Abstract
Neridronate is an aminobisphosphonate, licensed in Italy for the treatment of osteogenesis imperfecta (OI) and Paget’s disease of bone (PDB). A characteristic property of neridronate is that it can be administered both intravenously and intramuscularly, providing a useful system for administration in homecare. In this review, we discuss the latest clinical results of neridronate administration in OI and PDB, as well as in osteoporosis and other conditions. We will focus in particular on the latest evidence of the effect of neridronate on treatment of complex regional pain syndrome type I.
Background
Findings of a recent study are shedding new light on neridronate (6-amino-1-hydroxy-hexylidene-1,1-bisphosphonate), an aminobisphosphonate widely used in the treatment of bone diseases and with specific therapeutic indications for osteogenesis imperfecta (OI) and Paget’s disease of bone (PDB) in Italy. Differently from other bisphosphonates, neridronate can be administered both intravenously and intramuscularly. The latter regimen has demonstrated to be particularly relevant in particular patient groups as its administration does not require hospitalization and is suitable for homecare.
Results from a recent multicenter interventional trial have demonstrated its efficacy also in the treatment of the complex regional syndrome type I, thus opening the way to new therapeutic indications.
Here we review the potential clinical uses of neridronate in several bone-related diseases, in light of most recent literature. A systematic review of clinical studies and randomized controlled trials on neridronate in bone diseases was carried out. This study is upgrading our previous work by reviewing papers published after 2009.Citation1
Chemical features
A characteristic of all bisphosphonates is their P-C-P chain,Citation2 where the negative charge of the phosphate groups attributes the compounds a high affinity for the positively charged calcium ions on the surface of the bone. The other two side chains that the carbon atom bears determine the biological activity of the bisphosphonateCitation2 and its categorization in the two classes of nitrogen-containing (pamidronate, alendronate, ibandronate, neridronate, and olpadronate) or non-nitrogen containing (clodronate, etidronate, and tiludronate) drugs.
The structure and three-dimensional conformation of these side chains determine the biological activity of the bisphosphonate,Citation3 with the aminobisphosphonates having a higher potency than the non-aminobisphosphonates.
The aminobisphosphonates inhibit farnesyl pyrophosphate synthase, a key enzyme in the mevalonate pathway, which is required for the prenylation of proteins that play key roles in intracellular signaling pathways that regulate processes fundamental to osteoclast function.Citation4
Belonging to the aminobisphosphonates, neridronate distinguishes itself for its intramuscular or intravenous formulations, which altogether prevent a number of complications entailed by oral bisphosphonates. In fact, although oral bisphosphonates are the treatment of choice for a variety of bone diseases, their administration bears an uncomfortable burden for patients, with inevitable consequences on compliance to therapy. Notoriously oral bisphosphonates can cause local irritation and ulceration of the esophagus and stomach,Citation5 and for this reason they require a large amount of water when ingested and must be taken in an upright posture.
Pharmacokinetic studies have indicated that bisphosphonates have low oral absorption. The oral bioavailability of bisphosphonates is lower than 1%Citation6 and therefore must be taken when fasting and separated from intake of food or other drugs. These difficulties in oral administration have indeed favored the development of parenteral formulations of bisphosphonates.
Clinical use of neridronate
Over the years, neridronate has proven efficacious for off-label treatment of many clinical bone-related pathologies, other than OI and PDB.Citation1 The following sections are an overview of the bone-related pathologies in which neridronate has been used to date and the most relevant underlying studies.
Osteogenesis imperfecta
OI is a hereditary disease caused by a collagen defect, mostly caused by mutations of the genes coding the chains of collagen type 1 (gene COL1A1 or COL1A2), which lead usually to autosomal dominant OI. The disorder causes increased bone fragility and low bone mass and deformity, and the severity of the disease varies extensively among patients.
Treatment with bisphosphonates, in particular oral treatment with pamidronate, was first reported on a 12-year-old child in 1987 by Devogelaer et al.Citation7 Since then, bisphosphonates (generally pamidronate) administered intravenously have become the common treatment in children,Citation8 with clinical evidence of significant increases in bone mineral density (BMD) and decrease in fracture incidence.Citation9–Citation11
Neridronate has been extensively investigated in patients with OI. In growing children, the neridronate treatment induces a rapid increase in BMD and a significant 64% decrease in fracture numbers,Citation12 and similar results have been obtained also in newborns (<12 months old) affected by the more severe forms of the disease, with some evidence of improvement in the rate of skeletal growth.Citation13 The treatment has also been tested in adults with OI, with evidence of efficacy in lowering fracture rate.Citation14
The drug was administered intravenously every 3 months diluted in 250 mL of saline solution and infused intravenously over 30 minutes.Citation12–Citation14 The dose was 2 mg/kg bodyweight, until a maximum dosage of 100 mg neridronate in adult patients.
