Abstract
Purpose
The aim of this study was to investigate whether the consequences of neurologic lesions are underestimated when the Barthel Index (BI) is used to assess the clinical outcome of botulinum toxin injection.
Patients and methods
The records for all in- and outpatients with various neurologic lesions (stroke, multiple sclerosis, spinal cord injury, traumatic brain injury, and so forth) who had been referred to the authors’ departments and who had received botulinum toxin type A (Botox®) for spasticity within a 4-year period (2008–2011) were examined retrospectively. BI data were collected and analyzed.
Results
The BI score was found to have increased in follow-up assessments (P = 0.048). No correlation was found between the degree of spasticity and the BI score.
Conclusion
The specific injection of Botox in patients with neurologic lesions was not strongly correlated with a significant functional outcome according to the BI. The results of this study suggest that clinicians need to look at other measurement scales for the assessment of significant outcomes of Botox in the rehabilitation process after neurologic lesions.
Introduction
Spasticity is a form of muscle overactivity originating from damage to the central nervous system. It is defined as “a motor disorder characterized by velocity dependent increase in tonic stretch reflexes with exaggerated tendon jerks, resulting from hyper excitability of the stretch reflex.”Citation1 The occurrence, pathophysiology, and impact of spasticity are still subjects of interesting debate.Citation1 Moreover, there is no consensus concerning the number of patients developing spasticity. In the few studies that cover the subject, spasticity seems to be present in disabled subjects with stroke and multiple sclerosis (MS) at a rate of 19%–40%, depending on the spasticity measurement scale used.Citation2–Citation8
There are several methods for management of spasticity, including pharmacotherapy, physical therapy, chemical neurolysis, and surgery, but the results are unsatisfactory. There is adequate evidence for selective dorsal rhizotomy for spasticity in cerebral palsy. Nevertheless it is irreversible, and patients may experience deterioration in walking ability or bladder function, and later complications including spinal deformity.Citation9 Oral antispasticity drugs are nonselective in their action and may cause fatigue and functional loss. Furthermore, these drugs have severe adverse effects because of the high doses required to reduce spasticity.Citation10 Physical therapy is useful, but studies regarding this treatment are not conclusive. Chemical neurolysis with alcohol or phenol injections may cause loss of sensation in the skin and dysesthesia; furthermore, tolerance develops with repeated treatment, diminishing their effect.Citation11 Recently, chemodenervation with botulinum toxin type A (BoNT-A) has been shown to be an effective antispasticity agent.Citation12–Citation14 The use of BoNT-A for the management of upper and lower limb spasticity has advantages. It can be performed as an outpatient procedure without anesthesia, and the toxin does not cause loss of sensation in the skin or dysesthesia. BoNT-A has been found to be a highly effective and cost-effective agent for many of the common forms of spasticity and muscle overactivity, and it has a profile that is favorable when compared with systemic agents and other focal therapies.Citation1
No universally accepted clinical measure of spasticity exists. Both the Ashworth Scale (AS) and the Modified Ashworth Scale have a strong association with objective measures of resistance to passive movement, although their association with results from reflex-related electromyographic parameters is measurable but weak. The AS and modified Ashworth Scale (MAS) can be used with ease. However, resistance to passive movement is a complex issue. Some studies suggest that AS may be more reliable than the MAS.Citation15,Citation17 The final decision which scales to use should be based on the experience of the clinician.
