Abstract
Various drugs and surgical procedures have been utilized for the treatment of trigeminal neuralgia (TN). Despite numerous available approaches, the results are not completely satisfying. The need for more contemporaneous drugs to control the pain attacks is a common experience. Moreover, a number of patients become drug resistant, needing a surgical procedure to treat the neuralgia. Nonetheless, pain recurrence after one or more surgical operations is also frequently seen. These facts reflect the lack of the precise understanding of the TN pathogenesis. Classically, it has been related to a neurovascular compression at the trigeminal nerve root entry-zone in the prepontine cistern. However, it has been evidenced that in the pain onset and recurrence, various neurophysiological mechanisms other than the neurovascular conflict are involved. Recently, the introduction of new magnetic resonance techniques, such as voxel-based morphometry, diffusion tensor imaging, three-dimensional time-of-flight magnetic resonance angiography, and fluid attenuated inversion recovery sequences, has provided new insight about the TN pathogenesis. Some of these new sequences have also been used to better preoperatively evidence the neurovascular conflict in the surgical planning of microvascular decompression. Moreover, the endoscopy (during microvascular decompression) and the intraoperative computed tomography with integrated neuronavigation (during percutaneous procedures) have been recently introduced in the challenging cases. In the last few years, efforts have been made in order to better define the optimal target when performing the gamma knife radiosurgery. Moreover, some authors have also evidenced that neurostimulation might represent an opportunity in TN refractory to other surgical treatments. The aim of this work was to review the recent literature about the pathogenesis, diagnosis, and medical and surgical treatments, and discuss the significant advances in all these fields.
Introduction
Trigeminal neuralgia (TN) is a facial pain syndrome characterized by paroxysmal, shock-like pain attacks located in the somatosensory distribution of the trigeminal nerve. The prevalence of TN in the general population is 0.015%.Citation1 Facial pain has a considerable impact on quality of life. It has been recently shown that TN is the most frequent type of facial painCitation2 and that, among facial pain syndromes, the overall incidence of TN has remained constantCitation3 ranging from 12.6/100,000/yearCitation2 to 27/100,000/year.Citation3 TN is uncommon in population younger than 40 years (overall incidence of 0.2/100,000/year) and increases in incidence with advancing age, occurring in 25.9/100,000/year in individuals older than 80 years.Citation4 TN appears to be slightly more common among women and has both classical and symptomatic (~15% of cases) subtypes with the former most often associated with a neurovascular conflict of the trigeminal nerve in the prepontine cistern.Citation5 The right side is more frequently involved.Citation6 When TN occurs in young age or presents with bilateral symptoms, lack of triggered pain, absence of a refractory period, or an abnormal neurologic examination, secondary causes such as multiple sclerosis (MS) should be suspected.Citation5 Bilaterality may be seen in 5% of classical cases, but even in these cases, synchronous pain is not observed. Patients with bilateral TN often have a positive family history.Citation7 In patients affected by MS, prevalence is higher, ranging from 1%Citation8 to 6.3%.Citation9 In these patients, in addition to the episodic pain, a constant pain component is often complained. Although in these patients, pain is mainly unilateral, bilateral involvement can occur up to 31% of patients.Citation10
Advances in diagnosis
Clinical criteria for TN
The International Headache Society recently defined strict clinical criteria for TN diagnosis.Citation11 According to these criteria, a diagnosis of TN can be made when at least three attacks of unilateral facial pain occur fulfilling these criteria: 1) occurring in one or more divisions of the trigeminal nerve, with no radiation beyond the trigeminal distribution and 2) pain with at least three of the following four characteristics: a) recurring in paroxysmal attacks lasting from a fraction of a second to 2 minutes; b) severe intensity; c) electric shock-like, shooting, stabbing, or sharp in quality; and d) precipitated by innocuous stimuli to the affected side of the face. Important criteria for clinical diagnosis are also the lack of evident neurologic deficit and a pain that cannot be attributed to another disorder. Moreover, to rationalize the different subtypes of facial pain, a new classification scheme that divides facial pain into several distinct categories has been recently introduced.Citation12
More specifically, in this new classification,Citation12 it has been proposed to differentiate TN into: 1) type 1 (previously referred to as classic or typical TN), which is an idiopathic episodic pain with the previously reported clinical characteristics, lasting several seconds, with pain-free intervals between attacks and 2) type 2, describing idiopathic trigeminal facial pain that is aching, throbbing, or burning for more than 50% of the time and is constant in nature (constant background pain being the most significant attribute) with a minor component of sharp, episodic pain. It has also been theorized that TN type 1 can progress toward TN type 2 and that in this second type, the likelihood of detecting a structural abnormality such as a tumor or a vascular malformation is higher.Citation12
Nonetheless, the neurophysiological recording of trigeminal reflexes represents a useful and reliable test for the TN diagnosis, according to the European Federation of Neurological Societies (EFNS) guidelines on neuropathic pain assessmentCitation13 and the American Academy of Neurology–EFNS guidelines on TN management ().Citation14,Citation15 In cases of symptomatic TN, neurophysiological testing of trigeminal reflexes seems to provide the same sensitivity (95%) and specificity (93%) as magnetic resonance imaging (MRI).Citation16 Advances in MRI have been playing an important role in the diagnostic setting, especially in the presurgical evaluation of TN patient in order to identify secondary causes of TN and/or the neurovascular conflict. Studies have been publishedCitation17,Citation18 on the usefulness of three-dimensional fast imaging employing steady-state acquisition and magnetic resonance angiography (MRA) in surgical planning and prediction of surgical findings during micro-vascular decompression (MVD). A correlation more than 95% between this method and surgical findings has been demonstrated.Citation17,Citation18
Table 1 AAN–EFNS guidelines on TN management
Pathophysiological theories
Classically, TN has been related to a neurovascular compression in the prepontine cistern at the nerve root entry-zone due to an abnormal artery or vein, arteriovenous malformation, vestibular schwannoma, meningioma, epidermoid cyst, tuberculoma, various other cysts and tumors, aneurysm, vessels aggregation, and arachnoiditis.Citation19–Citation21 MS, diabetes mellitus, odontogenic inflammatory diseases, and otolaryngological pathology, such as sinusitis, have also been proposed as causes of TN.Citation22–Citation31
From a pathogenic point of view, TN shows a high complexity related to the involvement of various underlying neurophysiological mechanisms. Activation of peripheral receptor, transmission and projection of nociceptive information, and convergence of nociceptive afferents onto common central neurons,Citation32 as well as the interaction of a multitude of neurotransmitters and neuromodulators, may play a key role in the perception of pain.Citation33
Trigeminal convergence-projection theory