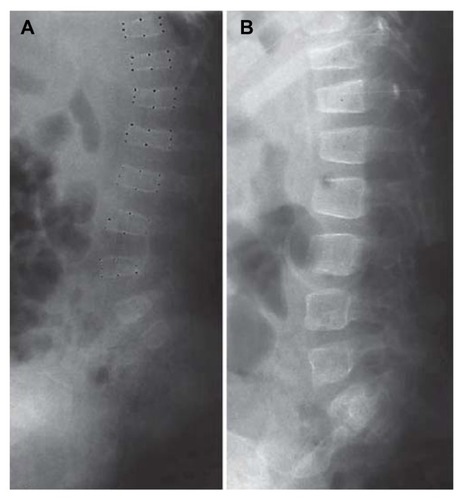
Recently, Semler et al,Citation15 in a study involving 28 children with severe or moderate OI, compared the treatment effect of the bisphosphonates pamidronate and neridronate. Both bisphosphonates are administered parenterally, the former given on 3 consecutive days every 4 months, the latter once every 3 months. To evaluate the effect of the bisphosphonates on the skeletal structure of OI patients, the authors focused on changes of the vertebral morphometry.
Pamidronate and neridronate proved equally efficient in improving vertebral area and vertebral indices of patients with OI (). The huge benefit in treating patients with neridronate instead of pamidronate is the reduced time of hospitalization. This is extremely important for the patients and their families and should lead to an increased quality of life in these severely handicapped children.
Figure 1 Vertebral bodies before (A) and after (B) treatment for 14 months for a child with Type I osteogenesis imperfecta.
As an agent increasing bone apposition, or increasing statural growth, growth hormone (GH) may play a therapeutic role in the treatment of OI. However, only few studies have been performed treating OI patients with GH.Citation16–Citation18
Recently, Antoniazzi et al have conducted a randomized controlled clinical trial evaluating the effects of a combined treatment of neridronate and recombinant GH (rGH) in children with mild and moderate OI.Citation19 The goal of the study was to investigate whether the combination of neridronate and rGH can further improve bone metabolism of children with OI who are already receiving treatment with neridronate. In this study, 30 prepubertal children, already in therapy with neridronate, were randomized to continue treatment with neridronate alone or to initiate a 12-month treatment combining neridronate and rGH. The effect of the combination of drugs was encouraging, as BMD at the lumbar spine and wrist as well as in the lumbar spine projected area increased significantly (P < 0.05). Moreover, the rate of linear growth velocity increased significantly with respect to the neridronate alone group.
A potential negative effect and serious complication of bisphosphonate therapy is osteonecrosis of the jaw, more commonly associated with intravenous administration of the drug.Citation20 However, although unusual cases have been reported in adults using aminobisphosphonates, no case of osteonecrosis of the jaw has been reported in patients treated with neridronate. Maines et al studied the occurrence of osteonecrosis of the jaw in pediatric patients in treatment with neridronate for a period ranging from 1.0 to 12.9 years. No evidence of osteonecrosis of the jaw could be demonstrated, even in those patients who had been treated with neridronate for the longer period.Citation21
Paget’s disease of bone
PDB is the most common metabolic bone disease after osteoporosis. To date, the etiology of the disease is still unclear, and both environmental factors and genetic susceptibility are thought to be involved.Citation22 The disease is characterized by focal increase of bone remodeling and disorganization of normal lamellar structure of the tissue, with consequent susceptibility to bone fractures, pain, and deformities.
PDB may involve one or more bones, and its distinctive feature is the large osteoclasts actively resorbing the bone. These cells seem to be adequately responding to bisphosphonate therapy, which therefore represents the treatment of choice. In particular, aminobisphosphonates are the most used PDB treatment. The response to treatment has been based on the changes in bone alkaline phosphatase (bAP) and variably defined by the full normalization of bAP or a decrease by .75%.
Neridronate is licensed in Italy for the treatment of Paget’s disease at the dose of 100 mg dissolved in 250–500 mL of saline solution given intravenously for 2 consecutive days. The registration was achieved after a multicenter clinical trial testing four different doses: 25, 50, 100, and 200 mg.Citation23 The highest dose was the most effective and was associated with a 65% rate of full remission and a biochemical response (decrease of .75%) in 95% of the patients.Citation23
Only few randomized clinical trials have compared the safety and efficacy of different bisphosphonates in PDB treatment. Namely, a comparison between oral alendronate and intravenous pamidronate proved that bisphosphonates had similar efficacy in achieving biochemical remission in previously untreated patients, but in those who had formerly been treated with pamidronate, alendronate was more effective.Citation24 In two other studies, a single intravenous dose of zoledronic acid proved more effective than a 2-month course of oral administration of risedronate in controlling bone turnover in the shortCitation25 and longCitation26 term.
