the association between heart rate and new-onset PAD is unknown, including among populations in China. Therefore, in this longitudinal cohort study with a 2.3-year follow-up, we investigated whether heart rate predicted the risk of new-onset PAD in a community-based population in China without PAD at baseline.
Patients and Methods
Study Population
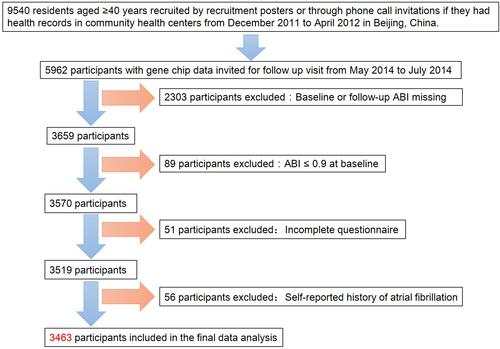
Participants were drawn from an atherosclerosis cohort survey in Gucheng community and Pingguoyuan community of Shijingshan District of Beijing, China.Citation22 First, we recruited 9540 residents aged ≥40 years either through study recruitment posters or phone call invitations if they had health records in community health centers from December 2011 to April 2012. Among the survey participants, we invited 5962 with gene chip data for a follow-up visit from May 2014 to July 2014. A total of 3659 participants (61.4% of the 5962 invited participants) attended the onsite follow-up visit with baseline or follow-up ABI date. Among these 3659 participants, we excluded 89 participants who had an ABI ≤ 0.9 at baseline. Moreover, we further excluded 51 participants who did not complete the questionnaire and 56 participants with a self-reported history of atrial fibrillation in 2012 and 2014 as well. Ultimately, the analysis consisted of 3463 eligible participants. The schematic diagram of recruitment and excluding procedure is shown in . This study obtained approval by the ethics committee of Peking University First Hospital, and we obtained written informed consent from each participant. We adhered to the principles of the Declaration of Helsinki.
Data Collection
Trained research staff was responsible for collecting baseline data according to standard operating procedures. A standardized questionnaire, including sociodemographic status, occupation, lifestyle, education, health behavior, diet, and medical history, was used to acquire basic information. We used an Omron HEM-7117 electronic sphygmomanometer, with the standard method of calibration and appropriate-sized cuffs to obtain the seated brachial blood pressure (BP) and pulse rate for each participant after a 5-minute rest. Triplicate measurements were taken on the right arm with intervals ≥1 minute between successive readings. For the measurement of each participant’s heart rate in the analysis, we calculated the mean pulse rate from the three consecutive measurements. In the same way, each participant’s systolic BP and diastolic BP were the mean of three consecutive measurements. Body mass index (BMI) was assessed as weight (kg) divided by height (m) squared.
After an overnight fast of 12 hours at minimum, we obtained a venous blood sample from each participant’s forearm. All laboratory variables at baseline, including fasting blood glucose, a standard 75 g oral glucose tolerance test, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, total cholesterol, triglycerides, and creatinine, were measured on the Roche C8000 Automatic Analyzer (Basel, Switzerland).
Current smoking in this analysis was smoking ≥1 cigarette per day for half a year at minimum. Current drinking in this analysis was drinking alcohol ≥1 time per week for half a year at minimum. Hypertension in this analysis was any self-reported history of hypertension, systolic BP ≥ 140 mm Hg, diastolic BP ≥ 90 mm Hg, or use of any antihypertensive drugs. Diabetes mellitus in this analysis was any self-reported history of diabetes, fasting blood glucose (FBG)≥7.0 mmol/L or a 2-hour plasma glucose value ≥11.1 mmol/L in an oral glucose tolerance test (OGTT), or use of any hypoglycemic drugs. Dyslipidemia in this analysis was any self-reported history of dyslipidemia, triglycerides (TG) >1.7 mmol/L (150mg/dL), total cholesterol (TC) >5.18 mmol/L (200 mg/dL), low-density lipoprotein cholesterol (LDL-C) >3.37 mmol/L (130 mg/dL), high-density lipoprotein cholesterol (HDL-C) <1.04 mmol/L (40 mg/dL), or use of any lipid-lowering drugs. Cardiovascular disease (CVD) in this analysis was any self-reported history of stroke or coronary heart disease.
