Abstract
Intermittent claudication is a serious symptom in patients with peripheral arterial disease, and severely limits activities of daily living. Conservative treatment (optimal medical therapy and exercise rehabilitation programs) and revascularization procedures (endovascular treatment [EVT] or open bypass surgery) can relieve intermittent claudication. Among these treatment options, EVT has developed dramatically during the past decade, and has enabled physicians to offer less invasive treatment options with increasing durability. EVT for aortoiliac lesions has matured, and its long-term patency now approaches that of open bypass surgery. The latest EVT technologies include drug-eluting stents, stent grafts, drug-coated balloons, and bioresorbable stents. The recently reported patency of stent grafts in the femoropopliteal lesions was comparable with that of the prosthetic bypass graft. In the course of the paradigm shift from bypass surgery to EVT, evidence of any long-term benefit of EVT compared with supervised exercise is still inconclusive. EVT could improve walking performance in the short-term, while supervised exercise could improve walking performance more efficiently in the long-term. Combined treatment with EVT and exercise may offer the most sustainable and effective symptom relief. This paper reviews the relevant literature on the treatment of intermittent claudication, focusing on the latest EVT technologies, and outlines a strategy for achieving long-term benefits.
Introduction
Intermittent claudication is a common clinical manifestation of peripheral arterial disease (PAD), and affects 25%–33% of patients with the condition.Citation1 Patients suffer from significant impairment in ambulatory function due to pain in the thighs, buttocks, and calves during walking, resulting in functional disability and significant lifestyle limitation.Citation2,Citation3 The functional limitations are associated with poorer quality of life, increased hospitalization rates, higher mortality, and higher medical costs.Citation4 Improving functional performance with claudicants is mandatory for these patients. Optimal medical therapy, an exercise rehabilitation program, endovascular treatment (EVT), or open bypass surgery can relieve the above symptoms.Citation5–Citation7 Among the treatment options, EVT has an increasing role in relieving intermittent claudication in patients with PAD.Citation8 This is due to the advent of novel EVT technologies, which enable physicians to deal with complex arterial lesions previously requiring open bypass surgery.Citation9 EVT directly improves arterial flow and has been shown to achieve rapid regression of ischemic symptoms in a significant number of patients.Citation10 However, it remains controversial whether EVT has a functional benefit for patients when compared with supervised exercise therapy.Citation11 This paper reviews the relevant literature on the treatment of intermittent claudication, focusing on the latest EVT technologies, and outlines a strategy for achieving long-term benefits.
Endovascular treatment
Endovascular revascularization in patients with PAD has developed rapidly during the past decade. A considerable number of patients can now be offered this less invasive treatment option. According to the Inter-Society Consensus for the Management of Peripheral Arterial Disease,Citation7 the revascularization method, ie, EVT or open surgical bypass, should be chosen based on anatomical complexity and the patient’s general condition. The Inter-Society Consensus for the Management of Peripheral Arterial Disease recommends endovascular treatment for simple lesions, such as stenotic or short occlusions, and surgical bypass for complex lesions, such as diffuse, long occlusions.Citation7 The technical success rate using EVT, even for long occlusions, now exceeds 90% due to the development of endovascular devices and increased operator expertise. In addition, EVT is less invasive and has fewer cardiovascular risks, so surgical bypass has been replaced by EVT as a primary revascularization procedure.Citation8,Citation9,Citation12,Citation13 With regard to the culprit lesion for intermittent claudication, approximately 30% of the arterial lesions are located in the iliac arteries, and 70% are located in the femoropopliteal and tibial tract.Citation14
EVT for aortoiliac lesions
The technical success rate using EVT for aortoiliac lesions has been reported to be 92%–99%.Citation12,Citation13,Citation15–Citation17 Even for long iliac occlusions, the technical success rate can reach 98%.Citation12 Complications and 30-day mortality after EVT have been reported to be 4.8%–13.4% and 0%–0.7%, respectively, which are significantly lower than the rates for bypass surgery (18% and 2.6%).Citation17 Higher primary patency could be achieved by placement of a bare metal stent (BMS), particularly for long occlusive lesions, than could be obtained using percutaneous transluminal angioplasty (PTA).Citation18 Indes et al reported a meta-analysis comparing the clinical outcomes between EVT and surgical bypass. Primary patency rates at 5 years were superior in the open bypass group (82.7%) compared with the EVT group (71.4%).Citation17 However, Soga et al reported that secondary patency, which included patency restored with reintervention, was 98.5% at 5 years, meaning that a high patency rate can be achieved with catheter-based reintervention.Citation13