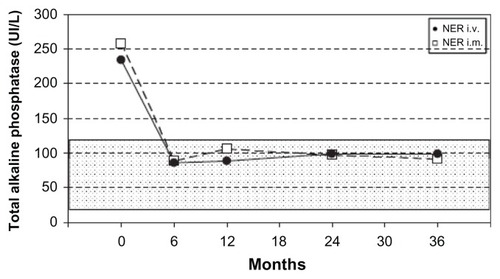
In a study conducted in 2007, Merlotti et al demonstrated that either zoledronic acid (4 mg) administered as a single intravenous regimen or neridronate (200 mg) given intravenously at the dose of 100 mg for 2 consecutive days showed a similar short-term efficacy in achieving biochemical remission at 6 and 9 months in up to 90% of patients with PDB, who did not respond to pamidronate therapy.Citation27 On the basis of the results obtained in this study, the authors consequently performed a randomized open-label study evaluating the long-term efficacy of the same neridronate dose (200 mg), either administered as an intravenous or intramuscular regimen, in 56 patients with PDB, with a 3-year follow-up.Citation28 After 6 months, 92.6% of patients receiving intravenous neridronate and 96.5% of patients with intramuscular regimen achieved a therapeutic response in terms of normalization of alkaline phosphatase levels or reduction of at least 75% in total alkaline phosphatase excess (). Response rates were maintained at 12 months but decreased progressively at 24 and 36 months in both neridronate regimens. At 6 months from treatment, 77.8% of patients in the intravenous regimen, and 86.2% of those in the intramuscular one, reported a decrease of bone pain at pagetic sites.
Figure 2 Mean serum alkaline phosphatase levels during the 36-month study for the intramuscular and intravenous neridronate regimens.
Abbreviations: NER, neridronate; i.v., intravenous; i.m., intramuscular.
Overall, the long-term tolerability of both neridronate regimens was excellent and comparable with that of the previous short-term studies with intravenous infusion. The intramuscular regimen in particular was associated mainly with fever as the major symptom of the acute-phase reaction, while intravenous administration was associated more frequently with fever and muscular pain. Both routes of administration of the 200 mg neridronate proved to have similar efficacy in the treatment of PDB. The intramuscular course of administration could be particularly useful for those patients unwilling or unable to undergo intravenous infusions or to take oral bisphosphonates, and represents an advantage as home treatment without major complications.
Postmenopausal osteoporosis
Bisphosphonates are the treatment of choice for postmenopausal as well as male and glucocorticoid (GC)-induced osteoporosis. Two pilot randomized-controlled studies evaluated the effect of neridronate on BMD. In the first study, performed in 78 women with postmenopausal osteoporosis,Citation29 neridronate (50 mg) was administered intravenously once every 2 months over 2 years and induced a significant and relevant increase in BMD (+7.4% and +5.8% at the lumbar spine and femoral neck, respectively). Similar results were obtained in the second pilot study, in which 40 postmenopausal women were treated with 25 mg neridronate monthly intramuscularly. The densitometric changes were 6.6% and 4.2% at the spine and hip, respectively.Citation30
A dose-finding clinical, multicenter trialCitation31 in 188 postmenopausal osteoporotic women randomized to intramuscular treatment with 25 mg neridronate every 2 weeks, neridronate 12.5 or 25 mg every 4 weeks, or placebo showed a significant dose-response relationship over the three doses for the BMD changes at the total hip and for serum C-terminal telopeptide of type I collagen changes but not for spine BMD and bAP changes. These results indicate that the 25 mg neridronate given intramuscularly monthly is probably the dose providing the maximum effects.
A recent ancillary study of this randomized controlled trial evaluated the changes in serum levels of the Wnt signaling antagonists sclerostin or dickkopf-1 (DKK1) during monthly intramuscular neridronate (12.5, 25.0, or 50.0 mg) treatment.Citation32 While serum DKK-1 remained stable during the entire 12-month study period in the three groups, serum sclerostin increased versus placebo group gradually and significantly only in patients treated with 25 or 50 mg neridronate monthly. The changes in serum sclerostin at 12 months were negatively correlated (P < 0.001), with changes in bAP (a bone-formation marker) even when data were adjusted for serum C-terminal telopeptide of type I collagen (a bone-resorption marker) changes and only when treated patients were included. This observation supports the hypothesis that the decrease in bone formation seen after several months of bisphosphonate therapy is associated with an increase in serum levels of sclerostin.Citation32 These results suggest that Wnt signaling may play a role in the coupling between resorption and formation as it also emerges from studies on other osteometabolic drugs such as teriparatideCitation33 and denosumab.Citation34
GC-induced osteoporosis
Another type of osteoporosis is that induced by GCs. As a matter of fact, the main effect of GCs on bone is the inhibition of osteoblast function leading to a decrease in bone formation, together with the interaction with biological membranes. Several studies and reports show a decrease in BMD and an increased risk of fractures during GC use. Bone loss, which takes place from the first phase of GC treatment and mainly occurs in the first 6 months, is predominant in bone with a high trabecular content (eg, vertebrae).Citation35 A long-standing use of GCs is typically indicated for rheumatic diseases such as rheumatoid arthritis, polymyalgia rheumatica, Sjögren’s syndrome, systemic lupus erythematosus, and other types of arthritis.