The reduction of spasticity in the clinical setting is supported by evidence demonstrating the effectiveness of botulinum toxin at impairment level,Citation16 although the evidence for functional benefit is still under investigation.Citation12,Citation13,Citation18,Citation19 Ashford and Turner-StokesCitation20 published an explanation for this paradox, reporting that either this was a methodological problem of the studies, which were inadequately powered to demonstrate functional gains, or the measures used were insufficiently sensitive to change following focal intervention with BoNT-A.Citation20 Precise assessment of activities of daily living in disabled subjects is important for quality care and for measuring the outcomes of botulinum toxin injection. The Barthel Index (BI) is user-friendly and multiple studies support its reliability and validity.Citation21 The BI comprises 10 questions measuring disability and functional independence in a person’s activities of daily living. It is rated from observation, and the questions are divided as follows: two items on a two-point scale, six items on a three-point scale, and two items on a four-point scale. Although validated and used globally, the BI has limitations in its application and evaluation: the BI is limited to numeric increases or decreases in total score, making it difficult to explain the clinical meaning of the scores or changes in scores.Citation22
The main aim of this study was to investigate whether the consequences of neurologic lesions are underestimated when the BI is used to assess the clinical outcome after BoNT-A injection. Secondary aims were to investigate functional outcomes of BoNT-A injection in patients with neurologic lesions and to correlate the outcomes with the units injected, the regions of selected muscles, and the reduction of degree of spasticity.
Material and methods
Records for all in- and outpatients with neurologic lesions who received BoNT-A (Botox®; Allergan Inc, Irvine, CA) for spasticity (35 males and 19 females; mean age, 42 ± 12 years) within a 4-year period (2008–2011) were examined retrospectively in the rehabilitation and neurologic departments of Rhodes General Hospital, Rhodes, Dodecanese, Greece, and the Rehabilitation Center Amyntaio, General Hospital of Florina, Amyntaio, Florina, Greece.
All subjects were clinically evaluated for spasticity and whether it would be useful to offer focal therapy through a physical medicine and rehabilitation physician (YD), and the assessment of spasticity was graded using the AS (range, 0–4) before and 1 month after the first injection session.Citation23 All injection sessions were performed under electromyographic monitoringCitation24 by the same physician (YD) and in muscles of the upper (pectoralis major, biceps, brachialis, brachioradialis, pronator teres, flexor carpi radialis and flexor carpi ulnaris, and flexor digitorum superficialis and profundus) and lower limbs (adductors, gastrocnemius, soleus, and tibialis posterior), depending on clinical examination.
To measure disability the authors used the Greek version of the BI in the original 100-point format. Item scores are summed to generate a total score, where 0 indicates maximum dependence and 100 indicates maximum independence.Citation25 The authors set a 20-point change as being clinically significant, following the most stringent recommendations of Collin et al.Citation26 The BI score was evaluated by two residents in neurology (Karvouni A and Kiourtidis D) before and 1 month after each session.
The Greek version of the BI has been used in a multicenter European study.Citation27 It is likely the translation did not undergo a full linguistic validation process when first compiled and therefore it may require further work in the future. The authors certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research. No financial support was received from Allergan Inc.
Data available on file for injected muscles (muscle region, BoNT-A dosage, number of sessions, and degree of spasticity) and pain and clonus before and after injection were collected and analyzed. Spasticity, clonus and pain are parameters which interfere in the rehabilitation process and influence the functional outcome. Patients were selected according to the following clinical and functional criteria: spasticity with an AS score ≥ 3, over 20 years of age, painful muscle spasms and clonus, no contractures. Spasticity was severe, causing difficulties in performing daily activities and in use of orthoses. Most (90%) of the patients involved in this study followed rehabilitation programs immediately after the injection sessions.
Statistical analysis
All quantitative data were represented by number of patients, mean value, and standard deviation; all qualitative data were represented by number of patients and percentage. The Kolmogorov- Smirnov test was used to monitor the regularity of the distributions. Quantitative variables were analyzed using the one- and two-way analysis of variance models. To control interaction between the variables of time and disease, a two-way mixed analysis of variance model was used; comparisons of absolute values of variables between groups were also performed with this model. Analysis of covariance was used to compare values of variables in follow-up assessment. Comparisons of differences in percentage for variables between groups were performed using the Kruskal-Wallis test and the Mann-Whitney U test (for pair-wise comparisons). Qualitative variables were analyzed using the chi-square test. Statistical analysis was performed using SPSS software (v 13.00; SPSS Inc, Chicago, IL). All tests were two-sided, and a P-value of 0.05 was considered statistically significant.