In the trigeminal convergence-projection theory, it has been hypothesized that continuous or recurrent nociceptive inputs from head and neck converge on spinal trigeminal nucleus (subnucleus caudalis), where the release of neurotransmitters and vasoactive substances may be promoted.Citation34,Citation35 This release decreases the threshold of adjacent second-order neurons that receive input from sites other than nociceptive sources. The signals from these excited second-order neurons may be transmitted to the thalamus, limbic system, and somatosensory cortex and interpreted as pain.Citation36
Bioresonance hypothesis
Recently, the bioresonance hypothesis for TN pathogenesis has been proposed. This theory states that when the vibration frequency of a structure surrounding the trigeminal nerve becomes close to its natural frequency, the resonance of the trigeminal nerve occurs. The bioresonance can damage trigeminal nerve fibers and lead to the abnormal transmission of the impulse, which may finally result in facial pain.Citation37
Ignition hypothesis
According to the ignition hypothesis, based on recent advances in the understanding of the electrical behavior of injured sensory neurons and on findings from histopathologic observations obtained from patients undergoing MVD, injury of trigeminal afferent neurons in the trigeminal root or ganglion makes these axons and axotomized somata hyperexcitable, giving rise to pain paroxysms as a result of synchronized afterdischarge activity.Citation38
Pathogenesis possibilities from brain imaging studies
New insights about the pathogenesis of TN have been coming from new MRI studies, such as voxel-based morphometry,Citation39 diffusion tensor imaging (DTI),Citation40 three-dimensional time- of-flight (TOF) MRA, and fluid-attenuated inversion recovery DTI-sequences.Citation41 Moreover, it has been evidenced that using functional MRI (fMRI), changes in brain activity associated with stimulation of the cutaneous trigger zone in patients with TN can be analyzed. Recently, Moisset et alCitation42 showed that painful stimuli in TN patients were associated with significantly increased activity in the spinal trigeminal nucleus, thalamus, primary and secondary somatosensory cortices, anterior cingulate cortex, insula, premotor/motor cortex, prefrontal areas, putamen, hippocampus, and brain-stem and that non-painful stimulation of the trigger zone activated all but three of these structures (spinal trigeminal nucleus, brainstem, and anterior cingulate cortex). This wide involvement of different neural structures also during non-painful stimulation of the trigger zone suggests a state of maintained sensitization of the trigeminal nociceptive systems. Interestingly, after a successful surgical treatment, the activation of the operated side was confined only to primary and secondary somatosensory cortices.Citation42 A gray matter volume reduction in TN patients was found, using voxel-based morphometry, in the primary somatosensory and orbitofrontal cortices, as well as in the secondary somatosensory cortex, thalamus, insula, cerebellum, and dorsolateral prefrontal cortex. Gray matter volume decreased within the anterior cingulate cortex, parahippocampus, and temporal lobe and correlated with increasing disease duration in TN, reflecting adaptation mechanism to chronic pain with regard to neuronal plasticity.Citation39 Similarly, using DTI, a lower fractional anisotropy, reflecting an abnormal tissue microstructure, was found in TN patients’ trigeminal nerves and in white matter in the brain, suggesting that trigeminal nerve structural abnormalities occur in TN, even if not apparent on gross imaging.Citation40 To investigate microstructural tissue changes of trigeminal nerve in patients with unilateral TN, Liu et alCitation41 used TOF MRA and fluid-attenuated inversion recovery DTI-sequences, and measured fractional anisotropy, mean diffusivity, axial diffusivity, and radial diffusivity on the involved trigeminal nerve. They found that the affected side showed significantly decreased fractional anisotropy and increased radial diffusivity, suggesting that demyelination without significant axonal injury is an important factor in TN pathogenesis.Citation41 Moreover, these new MRI techniques together with trigeminal tractography have also been utilized to identify microstructural changes in the trigeminal nerve after radiosurgery and possibly monitor the response to this treatment. In patients submitted to radiosurgery, a drop in fractional anisotropy values at the target with no significant change outside the target was evidenced, demonstrating highly focal changes after treatment. Radial diffusivity also changed markedly, suggesting that radiosurgery primarily affects myelin. Fractional anisotropy changes were detected regardless of trigeminal nerve enhancement, suggesting more sensitivity of tractography than conventional gadolinium-enhanced post-treatment MRI. In subjects with long-term follow-up, recovery of fractional anisotropy/radial diffusivity correlated with pain recurrence.Citation43
Advances in medical therapy
Historical and current medical therapy
Phenytoin was the first drug used for TN with reported positive effects.Citation44 However, according to the recent EFNS guidelines,Citation13 two drugs are considered as first-line therapy in TN: carbamazepine (CBZ; 200–1,200 mg/day) and oxcarbazepine (OXC; 600–1,800 mg/day). The effectiveness of CBZ was demonstrated in several studies.Citation45–Citation50 Specifically, CBZ has been found to reduce both the frequency and intensity of painful paroxysms and was equally efficacious on spontaneous and trigger-evoked attacks.Citation45 Nevertheless, frequent adverse event has been reported during CBZ therapy, especially in elderly patients.Citation51–Citation53 Thus, OXC is often used as initial treatment for TNCitation54 due to accepted greater tolerability and decreased potential drug interactions.Citation55 Three randomized controlled trials, comparing OXC (600–1,800 mg/day) to CBZ in TN patients,Citation56,Citation57 reported a reduction in the number of attacks and pain assessments equally good for both CBZ and OXC with more than 80% of patients responding to these drugs. Other drugs have been used in TN: baclofen was found to be superior to placebo in reducing the number of pain attacks.Citation58 Lamotrigine,Citation59 pimozide,Citation60 and tocainideCitation61 were reported to have good efficacy on pain attacks control. Lamotrigine in combination with CBZ or phenytoin was also found to be more effective than placebo.Citation59,Citation62 In patients having already undergone trigeminal surgery or taking concurrent medications, tizanidine was found to be better than placebo, but its effect decayed within 1–3 months.Citation63
Emerging medical therapy
It is common experience that TN can be difficult to treat and can recur after surgical treatments in patients under therapy with more drugs used in combination. Thus, new therapeutic modalities have been tried. More specifically, according to a recent overview,Citation64 gabapentin combined with regular ropivacaine injections into trigger sites improved pain control and quality of life, and pregabalin was found to be effective at 1 year follow-up in TN patients. Recently, Hu et alCitation65 systematically reviewed the therapeutic efficacy and safety of injection of botulinum toxin type A (BTX-A) in TN and found a response in approximately 70%–100% of patients with mean pain intensity and frequency reduced by approximately 60%–80% with no major adverse events reported. On these bases, they concluded that BTX-A may be effective in treatment of TN. These results are in agreement with Cruccu and Truini,Citation66 who recently reviewed the literature on the medical management of refractory TN and found that there is increasing evidence that BTX-A injections are efficacious and may be offered to patients before surgery or to patients unwilling to undergo surgery. Although it represents a promising treatment of TN with favorable risk-to-benefit ratio, to investigate the optimal dose of BTX-A treatment, the duration of therapeutic efficacy, the side effects, and the time and indications for repeat injection, further well-designed, randomized, controlled, double-blinded trials are needed. As recently evidenced, a problem in TN is the treatment of the acute crisis, where local anesthesia, such as ropivacaine, injected into a trigger area, an 8% spray of lidocaine, and the intravenous infusion of fosphenytoin can provide temporary pain relief.Citation64