Noteworthy is that almost 30%–50% of rheumatic patients on long-term GC treatment develop GC-induced osteoporosis (GIO) and osteoporosis-related fractures.Citation35 In rheumatic patients, the diagnostic threshold in GIO, based on BMD measurements, is different from the established guidelines for postmenopausal osteoporosis. In fact, patients treated with GCs, even when showing BMD levels similar to those of nontreated subjects, have a higher risk of fractures.Citation36
Prevention of GIO preferably relies on treatment with oral bisphosphonates or with intravenous zoledronate, but in patients with gastric or esophageal disease, or in those intolerant to oral bisphosphonates, especially when hospitalization is difficult, neridronate might represent a possible alternative. In fact, neridronate (25 mg once monthly given intramuscularly) has also been tested in patients with GC-induced osteoporosis. Sixty-nine osteopenic and osteoporotic patients, affected by rheumatic diseases under chronic low-dose GC therapy and with gastric or esophageal conditions which contraindicated treatment with oral bisphosphonates, were randomly assigned to be treated with monthly intramuscular neridronate 25 mg or placebo. (All patients also took daily calcium at the dose of 1000 mg and vitamin D at the dose of 800 IU.)Citation37 The trial showed that a 12-month intramuscular neridronate treatment in this kind of patient improves lumbar and femoral BMD (+6.3% and +4.2% versus placebo at the lumbar and femoral neck respectively; P ≤ 0.01) and reduces the markers of bone resorption.Citation37 These results appear comparable to those obtained in rheumatic patients under GC therapy with the administration of oral risedronate and alendronate.
Thanks to the proven safety, as observed for pediatric patients affected by OI, and the high gastrointestinal tolerability related to the parenteral administration, neridronate can be considered a first-choice treatment for GIO occurring in inflammatory bowel disease patients. Diamanti et al reported the case of a pediatric Crohn’s disease patient who experienced GIO and related back pain 3 years after treatment with GCs.Citation38 Intravenous neridronate was started after the first dual-energy X-ray absorptiometry evaluation, diluted in 250 mL of saline solution and infused intravenously over 30 minutes. A total of three infusions were administered, which were well tolerated without side effects. After the third administration of neridronate, a total spine radiograph indicated an improved bone density and a recovered vertebral height. So far, back pain has not reappeared.Citation38
Complex regional pain syndrome type I (CRPS-1)
A plethora of names has been used to describe this syndrome (eg, reflex sympathetic dystrophy, causalgia, Sudeck’s atrophy, algodystrophy, neurodystrophy, post-traumatic dystrophy). Since the underlying pathophysiology is poorly understood, and no treatment has been found to be so effective to obtain the indication for this disease, CRPS-I still remains a matter of debate. CRPS-I is a severely disabling pain syndrome characterized by allodynia, hyperalgesia, edema, signs of vasomotor instability, movement disorders, joint stiffness, and regional osteopenia that in most cases develop following a trauma or surgery. Recently, Tran et al, by reviewing the literature published between 1950 and 2009, identified 41 randomized controlled trials which were suitable for their inclusion criteria.Citation39 Eighteen of those studies included the use of pharmacological therapies. A single intravenous infusion of 7.5 mg alendronate,Citation40 intravenous clodronate (300 mg for 10 days),Citation41 intravenous pamidronate (60 mg once),Citation42 and 40 mg daily oral alendronate for 12–16 weeksCitation43 have been reported to be associated with positive results in controlling pain, edema, and functional impairment.Citation44 However, none of these studies provided conclusive evidence of efficacy and sufficient data to make the use of a bisphosphonate formally indicated for the treatment of CRPS-I, mainly because of their limited size, with 10–20 treated patients per study.