Results
Analyzing the study population
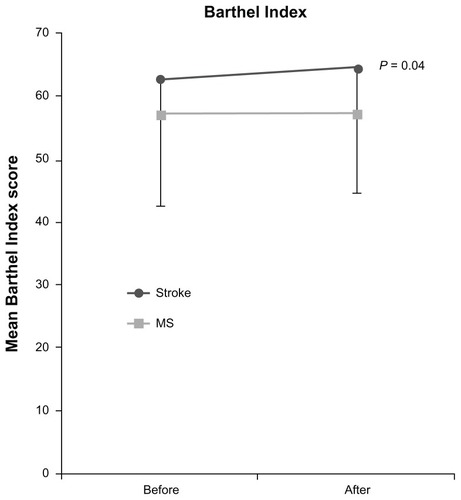
The results from patients with stroke and MS are presented in this study. Patients with traumatic brain injury, spinal cord injury, and cerebral palsy were excluded from any further analysis because of the small number of subjects. outlines the study population characteristics. There was a difference according to age between stroke and MS subjects (mean age, 62.42 ± 11.18 years and 44.83 ± 8.6 years, respectively; P < 0.001). A significant difference was found between stroke and MS subjects according to units injected in the upper and lower limbs (upper limbs: 212.78 ± 59 U and 100 ± 10 U, respectively, P = 0.013; lower limbs: 102.5 ± 20 U and 256 ± 93 U, respectively, P < 0.001) (). There was no significant interaction between groups, meaning the AS and BI scores changed over time in the same way in both groups [AS: F (2.21) = 0.380, P = 0.689; BI: F (2.26) = 0.682, P = 0.514] ().
Figure 1 Statistical difference between stroke and multiple sclerosis (MS) groups in Botox® units injected in upper and lower limbs.
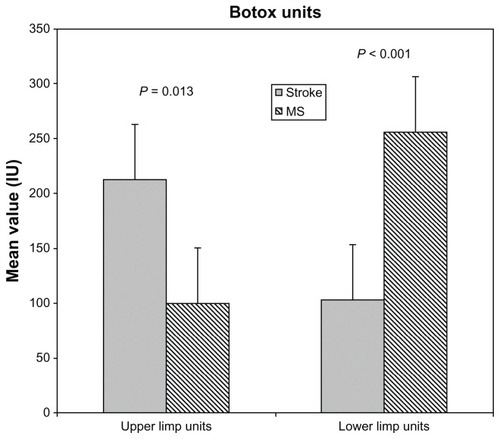
Figure 2 Interaction between stroke and multiple sclerosis (MS) groups: no statistically significant interaction was found between groups (F (2.21) = 0.380; P = 0.689); the Ashworth Scale score was found to change over time in the same way in both groups.
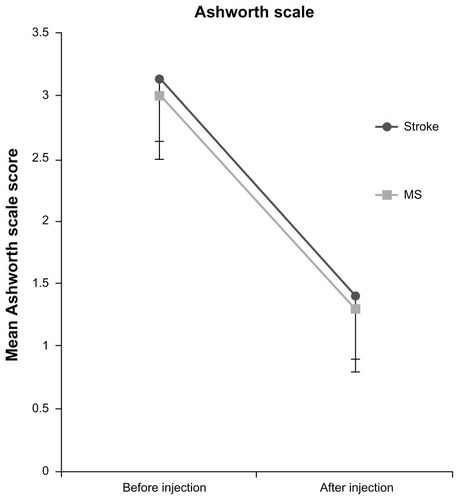
Table 1 The study population; separation according to the disease, and etiology of the neurologic lesion
BI score in stroke versus MS subjects
The mean BI score in stroke and MS subjects was 62.7 ± 24 and 57 ± 19, respectively, before injection (P = 0.18, not statistically significant) and was 64.6 ± 24 and 57.1 ± 19, respectively, after injection (P = 0.136, not statistically significant). The BI score increased significantly between groups during follow-up (P = 0.048). There was a small statistical difference in BI scores between stroke subjects (P = 0.04) but not between MS subjects (). No correlation was found between the degree of spasticity and BI score.
