Abstract
Granulomatosis with polyangiitis (GPA, formerly Wegener’s granulomatosis) is a multisystem autoimmune condition associated with anti-neutrophil cytoplasm antibodies. Management of GPA can be complex, owing to the sometimes fulminant and multisystem nature of the presentation, the age demographics of the affected population, and a significant incidence of disease relapse. In this paper, we discuss how some of the challenges in the management of GPA have been and continue to be addressed including: reducing the toxicity of induction therapy; developing biomarkers to determine who can safely stop maintenance immunosuppression; improving the efficacy of maintenance therapy for relapsing patients; managing localized disease; and management of disease and treatment-associated comorbidity. Consideration is also given to emerging therapeutics in the treatment of GPA.
Introduction
Granulomatosis with polyangiitis (GPA, formerly known as Wegener’s granulomatosis) is one of the anti-neutrophil cytoplasm antibody (ANCA)-associated small vessel vasculitides (AAV).Citation1 The other clinical syndromes associated with ANCA are microscopic polyangiitis (MPA) and eosinophilic granulomatosis with polyangiitis (formerly known as Churg Strauss syndrome).Citation2 Characteristic pathological features of GPA include arteritis of small-sized to medium-sized blood vessels, and granulomatous inflammation of the upper airways and lungs.Citation1 Renal involvement with a pauci-immune necrotizing glomerulonephritis is one of the commonest severe manifestations, occurring in 75% of patients during the course of the disease,Citation3,Citation4 and AAV is the commonest cause of a pulmonary-renal syndrome presenting with acute renal dysfunction and pulmonary hemorrhage.Citation5 AAV are rare diseases with a combined incidence of 20 per million per year and a prevalence of 144 per million in the UK.Citation6 Two thirds of cases of AAV in the UK are GPA.Citation6 AAV can occur at any age, including childhood, but is most common in older patients (peak age 55–70 years), and occurs equally in both sexes.Citation6 AAV causes significant morbidity and mortality, with end-stage renal failure occurring in more than 20% of patients at 5 years and a 5-year survival rate of approximately 75%.Citation7,Citation8 Prompt diagnosis and treatment is important to avoid mortality and/or permanent organ damage. With treatment, 85%–90% of patients will go into remission,Citation9 but the disease follows a relapsing-remitting course, with a 50% relapse rate within 5 years.Citation10 This review focuses on challenges in the management of GPA. In order to set the context for this, we first give a brief overview of the pathogenesis and clinical features of GPA in the context of AAV as a whole. More detailed information on these aspects can be obtained from other recent reviews.Citation11,Citation12
Pathogenesis of AAV
ANCA are directed against glycoprotein enzymes present in neutrophil granules and monocyte lysosomes, most notably proteinase 3 (PR3)Citation13 and myeloperoxidase (MPO).Citation14 Cytokine-primed neutrophils and monocytes express the target antigens on their surface.Citation15 Binding of ANCA to these antigens activates the cells, leading to a superoxide burst, and release of lytic enzymes and proinflammatory cytokines such as interleukin (IL)-8.Citation16–Citation18 ANCA binding to neutrophils promotes endothelial adhesion and cytotoxicity to cultured endothelial cells.Citation18,Citation19 There is in vivo evidence for the direct pathogenicity of MPO-ANCA from maternal-fetal transfer and from mouse and rat models.Citation20–Citation22 PR3 does not have a direct murine homolog, but immunoglobulin from PR3-ANCA-positive patients caused glomerulonephritis in a humanized mouse model.Citation23 T cells are also important, because effector memory T cells are present in chronic vasculitic lesions in GPACitation24,Citation25 and antigen-specific Th17 cells, and the cytokines IL-17 and IL-23 were raised in patients with active AAV.Citation26 There is recent evidence of defective T and B cell regulation in AAV.Citation27–Citation30 Emerging data suggest that activation of the alternative pathway of complement is important in AAV, and may be a therapeutic target.Citation31–Citation33
Genetics of AAV