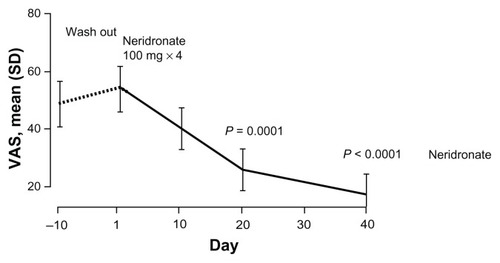
A recent multicenter, randomized, double-blind placebo-controlled trial tested the efficacy of neridronate in patients with CRPS-I.Citation45 The study involved 82 patients with CRPS-I at either hand or foot who were randomly assigned to intravenous infusion of 100 mg neridronate given every third day four times, starting from day 1 (first infusion) and ending on day 10 (fourth infusion) or placebo. After 50 days, the placebo group was treated with the same regimen of neridronate (open-extension phase). Within the first 20 days, the visual analog scale (VAS) score decreased significantly from baseline more in the neridronate group. In the following 20 days, VAS remained unchanged in the placebo group and further decreased in the active group (). A number of other indices of pain and quality of life were significantly improved with respect to the placebo group, and most of the patients in the active group were healed.Citation45 During the open-extension phase in the placebo group, the results of treatment were comparable to those seen during the blind phase in the active group (). A year later, none of the patients was referring symptoms linked to CRPS-I. These results provide conclusive evidence that the use of neridronate is associated with clinically relevant and persistent benefits and represents the treatment of choice for CRPS-I.Citation45
Figure 3 Double-blind phase: VAS trends from baseline to day 40 in patients with complex regional pain syndrome type I treated with neridronate or placebo.
Abbreviations: VAS, visual analog scale; SD, standard deviation.
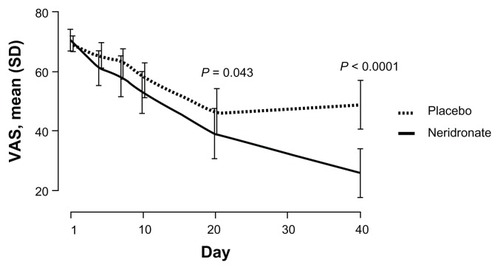
Figure 4 VAS values at the end of the follow-up period of the double-blind phase (day −10) and after the treatment course with intravenous neridronate in patients with complex regional pain syndrome type I.
Abbreviations: VAS, visual analog scale; SD, standard deviation.
Other conditions
Thalassemia
Osteopenia and osteoporosis are important causes of morbidity in patients with β-thalassemia major. In the pathogenesis of thalassemia-induced osteoporosis, genetic and acquired factors have been recognized.Citation46 Notwithstanding optimal therapeutic regimens, effective iron chelation therapy and adequate hormone replacement, unbalanced bone turnover and active bone resorption remain a major issue.Citation47–Citation49 The increased bone-turnover rate observed in thalassemic patients justifies the use of powerful antiresorptive drugs, such as bisphosphonates.
Treatment with intravenous neridronate was well tolerated and increased BMD in a large randomized trial involving 118 adults with β-thalassemia-induced osteoporosis.Citation50 Patients were randomized to calcium (500 mg) and vitamin D (400 UI) daily and neridronate (100 mg intravenously every 3 months) or calcium and vitamin D alone. Significant increases in BMD at the lumbar spine and total hip were noted in the neridronate group at 6 and 12 months from baseline (P < 0.001), and values were significantly higher than the control group at both time intervals. As expected, neridronate also induced a significant reduction of bone-turnover markers. Improvement in quality of life assessed as reduction in back pain and analgesic use became evident after only 3 months of therapy.Citation50
Primary hyperparathyroidism (PHPT)
The decrease in BMD and the moderate increase of fracture risk are often the only clinical manifestations of PHPT.Citation51 In those patients not suitable, or unwilling, to undergo surgery, treatment with antiresorptive agents is a reasonable option. In a recent study, Rossini et al tested the effect of intravenous administration of neridronate (100 mg every 2 months for 2 years) on BMD and biochemical markers of bone metabolism in postmenopausal women with PHPT.Citation51 Patients were followed up for 2 years after therapy discontinuation. Neridronate was well tolerated. BMD progressively rose to 6.6% ± 7.6%, 2.9% ± 4.5%, and 5.0% ± 3.9% at the lumbar spine, femoral neck, and total hip, respectively. The mean peak value was achieved 6 months after the last infusion at month 24. BMD values decreased during follow-up; however, 2 years after treatment discontinuation, lumbar spine BMD remained 3.9% ± 5.5% higher than baseline values (P < 0.01), while femoral BMD returned to baseline. As regards to bone-turnover markers, bAP and serum C-telopeptide of type I collagen decreased significantly within 6 months, the nadir value being after 18–24 months (P < 0.01). After 6 months of therapy discontinuation, all these bone-turnover markers returned to baseline.