The efficacy of BoNT-A on parameters that interfere with the rehabilitation process
An improvement in degree of spasticity (P < 0.001) was seen in both groups and did not differ significantly between sessions. Statistical difference was found between groups in Botox units injected in lower limbs (P < 0.001) (). The difference in degree of spasticity between Botox sessions and follow-up assessments was correlated with units injected in the upper limbs (r = 0.5). Pain was improved in most of the patients but this improvement was not significant (data not shown), while clonus showed a trend for reduction (P = 0.088). No local or systemic adverse events after BoNT-A injection were reported.
Discussion
The main finding of this study was a small increase in BI score, found to be slightly significant in stroke subjects and not significant in MS subjects. In contrast with this, the degree of spasticity was significantly reduced, and the other measured parameters of pain and clonus were also reduced.
The significant difference in BI scores among stroke subjects, which corresponds to a two-point BI difference before and after injection, is not significant according to the authors’ criteria (a 20-point change being considered clinically significant). In order to maintain the functional benefits, the session had to be repeated within a mean interval of 4 months. A possible explanation for poor functional improvement can be the choice of muscles injected; however, this was not the case in the present study because the authors used an individualized approach, based on the distribution of spasticity in each patient, rather than a standard protocol. Although the muscles to inject were not predetermined, the effect of BoNT-A on spasticity was demonstrated. Nevertheless, this could partially explain why an objective functional benefit that supports the necessity for designing case treatments tailored for the individual was not demonstrated. It is crucial to investigate whether the improved spasticity after BoNT-A treatment translates into a better quality of life.Citation28 For example, a minor improvement of spasticity, without significant effect on patient mobility, could eventually result to better quality of life if pain was reduced. Although, a significant improvement in physical findings without gain in functional status and mobility could have little or no impact on patients’ quality of life. Up to date, limited number of studies has used quality-of-life measures (eg, SF-36, EQ-5D) in order to evaluate the effect of botulinum toxin (Botox) treatment in patients with spasticity.Citation29–Citation31 On the other hand, the BI includes mobility and continence items that are unlikely to be affected by localized treatment of upper limb muscle spasticity, for example. This suggests that individualized goal-attainment scales (eg, the ability to put the spastic arm through a garment sleeve) are more relevant outcome measures in studies of this nature.Citation32
Pathak et alCitation33 have reviewed the evidence from randomized controlled trials regarding the use of BoNT-A therapy for spasticity due to various causes. All but one of the randomized controlled trials reviewed found improvements in upper and lower limb impairment with BoNT-A therapy. Similarly, results of the present study show lower degrees of spasticity in all patients, but the injected units were only correlated with a reduction of AS score in the upper limbs.Citation34 Another finding was that MS subject required double the dosing in the lower limbs and half the dosing in upper limbs of what the stroke patients required. There are differences in the degree of spasticity experienced by MS and stroke subjects (spastic versus flaccid paralysis). Moreover there are also differences according to the evolution or not of the lesion (ie, progressive MS or relapsing-remitting MS versus hemiplegia), residual mobility and functionality, the ability to walk and stand, and drug treatment (eg, corticosteroid therapy, interferon therapy in MS patients). In addition the element of fatigue and muscle weakness needs to be taken into account, as it reduces the mobility of both MS and stroke patients, but mostly MS patients significantly, increasing spasticity further. It is also obvious that patients with these disorders, but mostly MS patients which usually are younger, often face depression, potentially leading to mobility limitations.Citation35
Wissel et alCitation36 investigated the effects of local BoNT-A injections in 60 patients with acute (<12 months) and chronic spasticity and pain in a prospective multicenter study and found that intramuscular BoNT-A injections are a potent, well-tolerated treatment modality to significantly reduce spasticity-related local pain.Citation36 In line with this, the present study evaluated pain using a visual analog finding scale and found it to be reduced in 90% of the patients. This evaluation served as patient’s personal improvement. BoNT-A may help to control spasticity for months following injection.