EVT for femoropopliteal lesion
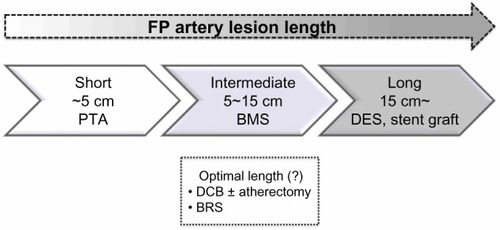
The femoropopliteal artery is one of the most challenging anatomies for EVT because of its longer lesion length and smaller diameter than the iliac artery, as well as the high stress arising from the surrounding muscle contractions.Citation19 Since the advent of the flexible, nitinol BMS, the primary patency rate for superficial femoral artery (SFA) lesions has improved. Several randomized controlled trials comparing BMS placement with PTA for 4–13 cm lesions showed a superior patency rate after BMS placement.Citation20–Citation22 Currently, primary nitinol stenting can be recommended as the first-line treatment for SFA lesions of intermediate length instead of PTA plus provisional stenting where stents are used only in the event of initial PTA failure (). However, as the lesion length becomes longer, the likelihood of intimal hyperplasia increases, which results in a loss of patency.Citation23,Citation24 Furthermore, Scheinert et al showed that the prevalence of stent fracture became higher when longer stents or a greater number of stents were used, which could eventually cause restenosis or pseudoaneurysm formation.Citation19
Figure 1 Considered stent type for each femoropopliteal lesion length. Short femoropopliteal lesions up to 5 cm can be treated with PTA, intermediate lesions 5–15 cm should be treated with BMS rather than only PTA, and a DES or stent graft can offer superior patency for longer lesions exceeding 15 cm compared with BMS. Other treatment strategies, such as DCB with/without atherectomy and BRS, are currently under investigation.
In an effort to combat neointimal hyperplasia, drug-eluting stents (DES) that enable local drug delivery to the SFA have been investigated as an alternative to BMSs. The Zilver® PTX® (Cook Medical, Bloomington, IA, USA) is the most widely used DES incorporating paclitaxel on its outer surface. Paclitaxel inhibits microtubule assembly and selectively inhibits smooth muscle proliferation and migration, as well as extracellular matrix deposition.Citation25 Dake et al have reported the 2-year results of their randomized trial comparing the primary patency rate for the Zilver-PTX with that of the BMS, in which patients were randomized to primary DES implantation or PTA.Citation26 Acute PTA failure occurred in 120 patients, who underwent secondary randomization to DES or BMS placement. Compared with the PTA group, the primary DES group demonstrated a significantly superior 2-year primary patency rate (74.8% versus 26.5%), and in addition, the provisional DES group showed superior 2-year primary patency compared with the provisional BMS group (83.4% versus 64.1%). Recently, Ansel presented the 4-year data for the randomized controlled Zilver-PTX trial. The 4-year primary patency rate for the paclitaxel group and the BMS group was 75% and 57.9%, respectively, representing a 41% reduction in restenosis due to the paclitaxel stent coating.Citation27 Cock et al reported that use of the Zilver-PTX could save health care costs due to the decreased need for reintervention.Citation28
A stent graft is another option for preventing in-stent restenosis. The membrane covering a stent could prevent direct contact between blood and the diseased arterial wall, and thus could mitigate intimal hyperplasia. Two randomized studies in which the expanded polytetrafluoroethylene/nitinol stent graft (Viabahn® endoprosthesis, WL Gore and Associates, Flagstaff, AZ, USA) was compared with bypass surgery using a synthetic graft showed noninferiority of the stent graft in terms of long-term clinical outcome and primary patency rate.Citation29,Citation30 The VIASTAR (Gore Viabahn® endoprosthesis with bioactive Propaten® surface versus bare nitinol stent in the treatment of TASC (Trans-Atlantic Inter-Society Consensus) B, C and D lesions in superficial femoral artery occlusive disease) trial, in which patients with long SFA lesions were randomized to either a Viabahn stent graft or a BMS, showed the stent graft to achieve significantly superior patency.Citation31 A summary of the results of the randomized trials for SFA stents is shown in .
Table 1 Randomized clinical trials of stent placement in the superficial femoral artery
While the primary patency after EVT has improved due to introduction of the DES and stent graft, the management of in-stent restenosis is of growing concern with the increasing numbers of stents implanted. As for DES, late thrombosis due to delayed endothelialization is still considered as a potential risk.Citation32 Stent grafts can also elicit severe inflammatory reactionsCitation33 and thrombus formationCitation34 because of direct contact between the polymer and blood. Recurrent failure rates after repeat EVT for in-stent restenosis remain higher than for primary EVT, possibly because of the reduced acute gain and the inflammatory response of the arterial wall to foreign bodies.Citation35 Therefore, “nothing left behind” in the artery could be the ideal strategy considering the repeat EVT or bypass surgery in the future.