Advances in surgical therapy
Various surgical approaches have been proposed for the treatment of drug-resistant TN. MVD is performed with the objective to resolve the neurovascular conflict between an abnormal vessel and the trigeminal nerve. On the other hand, percutaneous destructive procedures, involving a trans foramen ovale approach to the retrogasserian portion of the trigeminal nerve and gamma knife radiosurgery (GKRS) aiming at damaging the trigeminal nerve root with a high and concentrated dose of radiation, have been developed during the past years. While there is a wide literature about the surgical treatment of TN,Citation67,Citation68 the difficulty to evaluate the quality of published surgical reports is an emerging problemCitation69 as recently evidenced by international guidelines and systematic reviews.Citation67,Citation70
MVD
MVD is based on the assumption that a compression of trigeminal nerve by an abnormal vascular loop is the direct cause of TN.Citation71 Obviously, preoperatory radiological studies are mandatory in order to identify the abnormal vessels and the conflict with the nerve. Recently, three-dimensional fast imaging employing steady-state acquisition sequence that produces a very high-resolution T2-weighted MRI with an excellent contrast between structures, including cerebrospinal fluid, trigeminal nerve and adjacent blood vessels, and TOF MRA have been introduced. MVD has become one of the most common treatments for TN providing long pain relief. Unfortunately, not all patients achieve a good outcome after MVD.Citation14 The reported pain-free duration without medication after MVD ranges from 0.6 years to 10 years.Citation72 After 5 years, the percentage of patients free of pain ranges from 58% to 78%.Citation73,Citation74 It has been reported that patients with typical TN and immediate postoperative remission have more often an excellent/good postoperative outcome, being the immediate postoperative remission an independent predictive factor for good long-term outcome.Citation74 Unfortunately, as recently evidenced, no randomized controlled trials of reasonable quality have investigated the role of MVD in the TN treatment.Citation64,Citation70 Moreover, little is reported in the literature about the quality of life after MVD, but it has been evidenced as patients undergoing primary surgery with no recurrence and no complications show no evidence of depression and are very satisfied after MVD.Citation75 Described complications after this procedure are infections (), facial palsy, facial numbness, cerebrospinal fluid leak, and hearing deficit with a mortality of 0.1%.Citation76 Obviously, complications and side effects reduce satisfaction mainly after the partial sensory rhizotomy, which causing a sensory loss can lead to keratitis and eating difficulties, decreasing satisfaction of these patients compared to MVD patients.Citation75 It is a common experience that in some cases, identifying the neurovascular conflict cannot be easy during surgery. Recently, some authors reported the use of endoscope as a significant aid in patients with a bony ridge obscuring the view of the fifth nerve, with a very distal vascular compression, or if a combination of both occurs.Citation76,Citation77 Broggi et alCitation76 reported 8.5% of cases in whom conflict was not clearly visible with the microscope but revealed and solved with the endoscope. A fully endoscopic MVD has been describedCitation78 with pain outcome and rate of complications very similar to microscopic MVD.Citation77,Citation79
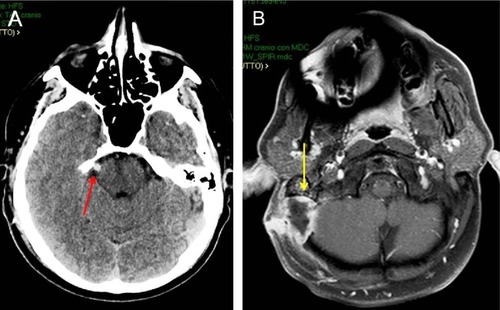
Figure 1 Postoperative CT scan of a 40-year-old man submitted to MVD for right TN (A; red arrow). Brain axial MRI after gadolinium administration (B) 2 months after MVD, showing an abscess at the site of operation (yellow arrow).
Percutaneous balloon compression
Percutaneous balloon compression (PBC) was introduced in the clinical setting by Mullan et alCitation79,Citation80 and has been extensively used in the treatment of TN due to low cost, simplicity, and the advantage of being the only percutaneous procedure performed with the patient under general anesthesia. There is a general consensus about the usefulness of PBC either in general populationCitation67 or in MS patients.Citation26,Citation68 PBC offers a good rate of immediate postoperative pain relief ranging from 80% to 90%Citation67,Citation81,Citation82 and a pain-free time without medication that ranges from 2 years to 3 years.Citation82,Citation83 However, no reports about the long-term quality of life of these patients are available in the literature. Some authors suggestedCitation84,Citation85 a lower efficacy in patients previously treated with other surgical procedures, in cases with positive history of MS and when a pear-like shape of the balloon at the operation is not obtained.Citation86 Complications can include numbness, dysesthesia, and, more rarely, masseter weakness that usually resolves within some months; meningitis and cranial nerve deficits are less common.Citation82,Citation87,Citation88 There are no standardized criteria concerning the compression time and the compression pressure. While experimental animal models suggest that a long compression time is associated with better outcome,Citation89 these data are not confirmed in the clinical setting where there are consistent data that a longer compression time does not affect the pain relief and only increase the complication rate.Citation86,Citation90,Citation91 Moreover, higher balloon pressures have been associated with higher rates of dysesthesia,severe numbness, and masseter weakness.Citation92 The variability of Meckel’s cave size has been advocated as another factor involved in the efficacy of the procedure; thus, cannulas of different sizes have been designed.Citation93 Technical failure to cannulate the foramen ovale using fluoroscopy can be a significant problem in some cases, but recently, intraoperative computed tomography with integrated neuronavigation has been safely used in reoperated patients due to prior failure under fluoroscopy.Citation94
Glycerol rhizotomy
The injection of glycerol in the trigeminal cistern determines pain relief in patients with TN due to demyelination and axonal fragmentation.Citation95,Citation96 Since its introduction,Citation97,Citation98 this technique has remained relatively unchanged with a reported initial pain relief more than 90%Citation99 and a rate of pain-free patients at 3 years of almost 50%.Citation100,Citation101 There are evidences that the success of glycerol rhizotomy depends on some degree of sensory loss postoperativelyCitation96,Citation102,Citation103 and that the chance of good outcome would be increased if facial pain was present during glycerol injection.Citation96 Dysesthesias, corneal numbness, masseter weakness, and herpes labialis have been reported as frequent complications of this procedure.Citation87,Citation96,Citation101 Recently, Goodwin et alCitation104 performed a MVD with injection of glycerol to the inferior third cisternal portion of the nerve, anterior to the root entry-zone, in 14 patients without neurovascular conflict on pre-operative MRI, reporting an 80% of good response at 3 months follow-up.