Dosing was based on the 1997 guidelines for BoNT-A.Citation37 In this study the authors found lower degrees of spasticity in all patients, but only in the upper limbs were the injected units significantly correlated with the reduction of the AS score – meaning that if a higher dose of botulinum toxin was injected, a greater reduction in spasticity occurred. This finding suggests the need to use the highest dose allowed in the upper limbs to increase the functional benefits. In addition, the adductors showed the minimum response to treatment possible, because the units injected in this muscle group (mean of 90 U for each limb per treatment session) were low (this result is also shown in other studiesCitation38), suggesting the need to inject more units in these muscles because of the central role of adductor muscles in lower limb spasticity (K Petropoulou, personal communication with YD, September 30, 2004). Furthermore, the optimal dose of BoNT-A for the treatment of upper limb spasticity has not been established. To date, only a single study has addressed this issue.Citation13 A group of BoNT-A experts has recommended a maximum dose of Botox of about 400–600 IU per session.
Stroke and MS are very different medical conditions, with different natural histories, so it is unusual to look at these groups of patients together. However, according to the authors’ analysis there was no significant interaction between groups – meaning the AS and BI score change over time in the same way in both groups – for this reason the authors chose to study these conditions together.
The whole country of Greece, which is divided into 51 counties, has fewer than 10 government rehabilitation clinics (almost 200 beds) and units, of which eight are located in the capital city, Athens (the authors are unable to provide the exact number of private rehabilitation clinics or to provide information regarding the use of BoNT-A in these private clinics, as there is no published information available on these subjects). The authors’ departments belong to peripheral general hospitals in the southeast and the northwest of Greece. Moreover, in the authors’ departments, patients from all around the Dodecanese (various islands) and the West Macedonian area (Kozani, Ptolemaida, and Florina) are treated. Long-term rehabilitation therapies were offered to inpatients only. Most of the patients followed therapy programs (ie, only physical therapy) in private at home or in physical therapy units around the counties and some followed therapy programs in organized private or government rehabilitation facilities (including physical therapy individualized home training programs, occupational therapy, speech therapy, and upper and lower limb orthosis).
Moreover, the authors believe the functional outcome would be better in an organized rehabilitation faculty where patients follow more individualized programs than in the authors’ departments. Because of this, it was impossible to follow a standard rehabilitation protocol in all subjects after injection. According to Baricich et al,Citation39 the treatment after injections could potentiate the pharmacological effect. The main limitations in the present study were the small sample size and the retrospective nature of the study. Another limitation was that subjects with different disabilities received different BoNT-A doses and a different number of injections (Botox sessions).
The authors are not aware of any other study examining the results of Botox injections in a clinical setting with stroke and MS at the same time. Most studies mainly investigate focal spasticity results (ie, the result in the spasticity of adductors or wrist flexors, and so forth) or the combined treatment of Botox injections with various other modalities.
Conclusion
The specific injection of Botox® in patients with neurogenic lesions was not strongly correlated with a significant functional outcome according to Barthel Index. On the other side the degree of spasticity was significantly reduced and all other measured parameters (pain, clonus) were also reduced. In the future more studies should investigate whether the improved spasticity after BoNT-A treatment translates into a better quality of life. The injection must be tailored to meet the patient’s needs. BoNT-A doses need to be individualized according to the patient’s profile in order to increase the patient’s capability to perform basic and functional tasks. In a clinical setting this reflects everyday practice.