Atherectomy has been investigated as one of the nothing left behind strategies, which could enable us to debulk the atherosclerotic plaque by cutting or grinding it away. Ambler et al conducted a meta-analysis to compare the efficacy of atherectomy versus PTA. In addition to the study being insufficiently powered, there was no evidence to support the superiority of atherectomy over PTA in terms of patency or target lesion revascularization.Citation36
Drug-coated balloons (DCB) have also been investigated as one of the nothing left behind strategies, offering a mechanism by which to deliver antiproliferative drugs directly to the diseased arterial wall without a stent scaffold. The majority of the DCB trials have used paclitaxel, which is highly lipophilic, allowing rapid intracellular uptake. Randomized controlled trials, such as THUNDER (ClinicalTrials.gov identifier NCT00156624),Citation37 FemPac (Femoral-Paclitaxel),Citation38 and LEVANT I (ClinicalTrials.gov identifier NCT00930813),Citation39 have shown significant reductions in late lumen loss and reintervention after treatment by DCB compared with conventional balloon angioplasty. However, severe calcification of the atherosclerotic lesion is responsible for a poor response to balloon dilation, due to significant acute vessel recoil and frequent flow-limiting dissections. Calcified plaque could also inhibit drug penetration into the arterial wall. Atherectomy has been combined with DCB in an attempt to reduce the plaque burden and achieve sufficient drug penetration. Cioppa et al investigated the combination of atherectomy and DCB for heavily calcified SFA lesions in 30 patients.Citation40 The minimal lumen diameter measured by intravascular ultrasound was 1.2±0.9 mm before procedure and 5.1±0.8 mm after atherectomy and DCB. The target lesion revascularization rate at 1 year was 10%. A combined strategy of atherectomy and DCB could offer sufficient acute gain of lesions, as well as suppression of intimal hyperplasia. Preliminary findings were recently presented for DEFINITIVE AR (Directional AthErectomy Followed by a PaclItaxel-Coated BallooN to InhibiT RestenosIs and Maintain Vessel PatEncy: A Pilot Study of Anti-Restenosis Treatment), in which patients were randomly allocated to treatment with a drug-coated balloon alone (n=54) or atherectomy followed by DCB (n=48). The technical success rate, defined as ≤30% residual stenosis of the target lesion, for atherectomy and DCB was significantly higher than that for DCB alone, and the flow-limiting dissection rate was significantly lower with atherectomy and DCB.Citation41 DCB has been demonstrated to offer superior patency when compared with a conventional balloon in femoropopliteal lesions; however, it should be noted that the published reports to date lack long-term data regarding the efficacy of DCB. Further, the recently presented INPACT-DEEP trial (ClinicalTrials.gov identifier NCT00941733) showed that DCB had no benefits in terms of avoiding target lesion revascularization and a higher prevalence of severe complications, such as major amputations, than a conventional balloon when DCB was used in patients suffering from critical limb ischemia due to a below-knee lesion.Citation42
Bioresorbable stents could be another possibility. These are designed to provide a temporary architectural scaffold for the arterial wall to avoid vessel recoil, eventually disappearing by bioabsorption. Disappearance of the rigid scaffold can facilitate recovery of vasomotion, adaptive shear stress, late luminal enlargement, and expansive remodeling. Once bioabsorption of the temporary scaffold has been completed, the potential risk of stent thrombosis could be reduced and there may be no requirement for long-term dual antiplatelet therapy.Citation43 Further, the nonmetallic scaffold does not restrict the use of noninvasive imaging such as computed tomography or magnetic resonance imaging. The most frequently used biodegradable polymer is poly-L-lactic acid, which is metabolized into lactic acid, carbon dioxide, and water.Citation44
The Igaki-Tamai stent (Kyoto Medical Planning Company, Kyoto, Japan) is the first bioresorbable stent to be implanted in human coronary arteries, with complete degradation taking 18–24 months. The first-in-human clinical study of the Igaki-Tamai stent involving 15 patients with 19 lesions demonstrated no major adverse cardiovascular events or stent thrombosis within 30 days, with one repeat percutaneous coronary intervention at the 6-month follow-up.Citation45 At 10-year clinical follow-up, freedom from cardiac death, noncardiac death, and major adverse cardiovascular events was 98%, 87%, and 48%, respectively.Citation46 Igaki-Tamai stents were also implanted in SFA lesions and evaluated in the PERSEUS study (ClinicalTrials.gov identifier NCT00484315), which recruited 45 patients with de novo SFA lesions.Citation47 The initial technical success rate was reported to be 100%, with no serious adverse events. The 6-month angiographic results revealed no reocclusions, although symptomatic restenosis occurred in 20% of patients. Use of bioresorbable stents in the SFA was demonstrated to be feasible and the results were very encouraging. Currently, the safety of the drug-eluting bioresorbable vascular scaffold is being evaluated in the ESPRIT I trial (ClinicalTrials.gov identifier NCT01468974), and restenosis has not been reported within the past 6 months.Citation48
Which is more effective for symptom relief: EVT for iliac or femoropopliteal lesions?