Radiofrequency thermocoagulation
Radiofrequency thermocoagulation is based on the attempt to electrocoagulate the trigeminal nerve and Gasserian ganglion rootlets.Citation105,Citation106 An initial pain relief more than 90% with a recurrence rate of up to 25% has been reported.Citation107,Citation108 The reported side effects, such as masticatory weakness, dysesthesia, and corneal numbness, seem to be related to significant individual variation of somatotopic organization of trigeminal nerve fibers and the irreversible damage of small, unmyelinated pain fibers.Citation109,Citation110 To overcome these limitations, a quadripolar electrode improving the accuracy of somatotopic identification, decreasing the lesion size, and reducing the unwanted injury has been developed.Citation111 A decrease in the incidence of masseter weakness and undesirable paresthesias and an improvement in the immediate pain relief rate using a curved tip electrode has previously been reported.Citation112 Moreover, the use of neuronavigator and computer tomography to improve needle localization seems to be associated with lower complications and recurrence rate compared to standard fluoroscopy in recent studies.Citation113,Citation114 Pulsed radiofrequency had been introduced with the aim to reduce the incidence of side effects; however, as recently reported in a prospective, randomized, double-blinded study comparing the effect of pulsed radiofrequency and conventional radiofrequency, although none of the patients in the pulsed radiofrequency group showed paresthesia, pain relief was not satisfactory as it was expected.Citation115
Gamma knife radiosurgery
GKRS has been used as a treatment modality in several centers for patients with concurrent medical illness who were poor candidates for MVD or who refuse more invasive surgery.Citation116,Citation117 Usually, the root entry-zone of the trigeminal nerve is used as a target and the dose protocols range from 70 Gy to 100 Gy.Citation118–Citation121 Nonetheless, considering that the underlying mechanisms are not fully understood, to date there is still uncertainty about the exact target and optimum dose to be used. In many clinical target volume definitions, the root entry-zone of the trigeminal nerve situated at 2–3 mm from the brainstem surface is chosen.Citation122 In a recent study, the radiosurgical target appeared to affect the duration of pain relief with the target closer to the brainstem, providing extended pain relief. However, the proximal radiosurgical target was also associated with an increased risk of mild to moderate facial numbness.Citation123 Alternative targets include the trigeminal nuclei in the brainstem or the centromedian nucleus of the thalamus.Citation124 In general, it has been reported that higher doses of radiation are related to better outcomes, but complications increase at doses greater than 90 Gy.Citation120,Citation125 In published long-term follow-up studies, the mean maximal radiation dose was approximately 80 Gy, and complications included facial numbness, which affected approximately 10% of the treated patients.Citation126–Citation128 Moreover, permanent dysesthesias and anesthesia dolorosa affecting the quality of life have been reported.Citation129 Although GKRS achieves relatively good outcomes on initial pain relief, the results suggest a rate of late failure, particularly among patients who performed GKRS following prior surgery.Citation130 Little et alCitation126 reported that 75% of patients with no previous surgery achieved long-term pain relief at 7 years compared with only 10% of patients with previous surgery. GKRS requires a delay before pain relief occurs. For this reason, some authors suggest that patients with extreme pain in need of fast relief should undergo other procedures.Citation131 Recently, it has been evidenced that overall pain relief following GKRS was comparable in patients with and without evidence of vascular compression on MRI. In the subgroup analysis of those with MRI evidence of vessel impingement of the affected trigeminal nerve, pain relief correlated with a higher dose to the point of contact between the impinging vessel and the trigeminal nerve.Citation132 Nonetheless, in a recent prospective cohort study comparing GKRS and MVD, the last one was significantly superior to GKRS in maintaining a pain-free status and provided similar early and superior longer-term patient satisfaction rates compared to GKRS.Citation133
Neuromodulation, really a chance for TN?
Two kinds of neuromodulation have been reported as optional treatments for chronic pain, refractory to conventional medical and surgical treatment: motor cortex stimulation (MCS) and deep brain stimulation (DBS). Chronic stimulation of the precentral cortex for the treatment of pain was first reported by Tsubokawa et alCitation134 in 1991, and several studies have documented excellent results of using MCS for the treatment of trigeminal neuropathic pain, with 75%–100% of patients achieving good to excellent pain relief.Citation135–Citation139 Nevertheless, these studies, mostly focusing on the use of MCS in pain syndrome, report few patients with idiopathic TN,Citation137–Citation139 with a limited follow-up.Citation139 On the other hand, DBS has been applied in the treatment of medically and surgically refractory chronic pain since 1997.Citation135,Citation140–Citation143 According to an interesting hypothesis, one of its main target, the posterior hypothalamus (pHyp), controls relationship between the neuropsychological circuits involved in pain behavior and the neurovegetative system. Franzini et alCitation144 reported the first series of chronic pHyp stimulation, and since then, many authors have proposed it to treat severe pain syndromes. In a systematic review,Citation145 the same authors reported that none of the four patients suffering from refractory neuropathic trigeminal pain benefited from the procedure, whereas all five patients affected with refractory TN due to MS and undergoing pHyp DBS experienced a significant decrease in pain attacks within the first trigeminal branch. Nevertheless, better results were obtained in chronic cluster headache and short, unilateral neuralgiform headache attacks with conjunctival injection and tearing. As for MCS, the limited number of studies and the relatively short follow-up make difficult to fully evaluate the efficacy of neuromodulation procedures. However, neurostimulation might represent an opportunity in TN refractory to other surgical treatments.