Acknowledgments
The authors would like to thank Dr Theodoros Loizidis for his useful comments in the preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- LanceJWSymposium synopsisFeldmanRGYoungRRKoellaWPSpasticity: Disordered Motor ControlChicago (IL)Year Book Medical Publishers1980485494
- BrownPPathophysiology of spasticityJ Neurol Neurosurg Psychiatry19945777737778021658
- O’DwyerNJAdaLNeilsonPDSpasticity and muscle contracture following strokeBrain1996119Pt 5173717498931594
- WelmerAKvon ArbinMWidén HolmqvistLSommerfeldDKSpasticity and its association with functioning and health-related quality of life 18 months after strokeCerebrovasc Dis200621424725316446538
- WatkinsCLLeathleyMJGregsonJMMooreAPSmithTLSharmaAKPrevalence of spasticity post strokeClin Rehabil200216551552212194622
- LeathleyMJGregsonJMMooreAPSmithTLSharmaAKWatkinsCLPredicting spasticity after stroke in those surviving to 12 monthsClin Rehabil200418443844315180128
- SommerfeldDKEekEUSvenssonAKHolmqvistLWvon ArbinMHSpasticity after stroke: its occurrence and association with motor impairments and activity limitationsStroke200435113413914684785
- RizzoMAHadjimichaelOCPreiningerovaJVollmerTLPrevalence and treatment of spasticity reported by multiple sclerosis patientsMult Scler200410558959515471378
- National Institute for Health and Clinical Excellence15122010NICE Publication type: Patient Information. Available from: http://www.nice.org.ukAccessed August 21, 2012
- KatrakPHColeAMPoulosCJMcCauleyJCObjective assessment of spasticity, strength, and function with early exhibition of dantrolene sodium after cerebrovascular accident: a randomized double-blind studyArch Phys Med Rehabil1992731491729971
- BakheitAMOBadwanDAHMcLellanDLThe effectiveness of chemical neurolysis in the treatment of lower limb muscle spasticityClin Rehabil1996104043
- BhaktaBBCozensJABamfordJMChamberlainMAUse of botulinum toxin in stroke patients with severe upper limb spasticityJ Neurol Neurosurg Psychiatry199661130358676154
- SimpsonDMAlexanderDNO’BrienCFBotulinum toxin type A in the treatment of upper extremity spasticity: a randomized, double-blind, placebo-controlled trialNeurology1996465130613108628472
- WardABA summary of spasticity management: a treatment algorithmEur J Neurol20029Suppl 14852 discussion 53–6111918650
- PandyanADJohnsonGRPriceCICurlessRHBarnesMPRodgersHA review of the properties and limitations of the Ashworth and modified Ashworth Scales as measures of spasticityClin Rehabil199913537338310498344
- Van KuijkAAGeurtsACBevaartBJvan LimbeekJTreatment of upper extremity spasticity in stroke patients by focal neuronal or neuromuscular blockade: a systematic review of the literatureJ Rehabil Med2002342516112019580
- MutluALivaneliogluAGunelMKReliability of Ashworth and Modified Ashworth scales in children with spastic cerebral palsyBMC Musculoskelet Disord2008994418215332
- BrashearAWattsMWMarchettiAMagarRLauHWangLDuration of effect of botulinum toxin type A in adult patients with cervical dystonia: a retrospective chart reviewClin Ther200022121516152411192142
- SmithSJEllisEWhiteSMooreAPA double-blind placebo-controlled study of botulinum toxin in upper limb spasticity after stroke or head injuryClin Rehabil200014151310688339
- AshfordSTurner-StokesLGoal attainment for spasticity management using botulinum toxinPhysiother Res Int2006111243416594313
- MahoneyFIBarthelDWFunctional evaluation: the Barthel IndexMd State Med J196514616514258950
- KwonSHartzemaAGDuncanPWMin-LaiSDisability measures in stroke: relationship among the Barthel Index, the Functional Independence Measure, and the Modified Rankin ScaleStroke200435491892314976324