Periodontitis
Periodontitis is a multifactorial chronic infectious disease characterized by a loss of the connective tissue attachment to the teeth and the resorption of the alveolar bone due to the inflammatory processes. The etiology of periodontal disease is reducible to bacterial infection, which results in an inflammatory reaction. Tissue damage is generated by collagenolytic enzymes such as matrix metalloproteinases (MMPs) which significantly contribute to periodontal tissue damage. Therapies aimed at blocking tissue damage mediated by MMPs and at blocking alveolar bone destruction are actively being sought. With this scope, bisphosphonates, due to their action on bone metabolism, and in some cases to their downregulation of MMPs enzymes, are currently being investigated as adjunctive therapy in the management of periodontal disease.
In an open-label, randomized, clinical trial, Graziani et al investigated the effect of the addition of intramuscular administration of neridronate (12.5 mg weekly for 3 months) in patients with advanced generalized chronic periodontal disease treated with conventional nonsurgical periodontal therapy as compared with the one obtained with conventional treatment alone.Citation52 After discontinuation of treatment, patients were followed-up for 3 months. Although at the end of the study both groups showed improvements at sites with deep pocket depth, no difference was observed between patients treated with conventional nonsurgical therapy and conventional nonsurgical therapy with addition of neridronate. As argued by the authors, one of the possible reasons for the lack of clinical effects 3 months after the end of drug intake could be related to an insufficient dosage used to expect an effective action on the osseous metabolism of the alveolar bone.
Malignancy
Bisphosphonates had been used for years as standard therapy of bone diseases related to malignancy. In breast cancer patients with bone metastasis, several bisphosphonates demonstrated clinical efficacy. Zoledronic acid was the most extensively studied bisphosphonate in patients with bone metastases from prostate cancer or other solid tumors, and it remains the only bisphosphonate registered worldwide for these indications.Citation53 In patients with multiple myeloma, bisphosphonate treatment reduces the risk of pathological vertebral fractures, skeletal-related morbidity, and pain.Citation54 The bisphosphonates most often used are ibandronate, pamidronate, and zoledronate. The data on neridronate are rather limited. In seven patients with bone lesions for multiple myeloma, 100 mg intravenous neridronic acid, monthly over a year, was associated with significant increases in spine BMD and decreases in bone-turnover markers.Citation55 In patients with hypercalcemia owing to solid metastatic tumors, an infusion of 125 mg neridronic acid (dissolved in 500 mL of saline solution) significantly decreased serum calcium inducing to normalization in 65% of the patients.Citation56
Currently, the data are insufficient to extend the use of neridronate for these indications, although in-vitro studiesCitation57,Citation58 and preliminary data on pediatric populationsCitation59 could open new opportunities in the future.
Conclusion
Neridronate is an aminobisphosphonate licensed for the treatment of OI and PDB. Its chemical structure and potency is very close to that of pamidronic acid. The results of a recent randomized, well powered clinical trial for the treatment of CRPS-I were very encouraging and should soon lead to a license for a new indication. Neridronate may be administered both intravenously and intramuscularly. This latter regimen is of particular interest and so far unique, allowing homecare treatments. According to the accumulated experiences from studies carried out in children with OI, this is to date the only bisphosphonate that can be used in children and in newborns.
Disclosure
Davide Gatti and Maurizio Rossini have received speaking fees from Amgen, Abiogen, Merck, Eli-Lylli, and Pfizer. Ombretta Viapiana has received speaking fees from Abiogen, Merck, and Pfizer. Luca Idolazzi has received speaking fees from Novartis. Silvano Adami is consultancy honoraria for Merck, and Amgen, and has received speaking fees from Amgen, Abiogen, Merck, Eli-Lylli, Pfizer, Novartis, and GSK.