Conclusion
The treatment of TN is a challenge both for neurologists and neurosurgeons. The lack of a full comprehension of the complex pathogenesis at the basis of TN remains a key factor explaining the results that are not always satisfying with the medical therapy. Progress has been made in the recent years both for the pathogenesis and surgical treatment due to implementation of neuroradiological techniques. Surgery has also taken advantage from the introduction of the endoscope and neuronavigation in the operating room. New drugs, such as BTX-A, may be offered to patients before surgery or to patients unwilling to undergo surgery. Better definition of GKRS targets would improve the results of this technique. Neurostimulation might represent an opportunity in patients refractory to other surgical treatments, but further studies are needed due to the few cases treated.
Disclosure
The authors reported no conflicts of interest.
References
- PenmanJTrigeminal neuralgiaVinkenPJBruynGWHandbook of Clinical Neurology5AmsterdamNorth-Holland Publishing Company1968296322
- KoopmanJSDielemanJPHuygenFJde MosMMartinCGSturkenboomMCIncidence of facial pain in the general populationPain20091471–312212719783099
- HallGCCarrollDMcQuayHJPrimary care incidence and treatment of four neuropathic pain conditions: a descriptive study, 2002–2005BMC Fam Pract200892618460194
- KatusicSBeardCMBergstralhEKurlandLTIncidence and clinical features of trigeminal neuralgia, Rochester, Minnesota, 1945–1984Ann Neurol199027189952301931
- GronsethGCruccuGAlksneJPractice parameter: the diagnostic evaluation and treatment of trigeminal neuralgia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the European Federation of Neurological SocietiesNeurology200871151183119018716236
- MaarbjergSGozalovAOlesenJBendtsenLTrigeminal neuralgia – a prospective systematic study of clinical characteristics in 158 patientsHeadache201454101574158225231219
- PollackIFJannettaPJBissonetteDJBilateral trigeminal neuralgia: a 14-year experience with microvascular decompressionJ Neurosurg19886845595652450974
- RugeDBrochnerDDavisLA study of the treatment of 637 patients with trigeminal neuralgiaJ Neurosurg195815552853613576196
- PutzkiNPfriemALimmrothVPrevalence of migraine, tension-type headache and trigeminal neuralgia in multiple sclerosisEur J Neurol200916226226719138330
- O’ConnorABSchwidSRHerrmannDNMarkmanJDDworkinRHPain associated with multiple sclerosis: systematic review and proposed classificationPain200813719611117928147
- Headache Classification Committee of the International Headache Society (IHS)The international classification of headache disorders, 3rd edition (beta version)Cephalalgia201333962980823771276
- EllerJLRaslanAMBurchielKJTrigeminal neuralgia: definition and classificationNeurosurg Focus2005185E315913279
- CruccuGSommerCAnandPEFNS guidelines on neuropathic pain assessment: revised 2009Eur J Neurol20101781010101820298428
- CruccuGGronsethGAlksneJAmerican Academy of Neurology Society; European Federation of Neurological SocietyAAN-EFNS guidelines on trigeminal neuralgia managementEur J Neurol200815101013102818721143
- BowsherDTrigeminal neuralgia: a symptomatic study on 126 successive patients with and without prevoius interventionPain Clin20001293101
- CruccuGBiasiottaAGaleottiFDiagnostic accuracy of trigeminal reflex testing in trigeminal neuralgiaNeurology200666113914116401867
- ZengQZhouQLiuZLiCNiSXueFPreoperative detection of the neurovascular relationship in trigeminal neuralgia using three-dimensional fast imaging employing steady-state acquisition (FIESTA) and magnetic resonance angiography (MRA)J Clin Neurosci201320110711123098388
- ZhouQLiuZLQuCCNiSLXueFZengQSPreoperative demonstration of neurovascular relationship in trigeminal neuralgia by using 3D FIESTA sequenceMagn Reson Imaging201230566667122405984
- KanoHAwanNRFlanneryTJStereotactic radiosurgery for patients with trigeminal neuralgia associated with petroclival meningiomasStereotact Funct Neurosurg2011891172421124048
- JamjoomABJamjoomZAal-FehailyMel-WatidySal-MoallemMNain-Ur-RahmanTrigeminal neuralgia related to cerebellopontine angle tumorsNeurosurg Rev19961942372419007886
- GuoZOuyangHChengZSurgical treatment of parapontine epidermoid cysts presenting with trigeminal neuralgiaJ Clin Neurosci201118334434621247769
- MarinkovićSTodorovićVGiboHThe trigeminal vasculature pathology in patients with neuralgiaHeadache20074791334133917927650
- SiqueiraSRTeixeiraMJSiqueiraJTClinical characteristics of patients with trigeminal neuralgia referred to neurosurgeryEur J Dent20093320721219756195
- LoveSCoakhamHBTrigeminal neuralgia: pathology and pathogenesisBrain2001124pt 122347236011701590
- SarlaniEGraceEGBalciunasBASchwartzAHTrigeminal neuralgia in a patient with multiple sclerosis and chronic inflammatory demyelinating polyneuropathyJ Am Dent Assoc2005136446947615884316
- MontanoNPapacciFCioniBDi BonaventuraRMeglioMPercutaneous balloon compression for the treatment of trigeminal neuralgia in patients with multiple sclerosis. Analysis of the potentially prognostic factorsActa Neurochir (Wien)2012154577978322350443
- UrbanPPForstTLenfersMKoehlerJConnemannBJBeyerJIncidence of subclinical trigeminal and facial nerve involvement in diabetes mellitusElectromyogr Clin Neurophysiol199939526727210421997
- RobertsAMPersonPChandranNBHoriJMFurther observations on dental parameters of trigeminal and -atypical facial neuralgiasOral Surg Oral Med Oral Pathol19845821211296592503
- al-GailaniMHaidarZTrigeminal neuralgia: an unusual case of dental originOdontostomatol Trop1987103–42252273506211
- SawayaRATrigeminal neuralgia associated with sinusitisORL J Otorhinolaryngol Relat Spec200062316016310810262
- LinYWLinSKWengIHFatal paranasal sinusitis presenting as trigeminal neuralgiaHeadache200646117417816412169
- SvenssonEPain mechanisms in myogenous temporomandibular disordersPain Forum19976158165
- SiddallPJCousinsMJPain mechanisms and management: an updateClin Exp Pharmacol Physiol1995226796888575103