Patients with aortoiliac lesions are often more symptomatic than those with femoropopliteal lesions because those with aortoiliac lesions have more ischemic muscle mass with walking, and there may be no substantial collateral arteries. Thus, patients with aortoiliac lesions could have effective symptom relief after EVT. In the MIMIC (Mild to Moderate Intermittent Claudication) trial, 34 patients with occlusive aortoiliac disease and 93 patients with femoropopliteal disease were randomized to combined therapy of supervised exercise plus EVT or to supervised exercise only.Citation49 Compared with the supervised exercise group, the adjusted absolute walking distance was 38% greater in the supervised exercise plus EVT group for the femoropopliteal trial, and 78% greater in the supervised exercise plus EVT group for the aortoiliac trial. Although low recruitment and eligibility rates and high dropout rates could limit the impact of the results, this study was able to demonstrate the benefit of adjunctive EVT, particularly for aortoiliac lesions.
Chetter et al also demonstrated superior relief of claudication with iliac angioplasty when compared with SFA angioplasty.Citation49 Quality of life, particularly physical functioning, in patients with iliac lesions approached that in an age-matched population after EVT. Ichihashi et al evaluated improvement in intermittent claudication when only occlusive iliac artery disease was treated by EVT in patients having concomitant iliac and SFA lesions.Citation51 Despite the remaining SFA lesions, 87% of patients had symptomatic relief, translating into at least one category upgrade in the Rutherford classification after stent placement for iliac artery lesions. Further, 72% of patients were completely relieved of intermittent claudication. The indications for treatment of SFA lesions could be evaluated when symptom relief is not enough to improve patient quality of life after treatment of iliac lesions.
Relief of symptoms of intermittent claudication after EVT versus exercise therapy
In patients with PAD, exercise therapy is also effective in improving symptoms and increasing walking capacity. Supervised exercise increases maximal treadmill walking distance by 50%–200% in patients with PAD.Citation6,Citation52 Several trials have compared exercise therapy and EVT with regard to their ability to improve intermittent claudication. Spronk et al compared the cost-effectiveness of EVT with that of supervised exercise in patients with intermittent claudication. No significant difference was found between the two therapies, but the costs of EVT were significantly higher than for exercise therapy.Citation53 The recent CLEVER trial (ClinicalTrials.gov identifier NCT00132743) randomized 111 patients with occlusive aortoiliac disease to receive optimal medical care, optimal medical care plus supervised exercise, or optimal medical care plus EVT.Citation54 Supervised exercise resulted in superior treadmill walking performance compared with EVT; however, the greatest improvement in self-reported quality of life was observed in the EVT cohort. A systematic review of eight randomized controlled trials comparing supervised or unsupervised exercise therapies with EVT failed to demonstrate the superiority of any particular therapy with regard to walking distance and quality of life.Citation55 Another systematic review by Liu et al indicated that the independent effect of EVT could improve walking performance in the short-term, but not in the long-term, while supervised exercise could improve walking performance more efficiently in the long term.Citation56 Mazari et al reported the results of a randomized controlled trial comparing the effectiveness of EVT versus supervised exercise for symptomatic relief in patients with femoropopliteal lesions. EVT and supervised exercise showed equal effectiveness in symptom relief, and in addition, a combination of EVT and supervised exercise achieved more sustained clinical improvement.Citation57 However, supervised exercise is typically not covered by medical insurance, and involves regular transport to an exercise center. Thus, few patients with PAD can participate in consistent supervised treadmill exercise therapy.Citation58
Home-based walking exercise is a promising alternative to supervised exercise. GOALS (Group Oriented Arterial Leg Study) is a randomized controlled trial that investigated whether a home-based exercise regimen can improve functional performance in patients with PAD when compared with a health education control group.Citation59 Participants met once weekly for 90 minutes with other PAD participants for the entire 6 months of the intervention, and all participants were instructed in the benefits of walking exercise, how to exercise, and self-monitoring. After 6 months, the home-based walking exercise program significantly improved walking distance and speed in patients with PAD. Therefore, home-based walking programs should be recommended for patients with PAD who are not eligible for supervised exercise, when a weekly group-mediated cognitive behavioral intervention is conducted. Mays et al also reported in their review literature that community walking programs with regular feedback and monitoring result in improvements in walking performance.Citation60 The effectiveness of a combination of exercise and EVT was evaluated in the MIMIC trial,Citation49 the purpose of which was to investigate whether there was an adjuvant benefit from EVT over supervised exercise with optimal medical therapy in the treatment of intermittent claudication. EVT was demonstrated to confer adjuvant benefit over supervised exercise with optimal medical therapy in terms of walking distance. Further, Mazari et alCitation57 reported that a combination of EVT and supervised exercise achieved more sustained clinical improvement than EVT or exercise alone.