References
- GattiDViapianaOIdolazziLFracassiEAdamiSNeridronic acid for the treatment of bone metabolic diseasesExpert Opin Drug Metab Toxicol20095101305131119761412
- EbetinoFHHoganAMSunSThe relationship between the chemistry and biological activity of the bisphosphonatesBone2011491203321497677
- EbetinoFHBaylessAVAmburgeyJIbbotsonKJDansereauSEbrahimpourAElucidation of a pharmacore for the bisphosphonate mechanism of bone antiresorptive activityPhosphorus Sulfur Silicon Relat Elem19961091–4217220
- AminDCornellSAGustafsonSKBisphosphonates used for the treatment of bone disorders inhibit squalene synthase and cholesterol biosynthesisJ Lipid Res199233165716631464749
- GrahamDYMalatyHMGoodgameRPrimary amino-bisphosphonates: a new class of gastrotoxic drugs-comparison of alendronate and aspirinAm J Gastroenterol1997928132213259260798
- LinJHBisphosphonates: a review of their pharmacokinetic propertiesBone199618275858833200
- DevogelaerJPMalghemJMaldagueBNagant de DeuxchaisnesCRadiological manifestations of bisphosphonate treatment with APD in a children suffering from osteogenesis imperfectaSkeletal Radiol19871653603633629280
- RauchFGlorieuxFHOsteogenesis imperfectaLancet200436394181377138515110498
- GlorieuxFHBishopNJPlotkinHChabotGLanoueGTraversRCyclic administration of pamidronate in children with severe osteogenesis imperfectaN Engl J Med1998339149479529753709
- LandCRauchFMunnsCFSahebjamSGlorieuxFHVertebral morphometry in children and adolescents with osteogenesis imperfecta: effect of intravenous pamidronate treatmentBone200639490190616730480
- PlotkinHRauchFBishopNJPamidronate treatment of severe osteogenesis imperfecta in children under 3 years of ageJ Clin Endocrinol Metab20008551846185010843163
- GattiDAntoniazziFPrizziRIntravenous neridronate in children with osteogenesis imperfecta: a randomized controlled studyJ Bone Miner Res200520575876315824848
- AntoniazziFZamboniGLauriolaSDonadiLAdamiSTatòLEarly bisphosphonate treatment in infants with severe osteogenesis imperfectaJ Pediatr2006149217417916887429
- AdamiSGattiDColapietroFIntravenous neridronate in adults with osteogenesis imperfectaJ Bone Miner Res200318112623012510813
- SemlerOBeccardRPalmisanoDReshaping of vertebrae during treatment with neridronate or pamidronate in children with osteogenesis imperfectaHorm Res Paediatr201176532132721952409
- MariniJCBordenickSHeavnerGThe growth hormone and somatomedin axis in short children with osteogenesis imperfectaJ Clin Endocrinol Metab19937612512568421094
- AntoniazziFBertoldoFMottesMGrowth hormone treatment in osteogenesis imperfecta with quantitative defect of type I collagen synthesisJ Pediatr199612934324398804334
- MariniJCHopkinsEGlorieuxFHPositive linear growth and bone responses to growth hormone treatment in children with types III and IV osteogenesis imperfecta: high predictive value of the carboxyterminal propeptide of type I procollagenJ Bone Miner Res200318223724312568401
- AntoniazziFMontiEVenturiGGH in combination with bisphosphonate treatment in osteogenesis imperfectaEur J Endocrinol2010163347948720592128
- GuttaRLouisPJBisphosphonates and osteonecrosis of the jaws: science and rationaleOral Surg Oral Med Oral Pathol Oral Radiol Endod2007104218619317448709
- MainesEMontiEDoroFMorandiGCavarzerePAntoniazziFChildren and adolescents treated with neridronate for osteogenesis imperfecta show no evidence of any osteonecrosis of the jawJ Bone Miner Metab201230443443822065238
- FalchettiAMariniFMasiLAmedeiABrandiMLGenetic aspects of the Paget’s disease of bone: concerns on the introduction of DNA-based tests in the clinical practice. Advantages and disadvantages of its applicationEur J Clin Invest201040765566720658751
- AdamiSBevilacquaMBrogginiMShort-term intravenous therapy with neridronate in Paget’s diseaseClin Exp Rheum20022015558
- WalshJPWardLCStewartGOA randomized clinical trial comparing oral alendronate and intravenous pamidronate for the treatment of Paget’s disease of boneBone200434474775415050907
- ReidIRMillerPLylesKComparison of a single infusion of zoledronic acid with risedronate for Paget’s diseaseN Engl J Med2005353989890816135834
- HoskingDLylesKBrownJPLong-term control of bone turnover in Paget’s disease with zoledronic acid and risedronateJ Bone Miner Res200722114214817032148
- MerlottiDRendinaDGennariLComparison of different intravenous bisphosphonate regimens for Paget’s disease of boneJ Bone Miner Res200722101510151717605632
- MerlottiDRendinaDGennariLComparison of Intravenous and Intramuscular neridronate regimens for the treatment of Paget disease of boneJ Bone Miner Res201126351251820814970
- BragaVGattiDColapietroFIntravenous intermittent neridronate in the treatment of postmenopausal osteoporosisBone200333334234513678775
- CascellaTMusellaTOrioFEffects of neridronate treatment in elderly women with osteoporosisJ Endocrinol Invest200528320220815952402