Participants’ systolic BP was measured simultaneously in both arms and both ankles (brachial artery and posterior tibial arteries) using a BP-203RPE III device (Omron Healthcare) immediately after the participant had rested in a supine position for a minimum of 5 minutes. The instrument calculated the ABI; and the left and right ABI values were calculated as the ankle systolic BP for each side divided by the highest brachial systolic BP. We measured ABI twice during the study: once at baseline and once during the follow-up in 2014. New-onset PAD in this analysis was the lowest ABI value being ≤0.9 at the 2014 follow-up.
Statistical Analysis
Continuous variables were expressed as means ± standard deviations for data with normal distributions and median (interquartile range) for that with non-normal distributions. Categorical variables were presented as frequency (percentage). A heart rate threshold of 80 beats per minute (bpm) was used as the cut-off value based on several previous cohort studies.Citation8,Citation23–Citation26 Therefore, all eligible participants were divided into two groups by heart rate (≥80 bpm vs <80 bpm) for further analysis. We used the Student’s t-test to compare differences for normally distributed continuous variables and Kruskal–Wallis test for data with non-normal distribution. Pearson’s χ2 test was used to compare differences for categorical variables.
We applied a spline smoothing function to examine the relationship between heart rate and the risk of new-onset PAD using a generalized additive model. Then, piecewise linear regression was conducted to fit the smoothing curve, with adjustment for potential confounders.
We used multivariate logistic regression models to investigate the effect of heart rate (as a continuous variable and as a categorical variable) on the risk of new-onset PAD. We applied three sets of models to examine the association of heart rate with new-onset PAD risk. Model 1 was not adjusted for any other variables, model 2 was adjusted for age as well as sex, and model 3 was additionally adjusted for BMI, baseline ABI, current smoking and drinking, diabetes mellitus, hypertension, dyslipidemia, CVD, and use of lipid-lowering agents, antihypertensive agents, and hypoglycemic agents.
Interactions were also tested, to examine the relationships between heart rate and PAD risk among the analyzed subgroups including sex, age, BMI, current smoking and drinking status, diabetes mellitus, hypertension, dyslipidemia, CVD, and use of lipid-lowering agents, antihypertensive agents, and hypoglycemic agents.
All analyses were performed using Empower(R) (www.empowerstats.com, X&Y Solutions, Boston, MA, USA) and R (R 3.4.3; http://www.R-project.org). A P-value of 0.05 (two-sided) was considered statistically significant for all tests.
Results
Baseline Characteristics of Participants
Baseline characteristics of all participants are shown overall and divided into two groups by heart rate (≥80 bpm vs <80 bpm) in –. In total, 60.0% of participants had heart rate <80 bpm and 40.0% had heart rate ≥80 bpm. The mean age of participants was 56.67 ± 8.54 years, among which 36.12% were men. The mean heart rate was 78.15 ± 11.26 bpm, mean BMI was 26.00 ± 3.33 kg/m2, and mean ABI was 1.11 ± 0.08. The sample was made up of 18.57% (n = 643) current smokers and 23.16% (n = 802) current drinkers. A total 48.66% (n = 1685) of participants had hypertension, 23.74% (n = 822) had diabetes, 71.67% (n = 2482) had dyslipidemia, and 12.16% (n = 421) had a history of CVD. Participants in the higher-heart rate group were significantly older, had significantly higher BMI, TG, FBG, OGTT, lower ABI, and higher prevalence rates of diabetes, hypertension, and dyslipidemia than those in the lower-heart rate group. There was no significant difference in the prevalence of CVD between the two groups.
Table 1 Baseline Characteristics of All Eligible Participants
Table 2 Baseline Prevalence of Disease of All Eligible Participants
Table 3 Baseline Laboratory Variable of All Eligible Participants
Predictors of New-Onset PAD
The incidence of PAD among all participants was 2.97% (n = 103) after a 2.3-year (median: 2.34 years; 25th percentile–75th percentile: 2.28–2.39 years) follow-up.