Medical treatment as an adjunctive therapy for EVT
Only two medications, ie, pentoxifylline and cilostazol, are currently approved by the US Food and Drug Administration for the treatment of PAD-associated walking impairment. However, a recent systematic review concluded that pentoxifylline and cilostazol are associated with an improvement in maximal treadmill walking distance of only 15% and 25%, respectively.Citation61 In a meta-analysis of nine trials comparing cilostazol with placebo, cilostazol was associated with an absolute improvement in walking distance of 42.1 m compared with placebo.Citation62 Ramipril, one of the angiotensin-converting enzyme inhibitors, was demonstrated to be effective in improving walking performance in a randomized controlled trial.Citation63 However, a recent meta-analysis by Shahin et al, including four randomized trials, demonstrated no association between use of angiotensin-converting enzyme inhibitors and improved walking performance.Citation64 Medical therapy alone could be insufficient to improve symptoms, so adjunctive EVT with/ without exercise therapy is required. Momsen et al conducted a meta-analysis evaluating the efficacy of medical therapies in improving walking capacity. Lipid-lowering drugs seem to be the most efficient therapy, increasing maximal walking distance to 160 m.Citation5
Antiplatelet therapy has an important role in reducing thrombosis or restenosis after EVT. Most physicians favor indefinite aspirin therapy and a minimum 4-week course of a second thienopyridine anti-platelet agent after stent placement.Citation65 More recent experience with the paclitaxel-eluting stent suggests that 2 months of dual antiplatelet therapy followed by indefinite aspirin is associated with low rates of acute stent thrombosis.Citation66 Strobl et al conducted the randomized, single-center MIRROR study (ClinicalTrials. gov identifier NCT00163267) which randomized 80 patients to either aspirin plus clopidogrel or aspirin plus placebo for preinterventional and post-interventional therapy.Citation67 Only BMSs were used for stent placement. At 6 months, patients receiving clopidogrel had significantly lower rates of target lesion revascularization compared with placebo (5% versus 20%). However, after cessation of the clopidogrel/placebo at 6 months, there was no significant difference in target lesion revascularization at 12 months after treatment (25% versus 32.4%). Patients who are also at high risk for restenosis, such as those with long, heavily calcified lesions, would be potential candidates for prolonged or even indefinite dual antiplatelet therapy.
Cilostazol has been demonstrated to have the potential to reduce in-stent restenosis. STOP-IC (ClinicalTrials. gov identifier NCT00163267), a prospective, multicenter, randomized controlled trial involving 200 patients treated with femoropopliteal stents, showed that treatment with cilostazol significantly reduced the rate of in-stent restenosis when compared with placebo (20% versus 49%, respectively).Citation68 The duration and intensity of antiplatelet therapy must be balanced against the bleeding risk in each patient.
Conclusion
Advances in EVT have prompted many physicians to consider more liberal indications of EVT for intermittent claudication with PAD. EVT can achieve effective symptom relief and a patency rate almost comparable with that of bypass surgery in patients with aortoiliac lesions. For femoropopliteal lesions, there is still room for improvement with EVT because patency decreases with increasing length, poor runoff, and diabetes. The use of new technology such as the DES, DCB, stent graft, and bioabsorbable stent seems promising; however, more liberal use of EVT for femoropopliteal lesions should be delayed until its efficacy and durability for treatment of intermittent claudication is demonstrated by large-scale studies. To date, multiple randomized controlled trials have shown no significant difference in effectiveness with regard to symptom relief between EVT and exercise therapy. EVT should be combined with exercise therapy and optimal medical therapies, including cilostazol and statins, which could offer the most effective and sustainable symptom relief for patients.
Acknowledgments
The authors appreciate the excellent advice of Marian Pahud (Valkenburg, the Netherlands) in submitting this paper.