A genome-wide association study (GWAS) has confirmed that GPA and MPA are different diseases with different major histocompatibility complex associations.Citation34 In the GWAS, GPA was also associated with the gene for its autoantigen PR3, confirming the importance of this antigen in the pathogenesis of the disease. The genetic associations were stronger for PR3-ANCA and MPO-ANCA rather than for the clinical syndromes of GPA and MPA, prompting consideration of whether these biomarkers should be used to stratify patients.Citation35
Clinical features of GPA
Systemic GPA often presents with symptoms of weight loss, fever, fatigue, arthralgia, and myalgia.Citation3,Citation4 GPA is characterized by granulomatous upper airways involvement in about 90% of patients.Citation3 Upper respiratory tract symptoms include epistaxis, sinusitis, otitis media, deafness, hoarseness, stridor, and proptosis due to orbital involvement. The granulomatous inflammation can lead to local damage, including nasal septal perforation, saddle nose, and tracheal stenosis.Citation36 Lung involvement occurs in 85% of patients with GPA at some stage, and includes pulmonary nodules and cavities, pulmonary infiltrates, pleural effusion, and pulmonary fibrosis.Citation3 Alveolar hemorrhage occurs in approximately 20% of patients with active GPA.Citation37 Renal involvement is a common feature of GPA, with necrotizing and crescentic glomerulonephritis occurring in 75% of patients at some point in their disease.Citation3 Renal replacement therapy is required in 20%–30% of patients, either due to rapidly progressive glomerulonephritis, or to gradual loss of kidney function over time.Citation7 Almost any organ in the body can be involved, but in particular vasculitic skin lesions, peripheral neuropathy, mononeuritis multiplex, granulomatous meningeal involvement, cardiac disease, and gut involvement are well recognized in GPA.Citation3 Localized GPA refers to granulomatous vasculitis limited to the upper airways, sinuses and orbits, or lungs, without systemic involvement or constitutional symptoms, and occurs in about 5% of patients with GPA.Citation38,Citation39 However, as stated above, about 90% of patients with systemic GPA have granulomatous disease of the upper airways.Citation3 Localized GPA has a high propensity for relapse,Citation40 and can cause significant local damage over time,Citation36 as well as treatment-associated morbidity if recurrent courses of induction treatment are required.
By contrast, MPA is characterized by systemic vasculitis without granulomatous disease. Pauci-immune glomerulonephritis is very common in MPA;Citation4 however, upper airways granulomata and pulmonary nodules are not a feature, and chronic lung damage in MPA tends to present with a more fibrosing, restrictive pattern.Citation41 Pulmonary hemorrhage can occur in both active GPA and active MPA.Citation37
Relapse risk in GPA
The relapse risk of GPA is approximately 50% at 5 years.Citation10 GPA and PR3-ANCA-positive patients are significantly more likely to relapse than patients with MPO-ANCA or MPA.Citation40,Citation42,Citation43 Patients with granulomatous disease of the upper airways are also more likely to relapse than those without this manifestation, whilst those with renal impairment had a reduced relapse risk.Citation40,Citation43 A rise in ANCA titer is not strongly predictive of relapse at an individual patient level, so it is important to monitor clinical symptoms and signs, as well as inflammatory markers.Citation44 More information about predicting relapse in GPA is discussed in the section on management below.
Laboratory markers of GPA
PR3-ANCA has a high sensitivity and specificity for the diagnosis of active GPA (>90%), in the right clinical context, although ANCA-negative and MPO-ANCA- positive cases do occur.Citation45 However, 50% of patients with the clinical syndrome of localized GPA are ANCA-negative.Citation38 Inflammatory markers such as erythrocyte sedimentation rate and C-reactive protein are also usually raised in active AAV. The gold standard for diagnosis of AAV is the finding of small vessel vasculitis on biopsy. The kidney is usually the best site for diagnosis, where a pauci-immune necrotizing glomerulonephritis is seen. In GPA, granulomatous inflammation is present in the upper airways, but in practice this is often missed in small biopsies due to sampling errors, and nonspecific collections of inflammatory cells are often found in biopsies of the lung or upper airways.