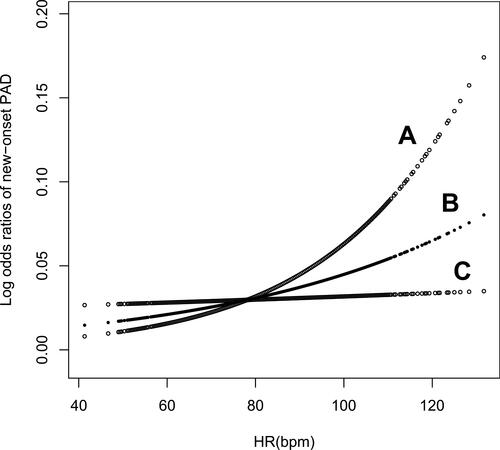
shows the smoothing curve of heart rate and new-onset PAD adjusted for sex, BMI, age, baseline ABI, current smoking and drinking status, diabetes mellitus, hypertension, dyslipidemia, cardiovascular disease, and use of lipid-lowering agents, antihypertensive agents, and hypoglycemic agents. The curve showed that the risk of PAD increased with elevated heart rate with no inflection points.
Figure 2 Smoothing curve of the risk of new-onset PAD by heart rate. Line B represents the smoothing curve for the association of heart rate and new-onset PAD. Lines A and C represent the 95% confidence interval for the risk of new-onset PAD. This relationship was adjusted for sex, age, body mass index, baseline ankle–brachial index, current smoking and drinking status, hypertension, diabetes mellitus, dyslipidemia, cardiovascular disease, and use of antihypertensive agents, lipid-lowering agents, and hypoglycemic agents.
The results of the multivariate regression models estimating the association of heart rate with new-onset PAD are shown in . Heart rate was significantly linked to the incidence of PAD (OR = 1.41, 95% confidence interval (CI): 1.20–1.64, P < 0.001), with every increase of 10 bpm associated with a 41% increase in the odds of developing new-onset PAD (Model 1). The relationship remained significant (OR = 1.22, 95% CI: 1.03–1.43, P = 0.020) after adjusting for sex, BMI, age, baseline ABI, current smoking and drinking status, diabetes mellitus, hypertension, dyslipidemia, CVD, and use of lipid-lowering agents, antihypertensive agents, and hypoglycemic agents. When participants were divided into two groups by heart rate (≥80 bpm vs <80 bpm), the incidence of new-onset PAD was consistently dose-dependently related to heart rate group in the multivariate regression models. The OR of being in the higher-heart rate group (≥80 bpm) for new-onset PAD was 2.24 (95% CI: 1.50–3.33, P < 0.001), compared with the lower-heart rate group (<80 bpm). In Model 3, after adjusting for various confounders and baseline ABI, this effect declined somewhat but remained significant (OR = 1.73, 95% CI: 1.14–2.63, P = 0.010).
Table 4 Logistic Regression Analysis of the Association of Heart Rate with New-Onset PAD
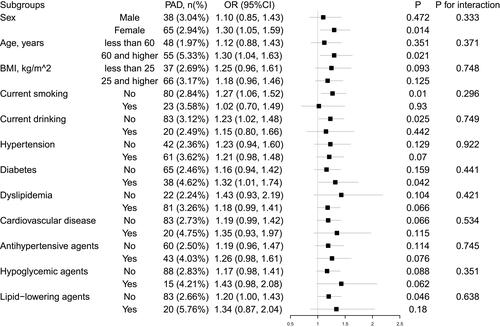
displays the results of the stratification and interaction analyses using a forest map. There was no significant heterogeneity among the analyzed subgroups in terms of sex, age (<60 vs ≥60 years), BMI (<25 vs ≥25 kg/m2), current drinking, current smoking, hypertension, dyslipidemia, diabetes mellitus, CVD, or use of lipid-lowering agents, antihypertensive agents, or hypoglycemic agents.
Figure 3 Subgroup analyses and interaction tests in different populations. The subgroup analyses were adjusted for sex, age, body mass index, baseline ankle–brachial index, current smoking and drinking status, hypertension, diabetes mellitus, dyslipidemia, cardiovascular disease, and use of antihypertensive agents, lipid-lowering agents, and hypoglycemic agents.
Discussion
Studies have confirmed that increased heart rate is of prognostic importance for all-cause mortality and cardiovascular mortality.Citation27 The main result of our study is that a higher heart rate was associated with a higher incidence of new-onset PAD in a community-based population. Consequently, these findings indicate we can use heart rate to predict new-onset PAD in the future.