- SessleBJHuJWMechanisms of pain arising from articular tissuesCan J Physiol Pharmacol1991696176261863912
- SessleBJHoJWYuXMNew trends in referred pain and hyperalgesia, pain research and clinical managementVecchietDAlbe-FessardDLimblomUBrainstem Mechanisms of Referred Pain and Hyperalgesia in the Orofacial and Temporomandibular Region7th edAmsterdamElsevier19935971
- BonicaJLThe Management of Pain2nd edMalvern, PALea and Febiger1990180
- JiaDZLiGBioresonance hypothesis: a new mechanism on the pathogenesis of trigeminal neuralgiaMed Hypotheses201074350550719900765
- DevorMAmirRRappaportZHPathophysiology of trigeminal neuralgia: the ignition hypothesisClin J Pain200218141311803297
- ObermannMRodriguez-RaeckeRNaegelSGray matter volume reduction reflects chronic pain in trigeminal neuralgiaNeuroimage20137435235823485849
- Aguiar de SousaDGeraldesRGil-GouveiaRNew daily persistent headache and radiologically isolated syndromeJ Neurol201326082179218123835633
- LiuYLiJButzkuevenHMicrostructural abnormalities in the trigeminal nerves of patients with trigeminal neuralgia revealed by multiple diffusion metricsEur J Radiol201382578378623265178
- MoissetXVillainNDucreuxDFunctional brain imaging of trigeminal neuralgiaEur J Pain201115212413120609605
- HodaieMChenDQQuanJLaperriereNTractography delineates microstructural changes in the trigeminal nerve after focal radiosurgery for trigeminal neuralgiaPLoS One201273e3274522412918
- SindrupSHJensenTSPharmacotherapy of trigeminal neuralgiaClin J Pain2002181222711803299
- CampbellFGGrahamJGZilkhaKJClinical trial of carbamazepine (tegretol) in trigeminal neuralgiaJ Neurol Neurosurg Psychiatry19662932652675327969
- KillianJMFrommGHCarbamazepine in the treatment of neuralgia. Use of side effectsArch Neurol19681921291364877400
- NicolCFA four year double blind study of tegretol in facial painHeadache19699154574896266
- RockcliffBWDavisEHControlled sequential trials of carbamazepine in trigeminal neuralgiaArch Neurol1996152129136
- WiffenPJDerrySMooreRAKalsoEACarbamazepine for chronic neuropathic pain and fibromyalgia in adultsCochrane Database Syst Rev20144CD00545124719027
- ZakrzewskaJMLinskeyMETrigeminal neuralgiaClin Evid (Online)2014101207
- McQuayHCarrollDJadadARWiffenPMooreAAnticonvulsant drugs for management of pain: a systematic reviewBMJ19953117012104710527580659
- WiffenPCollinsSMcQuayHCarrollDJadadAMooreAAnticonvulsant drugs for acute and chronic painCochrane Database Syst Rev2005203CD00113316034857
- WiffenPMcQuayHMooreRCarbamazepine for acute and chronic painCochrane Database Syst Rev20053CD00545116034977
- JensenTSAnticonvulsants in neuropathic pain: rationale and clinical evidenceEur J Pain20026suppl A616811888243
- KutluayEMcCagueKD’SouzaJBeydounASafety and tolerability of oxcarbazepine in elderly patients with epilepsyEpilepsy Behav20034217518012697143
- BeydounAClinical use of tricyclic anticonvulsants in painful neuropathies and bipolar disordersEpilepsy Behav200233SS18S2212609349
- BeydounASafety and efficacy of oxcarbazepine: results of randomized, double-blind trialsPharmacotherapy2000208 pt 2152S158S10937814
- FrommGHTerrenceCFChatthaASBaclofen in the treatment of trigeminal neuralgia: double-blind study and long-term follow-upAnn Neurol19841532402446372646
- ZakrzewskaJMChaudhryZNurmikkoTJPattonDWMullensELLamotrigine (Lamictal) in refractory trigeminal neuralgia: results from a double-blind placebo controlled crossover trialPain19977322232309415509
- LechinFvan der DijsBLechinMEPimozide-therapy for trigeminal neuralgiaArch Neurol198946909609632673161
- LindstromPLindblomUThe analgesic effect of tocainide in trigeminal neuralgiaPain198728145503103044
- WiffenPJDerrySMooreRALamotrigine for acute and chronic painCochrane Database Syst Rev20112CD00604421328280
- FrommGHAumentadoDTerrenceCFA clinical and experimental investigation of the effects of tizanidine in trigeminal neuralgiaPain19935332652718351156
- ZakrzewskaJMLinskeyMETrigeminal neuralgiaBMJ2014348g47424534115
- HuYGuanXFanLTherapeutic efficacy and safety of botulinum toxin type A in trigeminal neuralgia: a systematic reviewJ Headache Pain2013147223964790
- CruccuGTruiniARefractory trigeminal neuralgia. Non-surgical treatment optionsCNS Drugs2013272919623225488
- TatliMSaticiOKanpolatYSindouMVarious surgical modalities for trigeminal neuralgia: literature study of respective long-term outcomesActa Neurochir (Wien)2008150324325518193149
- MontanoNPapacciFCioniBDi BonaventuraRMeglioMWhat is the best treatment of drug-resistant trigeminal neuralgia in patients affected by multiple sclerosis? A literature analysis of surgical proceduresClin Neurol Neurosurg2013115556757222840414
- AkramHMirzaBKitchenNZakrzewskaJMProposal for evaluating the quality of reports of surgical interventions in the treatment of trigeminal neuralgia: the surgical trigeminal neuralgia scoreNeurosurg Focus2013353E323991816
- ZakrzewskaJMAkramHNeurosurgical interventions for the treatment of classical trigeminal neuralgiaCochrane Database Syst Rev20119CD00731221901707
- JannettaPJArterial compression of the trigeminal nerve at the pons in patients with trigeminal neuralgiaJ Neurosurg19672611591626018932
- GuWZhaoWMicrovascular decompression for recurrent trigeminal neuralgiaJ Clin Neurosci20142191549155324938387
- BroggiGFerroliPFranziniAServelloDDonesIMicrovascular decompression for trigeminal neuralgia: comments on a series of 250 cases, including 10 patients with multiple sclerosisJ Neurol Neurosurg Psychiatry2000681596410601403
- OesmanCMooijJJLong-term follow-up of microvascular decompression for trigeminal neuralgiaSkull Base201121531332222451832
- ZakrzewskaJMLopezBCKimSECoakhamHBPatient reports of satisfaction after microvascular decompression and partial sensory rhizotomy for trigeminal neuralgiaNeurosurgery200556613041311 discussion 131215918947
- BroggiMAcerbiFFerroliPTringaliGSchiaritiMBroggiGMicrovascular decompression for neurovascular conflicts in the cerebello-pontine angle: which role for endoscopy?Acta Neurochir (Wien)201315591709171623884611