Disclosure
The authors report no conflicts of interest in this work.
References
- RoseGABlackburnHCardiovascular survey methodsMonogr Ser World Health Organ1968561188
- RegensteinerJGHiattWRCollJRThe impact of peripheral arterial disease on health-related quality of life in the Peripheral Arterial Disease Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) ProgramVasc Med200813152418372434
- McDermottMMLiuKGreenlandPFunctional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptomsJAMA200429245346115280343
- McDermottMMMedications for improving walking performance in peripheral artery disease: still miles to goJAMA201330948748823385276
- MomsenAHJensenMBNoragerCBDrug therapy for improving walking distance in intermittent claudication: a systematic review and meta-analysis of robust randomized controlled studiesEur J Vasc Endovasc Surg20093846347419586783
- McDermottMMAdesPGuralnikJMTreadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trialJAMA200930116517419141764
- NorgrenLHiattWRDormandyJATASC II Working Group. Inter-society consensus for the management of peripheral arterial disease (TASC II)J Vasc Surg200745S5S6717223489
- RoweVLLeeWWeaverFAEtzioniDPatterns of treatment for peripheral arterial disease in the United States: 1996–2005J Vasc Surg20094991091719341885
- SchillingerMMinarEPercutaneous treatment of peripheral artery disease: novel techniquesCirculation20121262433244023147770
- O’Brien-IrrMSHarrisLMDosluogluHHDryjskiMLEndovascular intervention for treatment of claudication: is it cost-effective?Ann Vasc Surg20102483384020638623
- AhimastosAAPappasEPButtnerPGWalkerPJKingwellBAGolledgeJA meta-analysis of the outcome of endovascular and noninvasive therapies in the treatment of intermittent claudicationJ Vasc Surg2011541511152121958561
- IchihashiSHigashiuraWItohHSakaguchiSNishimineKKichikawaKLong-term outcomes for systematic primary stent placement in complex iliac artery occlusive disease classified according to Trans-Atlantic Inter-Society Consensus (TASC)-IIJ Vasc Surg20115399299921215582
- SogaYIidaOKawasakiDContemporary outcomes after endovascular treatment for aorto-iliac artery diseaseCirc J2012762697270422864278
- BalzerJOThalhammerAKhanVZangosSVoglTJLehnertTAngioplasty of the pelvic and femoral arteries in PAOD: results and review of the literatureEur J Radiol201075485620451340
- SixtSKrankenbergHMöhrleCEndovascular treatment for extensive aortoiliac artery reconstruction: a single-center experience based on 1712 interventionsJ Endovasc Ther201320647323391085
- OzkanUOguzkurtLTercanFTechnique, complication, and long-term outcome for endovascular treatment of iliac artery occlusionCardiovasc Intervent Radiol201033182419768500
- IndesJEPfaffMJFarrokhyarFClinical outcomes of 5358 patients undergoing direct open bypass or endovascular treatment for aortoiliac occlusive disease: a systematic review and meta-analysisJ Endovasc Ther20132044345523914850
- AbuRahmaAFHayesJDFlahertySKPeeryWPrimary iliac stenting versus transluminal angioplasty with selective stentingJ Vasc Surg20074696597017905559
- ScheinertDScheinertSSaxJPrevalence and clinical impact of stent fractures after femoropopliteal stentingJ Am Coll Cardiol20054531231515653033
- SchillingerMSabetiSLoeweCBalloon angioplasty versus implantation of nitinol stents in the superficial femoral arteryN Engl J Med20063541879188816672699
- BosiersMTorselloGGisslerHMNitinol stent implantation in long superficial femoral artery lesions: 12-month results of the DURABILITY I studyJ Endovasc Ther20091626126919642788
- LairdJRKatzenBTScheinertDNitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery: twelve-month results from the RESILIENT randomized trialCirc Cardiovasc Interv2010326727620484101
- SogaYIidaOHiranoKYokoiHNantoSNobuyoshiMMid-term clinical outcome and predictors of vessel patency after femoropopliteal stenting with self-expandable nitinol stentJ Vasc Surg20105260861520573476
- DohiTIidaOSogaYIncidence, predictors, and prognosis of in-stent occlusion after endovascular treatment with nitinol stents for femoropopliteal lesionsJ Vasc Surg20145910091015e124360237
- AxelDIKunertWGoggelmannCPaclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug deliveryCirculation1997966366459244237
- DakeMDAnselGMJaffMRSustained safety and effectiveness of paclitaxel-eluting stents for femoropopliteal lesions: 2-year follow-up from the Zilver PTX randomized and single-arm clinical studiesJ Am Coll Cardiol2013612417242723583245