Several studies have previously revealed an inverse correlation between heart rate and ABI,Citation18–Citation21 where ABI was decreased when the heart rate was accelerated through pacing among patients without significant organic heart disease. However, the studies above were all cross-sectional. To the best of our knowledge, our study is the first to report that increased heart rate was associated with the risk of new-onset PAD without inter-subgroup heterogeneity in a Chinese community-based population that did not have PAD at baseline after a 2.3-year follow-up, which further supports their potential association.
The incidence of new-onset PAD among all participants in our study was 3.07%. A cohort study in Taiwan enrolled 723,750 patients with no past history of PAD, rheumatic heart disease, or AF. The mean age of participants was 41.7 ± 16.8 years and the incidence of new-onset PAD was 3.4% after a 9-year-follow-up.Citation28 The participants in our study were older and had more risk factors of atherosclerosis, so the incidence of new-onset PAD was higher than that in other studies.
Based on clinical experience, beta blockers are contraindicated in severe PAD owing to the presumed peripheral hemodynamic consequences of beta blockers. However, there is no proof suggesting that beta blockers adversely affect PAD in current studies.Citation29 Moreover, studies have shown that the use of beta blockers was associated with better outcome in patients with coronary heart disease,Citation30 especially those with myocardial infarction.Citation31,Citation32 Our study found a correlation between heart rate and new-onset PAD, which leads to the question of whether beta blockers would affect the incidence of new-onset PAD. Unfortunately, there were too few patients who used beta blockers in our study to be able to reach any conclusions. However, we found no significant heterogeneity among the analyzed subgroups in terms of the use of antihypertensive agents. Future investigations are needed to answer that question.
There are several hypotheses describing the mechanism in the impact of heart rate on atherosclerosis. First, as early as 1998, increased heart rate was confirmed to be associated with sympathetic activity.Citation33 Sympathetic activation may mediate the vascular remodeling process.Citation34 Adrenergic receptors, a biomarker of the sympathetic reaction, are crucial in the regulation of vascular stiffness by influencing myocyte volume, the replication of smooth muscle cells, and collagen synthesis in peripheral arteries.Citation35 Second, separately from the effect of sympathetic activation, the pulsatile frequency alone can lead to endothelial function injuryCitation36 and inflammatory factor upregulation.Citation37 Additionally, increased heart rate causes vascular smooth muscle cells to create more fibronectin and collagen.Citation38 In summary, increased heart rate accelerates atherosclerosis via a few known pathophysiological mechanisms.
There were several limitations in our study. First, the measure of heart rate used here was the mean pulse rate. Pulse rate is not entirely equivalent to heart rate, especially for patients with atrial fibrillation. However, the relationship between pulse rate and new-on-set PAD remained after participants with a self-reported history of atrial fibrillation were excluded. Although we were unable to exclude patients who were unaware of the existence of asymptomatic atrial fibrillation, the incidence of atrial fibrillation in our study should be small, according to the epidemiology of atrial fibrillation.Citation39 Thus, the difference between pulse rate and heart rate is unlikely to influence the results of our study. Second, we obtained measures of ABI at only two time points. The accuracy of PAD diagnosis would be enhanced if based on measurements collected at multiple follow-up time points. Third, our study was based on a large community-based population in China who did not have PAD at baseline. Thus, it is difficult to generalize our findings to other populations.
Conclusions
Elevated heart rate was independently associated with the risk of new-onset PAD in a community-based population in Beijing. This indicates that heart rate may be crucial in PAD and supports the hypothesis that elevated heart rate accelerates the progression of atherosclerosis. Greater attention is needed to heart rate management for the purpose of PAD primary prevention.
Disclaimer
The views in the article belong to the authors and are not an official position of any institution or funder.
Acknowledgments
We thank all the staff of the Gucheng and Pingguoyuan Community Health Centers and the research coordinators who participated in this study.
Disclosure
The authors declare that they have no competing interests.