- AdamiSGattiDBertoldoFIntramuscular neridronate in postmenopausal women with low bone mineral densityCalcif Tissue Int200883530130718946626
- GattiDViapianaOAdamiSIdolazziLFracassiERossiniMBisphosphonate treatment of postmenopausal osteoporosis is associated with a dose dependent increase in serum sclerostinBone201250373974222178539
- GattiDViapianaOIdolazziLFracassiERossiniMThe waning of teriparatide effect on bone formation markers in postmenopausal osteoporosis is associated with increasing serum levels of DKK1J Clin Endocrinol Metab20119651555155921367927
- GattiDViapianaOFracassiESclerostin and DKK1 in postmenopausal osteoporosis treated with denosumabJ Bone Miner Res201227112259226322692843
- De NijsRNGlucocorticoid-induced osteoporosis: a review on pathophysiology and treatment optionsMinerva Med2008991234318299694
- CanalisEBilezikianJPAngeliAGiustinaAPerspectives on glucocorticoid induced osteoporosisBone200634459359815050888
- BenucciMSaviolaGBaiardiPEffects of monthly intramuscular neridronate in rheumatic patients in chronic treatment with low-dose glucocorticoidsClin Exp Rheumatol200927456757319772786
- DiamantiANotoCBassoMSPapadatouBBracciFCastroMEfficacy and safety of intravenous neridronate in pediatric bone loss associated to Crohn’s disease: a case reportClin Exp Rheumatol200927116519327248
- Tran deQHDuongSBertiniPFinlaysonRJTreatment of complex regional pain syndrome: a review of the evidenceCan J Anaesth201057214916620054678
- AdamiSFossaluzzaVGattiDBisphosphonate therapy of reflex sympathetic dystrophy syndromeAnn Rheum Dis1997562012049135227
- VarennaMZucchiFGhiringhelliDIntravenous clodronate in the treatment of reflex sympathetic dystrophy syndrome. A randomized, double blind, placebo controlled studyJ Rheumatol2000271477148310852274
- RobinsonJNSandomJChapmanPTEfficacy of pamidronatein complex regional pain syndrome type IPain Med2004527628015367305
- ManicourtDHBrasseurJPBoutsenYRole of alendronate in therapy for post-traumatic complex regional pain syndrome type I of the lower extremityArthritis Rheum2004503690369715529370
- BrunnerFSchmidAKisslingRBiphosphonates for the therapy of complex regional pain syndrome I – systematic reviewEur J Pain200913172118440845
- VarennaMAdamiSRossiniMTreatment of complex regional pain syndrome type I with neridronate: a randomized, double-blind, placebo-controlled studyRheumatology201352353454223204550
- HaidarRMusallamKMTaherATBone disease and skeletal complications in patients with β thalassemia majorBone201148342543221035575
- LascoAMorabitoNGaudioABuemiMWasniewskaMFrisinaNEffects of hormonal replacement therapy on bone metabolism in young adults with beta-thalassemia majorOsteoporos Int200112757057511527055
- KarimiMGhiamAFHashemiAAlinejadSSoweidMKashefSBone mineral density in beta-thalassemia major and intermediaIndian Pediatr2007441293217277428
- BaldiniMFortiSMarconAEndocrine and bone disease in appropriately treated adult patients with beta-thalassemia majorAnn Hematol201089121207121320582415
- ForniGLPerrottaSGiustiANeridronate improves bone mineral density and reduces back pain in β-thalassaemia patients with osteoporosis: results from a phase 2, randomized, parallel-arm, open-label studyBr J Haematol2012158227428222571408
- RossiniMViapianaOKalpakciogluBLong-term effects of neridronate and its discontinuation in patients with primary hyperparathyroidismCalcif Tissue Int2011891212821567168
- GrazianiFCeiSGuerreroALack of short-term adjunctive effect of systemic neridronate in non-surgical periodontal therapy of advanced generalized chronic periodontitis: an open label-randomized clinical trialJ Clin Periodontol200936541942719419443
- Mackiewicz-WysockaMPankowskaMWysockiPJProgress in the treatment of bone metastases in cancer patientsExpert Opin Investig Drugs2012216785795
- MhaskarRRedzepovicJWheatleyKBisphosphonates in multiple myeloma: a network meta-analysisCochrane Database Syst Rev20125CD00318822592688
- PittariGCostiDRaballoMIntravenous neridronate for skeletal damage treatment in patients with multiple myelomaActa Biomed200677818417172186
- O’RourkeNPMcCloskeyEVRosiniSTreatment of malignant hypercalcaemia with aminohexane bisphosphonate (neridronate)Br J Cancer1994699149178180023
- ChebbiIMigianu-GriffoniESainte-CatherineOLecouveyMSeksekOIn vitro assessment of liposomal neridronate on MDA-MB-231 human breast cancer cellsInt J Pharm20103831–211612219748562
- NicolinVNarducciPBareggiRRole of neridronate on MCF-7 estrogen dependent breast cancer model of bone metastasis: a preliminary studyInvest New Drugs201129118919119798468
- ClericoAPaianoMSuraciSMarucciGZambranoAVarrassoGIntravenous neridronate in children affected by malignancies: preliminary results; Pediatric Blood & Cancer39th Annual Conference of the International Society of Paediatric Oncology SIOP 2007Mumbai, India31 October–3 November 2007 SIOP Abstracts49Supplement 6 pages 5131102007