- AnselGZilver PTX randomized trial of paclitaxel-eluting stents for femoropopliteal artery disease: 4 year resultsAbstract presented at the 2013 Vascular Interventional Advances meetingLas Vegas, NV, USAOctober 8–11, 2013
- De CockESapovalMJuliaPde LissovoyGLopesSA budget impact model for paclitaxel-eluting stent in femoropopliteal disease in FranceCardiovasc Intervent Radiol20133636237023073560
- KedoraJHohmannSGarrettWMunschaurCTheuneBGableDRandomized comparison of percutaneous Viabahn stent grafts vs prosthetic femoral-popliteal bypass in the treatment of superficial femoral arterial occlusive diseaseJ Vasc Surg200745101617126520
- McQuadeKGableDPearlGTheuneBBlackSFour-year randomized prospective comparison of percutaneous ePTFE/nitinol self-expanding stent graft versus prosthetic femoral-popliteal bypass in the treatment of superficial femoral artery occlusive diseaseJ Vasc Surg20105258459020598480
- LammerJZellerTHauseggerKAHeparin-bonded covered stents versus bare-metal stents for complex femoropopliteal artery lesions: the randomized VIASTAR trial (Viabahn endoprosthesis with PROPATEN bioactive surface [VIA] versus bare nitinol stent in the treatment of long lesions in superficial femoral artery occlusive disease)J Am Coll Cardiol2013621320132723831445
- JonerMFinnAVFarbAPathology of drug eluting stents in humans: delayed healing and late thrombotic riskJ Am Coll Cardiol20064819320216814667
- FarhatniaYTanAMotiwalaACousinsBGSeifalianAMEvolution of covered stents in the contemporary era: clinical application, materials and manufacturing strategies using nanotechnologyBiotechnol Adv20133152454223305892
- SaxonRRChervuAJonesPAHeparin-bonded, expanded polytetrafluoroethylene-lined stent graft in the treatment of femoropopliteal artery disease: 1-year results of the VIPER (Viabahn Endoprosthesis with Heparin Bioactive Surface in the Treatment of Superficial Femoral Artery Obstructive Disease) trialJ Vasc Interv Radiol20132416517323369553
- RyerEJTrocciolaSMDeRubertisBAnalysis of outcomes following failed endovascular treatment of chronic limb ischemiaAnn Vasc Surg20062044044616865606
- AmblerGKRadwanRHayesPDTwineCPAtherectomy for peripheral arterial diseaseCochrane Database Syst Rev20143CD00668024638972
- TepeGZellerTAlbrechtTLocal delivery of paclitaxel to inhibit restenosis during angioplasty of the legN Engl J Med200835868969918272892
- WerkMLangnerSReinkensmeierBInhibition of restenosis in femoropopliteal arteries: paclitaxel-coated versus uncoated balloon: femoral paclitaxel randomized pilot trialCirculation20081181358136518779447
- ScheinertDDudaSZellerTThe LEVANT I (lutonix paclitaxel-coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: first-in-human randomized trial of low-dose drug-coated balloon versus uncoated balloon angioplastyJACC Cardiovasc Interv20147101924456716
- CioppaAStabileEPopusoiGCombined treatment of heavy calcified femoro-popliteal lesions using directional atherectomy and a paclitaxel coated balloon: one-year single centre clinical resultsCardiovasc Revasc Med20121321922322632996
- ZellerTDirectional atherectomy followed by a Paclitaxel-coated balloon to inhibit restenosis and maintain vessel patency: a pilot study of anti- restenosis treatmentAbstract presented at the 2013 Vascular Interventional Advances meetingLas Vegas, NV, USAOctober 8–11, 2013
- ZellerTDEB vs PTA for infrapopliteal revascularisation: 12 months results from the IN.PACT DEEP randomized trialAbstract presented at the Leipzig Interventional CourseLeipzig, GermanyJanuary 27–31, 2013
- OnumaYSerruysPWBioresorbable scaffold: the advent of a new era in percutaneous coronary and peripheral revascularization?Circulation201112377979721343594
- NairLSLaurencinCTBiodegradable polymers as biomaterialsProg Polym Sci200732762798
- TamaiHIgakiKKyoEInitial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humansCirculation200010239940410908211
- NishioSKosugaKIgakiKLong-term (>10 years) clinical outcomes of first-in-human biodegradable poly-L-lactic acid coronary stents: Igaki-Tamai stentsCirculation20121252343235322508795
- BiaminoGSchmidtAScheinertDTreatment of SFA lesions with PLLA biodegradable stents: results of the PERSEUS studyJ Endovasc Ther2005125
- LammerJEvaluation of Esprit BVS in the treatment of patients with occlusive vascular disease of the superficial femoral artery (SFA) or common or external iliac arteriesAbstract presented at the 2013 Vascular Interventional Advances meetingLas Vegas, NV, USAOctober 8–11, 2013