Additional information
Funding
References
- Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive Summary: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e686–e725. doi:10.1161/CIR.0000000000000470
- Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–386. doi:10.1056/NEJM199202063260605
- Steg PG, Bhatt DL, Wilson PW, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197–1206. doi:10.1001/jama.297.11.1197
- Lee JY, Lee SW, Lee WS, et al. Prevalence and clinical implications of newly revealed, asymptomatic abnormal ankle-brachial index in patients with significant coronary artery disease. JACC Cardiovasc Interv. 2013;6:1303–1313. doi:10.1016/j.jcin.2013.08.008
- Kurvers HAJM, van der Graaf Y, Blankensteijn JD, et al. Screening for asymptomatic internal carotid artery stenosis and aneurysm of the abdominal aorta: comparing the yield between patients with manifest atherosclerosis and patients with risk factors for atherosclerosis only1 1Competition of interest: none. J Vasc Surg. 2003;37:1226–1233. doi:10.1016/S0741-5214(02)75140-9
- Leertouwer TC, Pattynama PM, van den Berg-huysmans A. Incidental renal artery stenosis in peripheral vascular disease: a case for treatment? Kidney Int. 2001;59:1480–1483. doi:10.1046/j.1523-1755.2001.0590041480.x
- Mw G, Wb K, Belanger A, et al. Influence of heart rate on mortality among persons with hypertension: the Framingham Study. Am Heart J. 1993;125:1148–1154. doi:10.1016/0002-8703(93)90128-V
- King D, Everett C, Mainousiii A, Liszka H. Long-term prognostic value of resting heart rate in subjects with prehypertension. Am J Hypertens. 2006;19:796–800. doi:10.1016/j.amjhyper.2006.01.019
- Okin PM, Kjeldsen SE, Julius S, et al. All-cause and cardiovascular mortality in relation to changing heart rate during treatment of hypertensive patients with electrocardiographic left ventricular hypertrophy. Eur Heart J. 2010;31:2271–2279. doi:10.1093/eurheartj/ehq225
- Julius S, Palatini P, Kjeldsen SE, et al. Usefulness of heart rate to predict cardiac events in treated patients with high-risk systemic hypertension. Am J Cardiol. 2012;109:685–692. doi:10.1016/j.amjcard.2011.10.025
- Kolloch R, Legler UF, Champion A, et al. Impact of resting heart rate on outcomes in hypertensive patients with coronary artery disease: findings from the INternational VErapamil-SR/trandolapril STudy (INVEST). Eur Heart J. 2008;29:1327–1334. doi:10.1093/eurheartj/ehn123
- Lonn EM, Rambihar S, Gao P, et al. Heart rate is associated with increased risk of major cardiovascular events, cardiovascular and all-cause death in patients with stable chronic cardiovascular disease: an analysis of ONTARGET/TRANSCEND. Clin Res Cardiol. 2014;103:149–159. doi:10.1007/s00392-013-0644-4
- McAlister FA, Wiebe N, Ezekowitz JA, et al. Meta-analysis: beta-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med. 2009;150:784–794. doi:10.7326/0003-4819-150-11-200906020-00006
- Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. The Lancet. 2010;376:875–885. doi:10.1016/S0140-6736(10)61198-1
- Benetos A, Adamopoulos C, Bureau J-M, et al. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation. 2002;105:1202–1207. doi:10.1161/hc1002.105135
- Tomiyama H, Hashimoto H, Tanaka H, et al. Synergistic relationship between changes in the pulse wave velocity and changes in the heart rate in middle-aged Japanese adults: a prospective study. J Hypertens. 2010;28:687–694. doi:10.1097/HJH.0b013e3283369fe8
- Grassi G, Seravalle G, Quarti-Trevano F. The ‘neuroadrenergic hypothesis’ in hypertension: current evidence. Exp Physiol. 2010;95:581–586. doi:10.1113/expphysiol.2009.047381
- Abraham P, Desvaux B, Colin D, et al. Heart rate-corrected ankle-to-arm index in the diagnosis of moderate lower extremity arterial disease. Angiology. 1995;46:673–677. doi:10.1177/000331979504600805
- Su HM, Lee KT, Chu CS, et al. Effects of heart rate on brachial-ankle pulse wave velocity and ankle-brachial pressure index in patients without significant organic heart disease. Angiology. 2007;58:67–74. doi:10.1177/0003319706295481