- SandellTRingstadGAEidePKUsefulness of the endoscope in microvascular decompression for trigeminal neuralgia and MRI-based prediction of the need for endoscopyActa Neurochir (Wien)2014156101901190925008460
- HalpernCHLangSSLeeJYFully endoscopic microvascu-lar decompression: our early experienceMin Invasive Surg20132013739432
- MullanSDudaEEPatronasNJSome examples of balloon technology in neurosurgeryJ Neurosurg19805233213297359186
- MullanSLichtorTPercutaneous microcompression of the trigeminal ganglion for trigeminal neuralgiaJ Neurosurg1983596100710126631493
- BergenheimATAsplundPLinderothBPercutaneous retrogasserian balloon compression for trigeminal neuralgia: review of critical technical details and outcomesWorld Neurosurg201379235936822480980
- BrownJAMcDanielMDWeaverMTPercutaneous trigeminal nerve compression for treatment of trigeminal neuralgia: results in 50 patientsNeurosurgery19933245705737682678
- BaaborMGPerez-LimonteLPercutaneous balloon compression of the gasserian ganglion for the treatment of trigeminal neuralgia: personal experience of 206 patientsActa Neurochir Suppl201110825125421107968
- JellishWSBenedictWOwenKAndersonDFluderESheaJFPerioperative and long-term operative outcomes after surgery for trigeminal neuralgia: microvascular decompression vs percutaneous balloon ablationHead Face Med200841118597696
- SkirvingDJDanNGA 20-year review of percutaneous balloon compression of the trigeminal ganglionJ Neurosurg200194691391711409519
- MontanoNPapacciFCioniBDi BonaventuraRMeglioMThe role of percutaneous balloon compression in the treatment of trigeminal neuralgia recurring after other surgical proceduresActa Neurol Belg20141141596424338759
- LopezBCHamlynPJZakrzewskaJMSystematic review of ablative neurosurgical techniques for the treatment of trigeminal neuralgiaNeurosurgery2004544973982 discussion 982–98315046666
- LichtorTMullanJFA 10-year follow-up review of percutaneous microcompression of the trigeminal ganglionJ Neurosurg199072149542294184
- LiFHanSMaYYiFXuXLiuYOptimal duration of percutaneous microballoon compression for treatment of trigeminal nerve injuryNeural Reg Res201492179189
- MeglioMCioniBPercutaneous procedures for trigeminal neuralgia: microcompression versus radiofrequency thermocoagulation. Personal experiencePain19893819162780067
- ParkSSLeeMKKimJWJungJYKimISGhangCGPercutaneous balloon compression of trigeminal ganglion for the treatment of idiopathic trigeminal neuralgia: experience in 50 patientsJ Korean Neurosurg Soc200843418618919096641
- ZanussoMCurriDLandiAColomboFVolpinLCervelliniPPressure monitoring inside Meckel’s cave during percutaneous micro-compression of gasserian ganglionStereotact Funct Neurosurg199156137431947500
- GoerssSJAtkinsonJLKallmesDFVariable size percutaneous balloon compression of the gasserian ganglion for trigeminal neuralgiaSurg Neurol200971338839018291447
- GeorgiopoulosMEllulJChroniEConstantoyannisCMinimizing technical failure of percutaneous balloon compression for trigeminal neuralgia using neuronavigationISRN Neurol2014201463041824729892
- KondziolkaDLunsfordLDPercutaneous retrogasserian glycerol rhizotomy for trigeminal neuralgia: technique and expectationsNeurosurg Focus2005185E715913283
- PollockBEPercutaneous retrogasserian glycerol rhizotomy for patients with idiopathic trigeminal neuralgia: a prospective analysis of factors related to pain reliefJ Neurosurg2005102222322815739548
- BennettMHLunsfordLDPercutaneous retrogasserian glycerol rhizotomy for tic douloureux, part 2: results and implications of trigeminal evoked potential studiesNeurosurgery19841444314356728145
- LunsfordLDBennettMHPercutaneous retrogasserian glycerol rhizotomy for tic douloureux, part 1: technique and results in 112 patientsNeurosurgery19841444244306728144
- MahajanVKRanjanNSharmaSSharmaNLSpontaneous tooth exfoliation after trigeminal herpes zoster: a case series of an uncommon complicationIndian J Dermatol201358324423723511
- NorthRBKiddDHPiantadosiSCarsonBSPercutaneous retrogasserian glycerol rhizotomy: predictors of success and failure in treatment of trigeminal neuralgiaJ Neurosurg19907268518562338568
- SlettebøHHirschbergHLindegaardKFLong-term results after percutaneous retrogasserian glycerol rhizotomy in patients with trigeminal neuralgiaActa Neurochir (Wien)19931223–42312358372713
- BlomstedtPCBergenheimATTechnical difficulties and perioperative complications of retrogasserian glycerol rhizotomy for trigeminal neuralgiaStereotact Funct Neurosurg2002793–416818112890975
- BurchielKJPercutaneous retrogasserian glycerol rhizolysis in the management of trigeminal neuralgiaJ Neurosurg19886933613663404233
- GoodwinCRYangJXBettegowdaCGlycerol rhizotomy via a retrosigmoid approach as an alternative treatment for trigeminal neuralgiaClin Neurol Neurosurg2013115122454245624161889
- SweetWGProceedings: analgesia dolorosa after differential retrogasserian thermal or mechanical rhizotomy: tactics employed to decrease its influenceJ Neurol Neurosurg Psychiatry19753844071141935
- LiuJKApfelbaumRITreatment of trigeminal neuralgiaNeurosurg Clin N Am200415331933415246340
- KarolEAAgnerCTechnological advances in the surgical management of trigeminal neuralgiaCrit Rev Neurosurg199992707810087097
- KarolEASanzOPGonzalez La RivaFNReyRDA micrometric multiple electrode array for the exploration of gasserian and retrogasserian trigeminal fibers: preliminary report: technical noteNeurosurgery19933311541588355835
- KanpolatYOnolBExperimental percutaneous approach to the trigeminal ganglion in dogs with histopathological evaluation of radiofrequency lesionsActa Neurochir Suppl (Wien)1980303633666970511
- SmithHPMcWhorterJMChallaVRRadiofrequency neurolysis in a clinical model: neuropathological correlationJ Neurosurg19815522462537252548
- KarolEAKarolMNA multiarray electrode mapping method for percutaneous thermocoagulation as treatment of trigeminal neuralgia: technical note on a series of 178 consecutive proceduresSurg Neurol20097111117 discussion 17–1818328544