- ChetterICSparkJIKentPJBerridgeDCScottDJKesterRCPercutaneous transluminal angioplasty for intermittent claudication: evidence on which to base the medicineEur J Vasc Endovasc Surg1998164774849894486
- GreenhalghRMBelchJJBrownLCThe adjuvant benefit of angioplasty in patients with mild to moderate intermittent claudication (MIMIC) managed by supervised exercise, smoking cessation advice and best medical therapy: results from two randomised trials for stenotic femoropopliteal and aortoiliac arterial diseaseEur J Vasc Endovasc Surg20083668068819022184
- IchihashiSHigashiuraWItohHSakaguchiSKichikawaKIliac artery stent placement relieves claudication in patients with iliac and superficial femoral artery lesionsCardiovasc Intervent Radiol20133662362822692181
- FakhryFvan de LuijtgaardenKMBaxLSupervised walking therapy in patients with intermittent claudicationJ Vasc Surg2012561132114223026425
- SpronkSBoschJLden HoedPTCost-effectiveness of endovascular revascularization compared to supervised hospital-based exercise training in patients with intermittent claudication: a randomized controlled trialJ Vasc Surg2008481472148018771879
- MurphyTPCutlipDERegensteinerJGSupervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) studyCirculation201212513013922090168
- FransFABipatSReekersJALegemateDAKoelemayMJSystematic review of exercise training or percutaneous transluminal angioplasty for intermittent claudicationBr J Surg201299162821928409
- LiuJWuYLiZLiWWangSEndovascular treatment for intermittent claudication in patients with peripheral arterial disease: a systematic reviewAnn Vasc Surg20142897798224342830
- MazariFAKhanJACarradiceDRandomized clinical trial of percutaneous transluminal angioplasty, supervised exercise and combined treatment for intermittent claudication due to femoropopliteal arterial diseaseBr J Surg201299394822021102
- RegensteinerJGExercise rehabilitation for the patient with intermittent claudication: a highly effective yet underutilized treatmentCurr Drug Targets Cardiovasc Haematol Disord2004423323915379615
- McDermottMMLiuKGuralnikJMHome-based walking exercise intervention in peripheral artery disease: a randomized clinical trialJAMA2013310576523821089
- MaysRJRogersRKHiattWRRegensteinerJGCommunity walking programs for treatment of peripheral artery diseaseJ Vasc Surg2013581678168724103409
- StevensJWSimpsonEHarnanSSystematic review of the efficacy of cilostazol, naftidrofuryl oxalate and pentoxifylline for the treatment of intermittent claudicationBr J Surg2012991630163823034699
- PandeRLHiattWRZhangPHittelNCreagerMAMcDermottMA pooled analysis of the durability and predictors of treatment response of cilostazol in patients with intermittent claudicationVasc Med20101518118820385711
- AhimastosAAWalkerPJAskewCEffect of ramipril on walking times and quality of life among patients with peripheral artery disease and intermittent claudication, a randomized controlled trialJAMA201330945346023385271
- ShahinYMazariFChetterIDo angiotensin-converting enzyme inhibitors improve walking distance in patients with symptomatic lower limb arterial disease? A systematic review and meta-analysis of randomised controlled trialsInt J Surg2011920921321195215
- SobieszczykPEisenhauerAManagement of patients after endovascular interventions for peripheral artery diseaseCirculation201312874975723940389
- DakeMDAnselGMJaffMRPaclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study resultsCirc Cardiovasc Interv2011449550421953370
- StroblFFBrechtelKSchmehlJTwelve-month results of a randomized trial comparing mono with dual antiplatelet therapy in endovascularly treated patients with peripheral artery diseaseJ Endovasc Ther20132069970624093324
- IidaOYokoiHSogaYCilostazol reduces angiographic restenosis after endovascular therapy for femoropopliteal lesions in the Sufficient Treatment of Peripheral Intervention by Cilostazol studyCirculation20131272307231523652861
- KrankenbergHSchlüterMSteinkampHJNitinol stent implantation versus percutaneous transluminal angioplasty in superficial femoral artery lesions up to 10 cm in length: the femoral artery stenting trial (FAST)Circulation2007116328529217592075
- DickPWallnerHSabetiSBalloon angioplasty versus stenting with nitinol stents in intermediate length superficial femoral artery lesionsCatheter Cardiovasc Interv20097471090109519859954