- BohannonRWSmithMBInterrater reliability of a modified Ashworth Scale of muscle spasticityPhys Ther19876722062073809245
- O’BrienCFInjection techniques for botulinum toxin using electromyography and electrical stimulationMuscle Nerve Suppl19976S176S1809826989
- Van der PuttenJJHobartJCFreemanJAThompsonAJMeasuring change in disability after inpatient rehabilitation: comparison of the responsiveness of the Barthel Index and the Functional Independence MeasureJ Neurol Neurosurg Psychiatry199966448048410201420
- CollinCWadeDTDaviesSHorneVThe Barthel ADL Index: a reliability studyInt Disabil Stud198810261633403500
- CampbellSESeymourDGPrimroseWRfor ACMEplus Project TeamA multi-centre European study of factors affecting the discharge destination of older people admitted to hospital: analysis of in-hospital data from the ACMEplus projectAge Ageing200534546747516043443
- Contopoulos-IoannidisDGKarvouniAKouriIIoannidisJPReporting and interpretation of SF-36 outcomes in randomised trials: systematic reviewBMJ2009338a300619139138
- BorgJWardABWisselJBEST Study GroupRationale and design of a multicentre, double-blind, prospective, randomized, European and Canadian study: evaluating patient outcomes and costs of managing adults with post-stroke focal spasticityJ Rehabil Med2011431152221174051
- CatyGDDetrembleurCBleyenheuftCDeltombeTLejeuneTMEffect of simultaneous botulinum toxin injections into several muscles on impairment, activity, participation, and quality of life among stroke patients presenting with a stiff knee gaitStroke200839102803280818635841
- ThorleyMDonagheySEdwardsPEvaluation of the effects of Botulinum toxin A injections when used to improve ease of care and comfort in children with cerebral palsy whom are non-ambulant: a double blind randomized controlled trialBMC Pediatr201212112022873758
- DionyssiotisYKiourtidisDKarvouniAKalionzoglouAKliafasITreatment of spasticity and functional outcome after botulinum toxin A (Botox®) injection in patients with neurologic lesionsChristodoulouNProceedings of the 8th Mediterranean Congress of Physical and Rehabilitation MedicineSeptember 29–October 2, 2010Limassol, CyprusBologna, ItalyMedimond20104753
- PathakMSNguyenHTGrahamHKMooreAPManagement of spasticity in adults: practical application of botulinum toxinEur J Neurol200613Suppl 1425016417597
- We Move Spasticity Study GroupDose, administration, and a treatment algorithm for use of botulinum toxin type A for adult onset muscle overactivity in patients with an upper motoneuron lesionA We Move Self-Study CME Activity2002154165
- DionyssiotisYBone loss and fractures in multiple sclerosis: focus on epidemiologic and physiopathological featuresInt J Gen Med2011450550921845056
- WisselJMüllerJDressnandtJManagement of spasticity associated pain with botulinum toxin AJ Pain Symptom Manage2000201444910946168
- MayerNHSimpsonDMDosing, administration, and a treatment algorithm for use of botulinum toxin A for adult-onset muscle overactivity in patients with an upper motoneuron lesionMayerNHSimpsonDMSpasticity: Etiology, Evaluation, Management and the Role of Botulinum ToxinBronx (NY)We Move (Worldwide Education and Awareness for Movement Disorders)2002154165
- SnowBJTsuiJKBhattMHVarelasMHashimotoSACalneDBTreatment of spasticity with botulinum toxin: a double-blind studyAnn Neurol19902845125152252363
- BaricichACardaSBertoniMMadernaLCisariCA single-blinded, randomized pilot study of botulinum toxin type A combined with non-pharmacological treatment for spastic footJ Rehabil Med2008401087087219242626