- ToblerWDTewJMJrCosmanEKellerJTQuallenBImproved outcome in the treatment of trigeminal neuralgia by percutaneous stereotactic rhizotomy with a new, curved tip electrodeNeurosurgery19831233133176341871
- GusmãoSOliveiraMTazinaffoUHoneyCRPercutaneous trigeminal nerve radiofrequency rhizotomy guided by computerized tomography fluoroscopy. Technical noteJ Neurosurg200399478578614567619
- XuSJZhangWHChenTWuCYZhouMDNeuronavigator-guided percutaneous radiofrequency thermocoagulation in the treatment of intractable trigeminal neuralgiaChin Med J (Engl)2006119181528153516996006
- ErdineSOzyalcinNSCimenACelikMTaluGKDisciRComparison of pulsed radiofrequency with conventional radiofrequency in the treatment of idiopathic trigeminal neuralgiaEur J Pain200711330931316762570
- McNattSAYuCGiannottaSLZeeCSApuzzoMLPetrovichZGamma knife radiosurgery for trigeminal neuralgiaNeurosurgery200556612951301 discussion 1301–130315918946
- PollockBEPhuongLKGormanDAFooteRLStaffordSLStereotactic radiosurgery for idiopathic trigeminal neuralgiaJ Neurosurg200297234735312186463
- KondziolkaDLunsfordLDFlickingerJCStereotactic radio-surgery for trigeminal neuralgia: a multiinstitutional study using the gamma unitJ Neurosurg19968469409458847587
- KondziolkaDPerezBFlickingerJCHabeckMLunsfordLDGamma knife radiosurgery for trigeminal neuralgia: results and expectationsArch Neurol19985512152415299865796
- RégisJMétellusPLazorthesYPorcheronDPeragutJCEffect of gamma knife on secondary trigeminal neuralgiaStereotact Funct Neurosurg199870suppl 12102179782253
- YoungRFVermulenSPosewitzAGamma knife radiosurgery for the treatment of trigeminal neuralgiaStereotact Funct Neurosurg199870suppl 11921999782251
- LettmaierSRadiosurgery in trigeminal neuralgiaPhys Med201430559259524889155
- XuZSchlesingerDMoldovanKImpact of target location on the response of trigeminal neuralgia to stereotactic radiosurgeryJ Neurosurg2014120371672424313616
- KeepMFDeMarePAAshbyLSGamma knife surgery for refractory postherpetic trigeminal neuralgia: targeting in one session both the retrogasserian trigeminal nerve and the centromedian nucleus of the thalamusJ Neurosurg2005102suppl27628215662825
- PollockBEPhuongLKFooteRLStaffordSLGormanDAHigh-dose trigeminal neuralgia radiosurgery associated with increased risk of trigeminal nerve dysfunctionNeurosurgery20014915862 discussion 62–6411440460
- LittleASShetterAGShetterMEBayCRogersCLLong-term pain response and quality of life in patients with typical trigeminal neuralgia treated with gamma knife stereotactic radiosurgeryNeurosurgery2008635915923 discussion 923–92419005382
- RiesenburgerRIHwangSWSchirmerCMOutcomes following single-treatment gamma knife surgery for trigeminal neuralgia with a minimum 3-year follow-upJ Neurosurg2010112476677119780644
- UrgosikDLiscakRNovotnyJJrVymazalJVladykaVTreatment of essential trigeminal neuralgia with gamma knife surgeryJ Neurosurg2005102suppl293315662776
- LopezBCHamlynPJZakrzewskaJMStereotactic radiosurgery for primary trigeminal neuralgia: state of the evidence and recommendations for future reportsJ Neurol Neurosurg Psychiatry20047571019102415201363
- LeeJKChoiHJKoHCChoiSKLimYJLong term outcomes of gamma knife radiosurgery for typical trigeminal neuralgia-minimum 5-year follow-upJ Korean Neurosurg Soc201251527628022792424
- MathieuDEffendiKBlanchardJSéguinMComparative study of gamma knife surgery and percutaneous retrogasserian glycerol rhizotomy for trigeminal neuralgia in patients with multiple sclerosisJ Neurosurg2012117suppl17518023205807
- SheehanJPRayDKMonteithSGamma Knife radiosurgery for trigeminal neuralgia: the impact of magnetic resonance imaging-detected vascular impingement of the affected nerveJ Neurosurg20101131535819852545
- LinskeyMERatanatharathornVPeñagaricanoJA prospective cohort study of microvascular decompression and gamma knife surgery in patients with trigeminal neuralgiaJ Neurosurg2008109suppl16017219123904
- TsubokawaTKatayamaYYamamotoTHirayamaTKoyamaSChronic motor cortex stimulation for the treatment of central painActa Neurochir Suppl (Wien)1991521371391792954
- LevyRDeerTRHendersonJIntracranial neurostimulation for pain control: a reviewPain Phisician2010132157165
- FontaineDHamaniCLozanoAEfficacy and safety of motor cortex stimulation for chronic neuropathic pain: critical review of the literatureJ Neurosurg2009110225125618991496
- RaslanAMNasseriMBahgatDAbduEBurchielKJMotor cortex stimulation for trigeminal neuropathic or deafferentation pain: an institutional case series experienceStereotact Funct Neurosurg2011892838821293167
- TaneiTKajitaYNodaHEfficacy of motor cortex stimulation for intractable central neuropathic pain: comparison of stimulation parameters between post-stroke pain and other central painNeurol Med Chir (Tokyo)201151181421273738
- BuchananRJDarrowDMonsivaisDNadasdyZGjiniKMotor cortex stimulation for neuropathic pain syndromes: a case series experienceNeuroreport201425971571724780896
- BroggiGFranziniALeoneMBussoneGUpdate on neurosurgical treatment of chronic trigeminal autonomic cephalalgias and atypical facial pain with deep brain stimulation of posterior hypothalamus: results and commentsNeurol Sci200728suppl 2S138S14517508161
- RascheDRinaldiPCYoungRFTronnierVMDeep brain stimulation for the treatment of various chronic pain syndromesNeurosurg Focus2006216E817341052
- FranziniAMarrasCTringaliGChronic high frequency stimulation of the posteromedial hypothalamus in facial pain syndromes and behaviour disordersActa Neurochir Suppl200797pt 239940617691328
- CordellaRFranziniALa MantiaLMarrasCErbettaABroggiGHypothalamic stimulation for trigeminal neuralgia in multiple sclerosis patients: efficacy on the paroxysmal ophthalmic painMult Scler200915111322132819812115
- FranziniAFerroliPLeoneMBroggiGStimulation of the posterior hypothalamus for treatment of chronic intractable cluster headaches: first reported seriesNeurosurgery200352510951099 discussion 110112699552
- FranziniAMessinaGCordellaRMarrasCBroggiGDeep brain stimulation of the posteromedial hypothalamus: indications, long term results, and neurophysiological considerationsNeurosurg Focus2010292E1320672915