- Hsu PC, Lee WH, Chu CY, et al. Heart rate significantly influences the relationship between atrial fibrillation and ankle-brachial index. J Cardiol. 2015;66:143–147. doi:10.1016/j.jjcc.2014.10.011
- Schroll M, Munck O. Estimation of peripheral arteriosclerotic disease by ankle blood pressure measurements in a population study of 60-year-old men and women. J Chronic Dis. 1981;34:261–269. doi:10.1016/0021-9681(81)90031-X
- Fan F, Qi L, Jia J, et al. Noninvasive central systolic blood pressure is more strongly related to kidney function decline than peripheral systolic blood pressure in a Chinese community-based population. Hypertension. 2016;67:1166–1172. doi:10.1161/HYPERTENSIONAHA.115.07019
- Thomas F, Bean K, Provost J-C, et al. Combined effects of heart rate and pulse pressure on cardiovascular mortality according to age. J Hypertens. 2001;19:863–869. doi:10.1097/00004872-200105000-00005
- Palatini P, Thijs L, Staessen JA, et al. Predictive value of clinic and ambulatory heart rate for mortality in elderly subjects with systolic hypertension. Arch Intern Med. 2002;162:2313–2321. doi:10.1001/archinte.162.20.2313
- Saxena A, Minton D, Lee DC, et al. Protective role of resting heart rate on all-cause and cardiovascular disease mortality. Mayo Clin Proc. 2013;88:1420–1426. doi:10.1016/j.mayocp.2013.09.011
- Paul L, Hastie CE, Li WS, et al. Resting heart rate pattern during follow-up and mortality in hypertensive patients. Hypertension. 2010;55:567–574. doi:10.1161/HYPERTENSIONAHA.109.144808
- Courand PY, Lantelme P. Significance, prognostic value and management of heart rate in hypertension. Arch Cardiovasc Dis. 2014;107:48–57. doi:10.1016/j.acvd.2013.11.003
- Hsu PC, Chiu CA, Chu CY, et al. CHADS2 score and risk of new-onset peripheral arterial occlusive disease in patients without atrial fibrillation: a nationwide cohort study in Taiwan. J Atheroscler Thromb. 2015;22:490–498. doi:10.5551/jat.27284
- Paravastu SC, Mendonca DA, Da Silva A. Beta blockers for peripheral arterial disease. Cochrane Database Syst Rev. 2013;CD005508. doi:10.1002/14651858.CD005508.pub3
- Fihn SD, Gardin JM, Abrams J, et al. ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;2012(60):e44–e164.
- Kjekshus JK. Importance of heart rate in determining beta-blocker efficacy in acute and long-term acute myocardial infarction intervention trials. Am J Cardiol. 1986;57:43F–9F. doi:10.1016/0002-9149(86)90888-X
- Cucherat M. Quantitative relationship between resting heart rate reduction and magnitude of clinical benefits in post-myocardial infarction: a meta-regression of randomized clinical trials. Eur Heart J. 2007;28:3012–3019. doi:10.1093/eurheartj/ehm489
- Grassi GVS, Bertinieri G, Seravalle G, Stella ML, Dell’Oro R, Mancia G. Heart rate as marker of sympathetic activity. J Hypertens. 1998;16:1635–1639. doi:10.1097/00004872-199816110-00010
- Grassi G. Assessment of sympathetic cardiovascular drive in human hypertension: achievements and perspectives. Hypertension. 2009;54:690–697. doi:10.1161/HYPERTENSIONAHA.108.119883
- Mancia G, Grassi G, Giannattasio C, et al. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension. 1999;34:724–728. doi:10.1161/01.HYP.34.4.724
- Custodis F, Schirmer SH, Baumhakel M, et al. Vascular pathophysiology in response to increased heart rate. J Am Coll Cardiol. 2010;56:1973–1983. doi:10.1016/j.jacc.2010.09.014
- Himburg HA, Dowd SE, Friedman MH. Frequency-dependent response of the vascular endothelium to pulsatile shear stress. Am J Physiol Heart Circ Physiol. 2007;293:H645–53. doi:10.1152/ajpheart.01087.2006
- Stegemann JP, Hong H, Nerem RM. Mechanical, biochemical, and extracellular matrix effects on vascular smooth muscle cell phenotype. J Appl Physiol (1985). 2005;98:2321–2327. doi:10.1152/japplphysiol.01114.2004
- Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129:837–847. doi:10.1161/CIRCULATIONAHA.113.005119