Efficacy and side effects of current and historical management strategies
In the 1950s, the prognosis of generalized GPA was poor, with only about 10% survival at 2 years.Citation46 In the 1970s, a regimen of cyclophosphamide and glucocorticoids was introduced at the National Institutes of Health, leading to remission in 85%–90% of patients within 3–6 months, but high cumulative toxicity of cyclophosphamide.Citation46 Improvements in supportive management, including the availability of hemodialysis for renal failure and ventilatory support for pulmonary hemorrhage have also improved the prognosis of severe vasculitis. However, high cumulative doses of cyclophosphamide have been associated with a number of short-term and long-term side effects, including infections, leukopenia, impaired fertility, and cancer.Citation47–Citation51 Corticosteroids also have a number of well documented side effects, including weight gain, diabetes, osteoporosis, increased cardiovascular risk, psychological effects, and skin thinning. Studies have shown that there is a greater chance of death from infection than from uncontrolled disease within the first year of diagnosis of AAV.Citation52
Aims of treatment of GPA
AAV are believed to be autoimmune diseases and are treated by immunosuppression. Treatment of AAV is divided into two phases, ie, an induction phase and a remission maintenance phase. The aim of induction treatment is to rapidly reduce inflammation to control signs and symptoms of disease and prevent permanent tissue damage. In the remission maintenance phase, lower-dose immunosuppression is used to prevent relapse. Of course, these aims should be achieved with the minimum of toxicity. As a guide, lists the induction and maintenance strategies that are commonly in use for different severities of GPA, with reference to the evidence supporting them. lists the short-term and long-term side effects that have been associated with the drugs most commonly used to treat GPA. In this review, we have chosen to discuss particular challenges in managing GPA, namely: reducing the toxicity of induction therapy for GPA; the development of predictive tools to determine who can safely stop maintenance immunosuppression; improving the efficacy of remission maintenance strategies in GPA; managing localized GPA; and management of disease and treatment-related comorbidity. Finally, we discuss the possible future therapeutics in the management of AAV.
Table 1 Evidence for immunosuppression in GPA, based on disease severity
Table 2 Short-term side effects and long-term damage associated with treatment in GPA
The results of randomized trials of induction therapy for AAV discussed below have enabled a reduction in the intensity and duration of induction immunosuppression for GPA, and evidence would suggest that this has led to improvements in outcome over the past 30 years.Citation53,Citation54 Several of the trials have been carried out by the European Vasculitis Study Group (EUVAS). In their trials, EUVAS decided to subgroup vasculitis according to severity, to give high-intensity treatment to induce remission and low-intensity immunosuppression to prevent relapse, to agree on a standard regimen by consensus, to test against current best practice by randomized controlled trials, and to use standardized scoring systems for measuring outcome.
Reducing the toxicity of induction therapy for GPA
Induction therapy for GPA is effective for most patients, but the toxicity can be high, especially in elderly patients and those with severe renal impairment.Citation55 The two main approaches to reduce toxicity have been to reduce the cyclophosphamide exposure, and more recently, trials have been designed to reduce exposure to corticosteroids. lists the randomized controlled trials of induction therapy that have been carried out in AAV over the past 20 years and summarizes their main outcomes, and lists the induction trials currently ongoing or completed and not yet published. Most of these trials included patients with either GPA or MPA.
Table 3 Completed multicenter randomized controlled studies of induction therapy in AAV
Table 4 Trials of induction therapy in AAV in progress or completed and not yet published
Reducing the doses of cyclophosphamide
The CYCAZAREM (Cyclophosphamide vs azathioprine for early remission phase of vasculitis) study established that it was possible to switch from cyclophosphamide to azathioprine once remission was attained at 3–6 months, thereby reducing cyclophosphamide exposure.Citation56 The CYCLOPS (Randomized trial of daily oral versus pulse cyclophosphamide as therapy for ANCA-associated systemic vasculitis) trial showed that pulsed intravenous cyclophosphamide given every 2–3 weeks for 3–6 months until disease remission was attained was as effective in inducing remission as daily oral cyclophosphamide, whilst the total dose of cyclophosphamide administered was approximately half in the intravenous cyclophosphamide group.Citation57 Longer-term follow-up of patients from this study demonstrated a higher relapse rate in the intravenous cyclophosphamide group; however, there was no difference in mortality or renal function at the end of the study.Citation58 Due to the toxicity of cyclophosphamide, lower-dose therapy is generally preferred.
Assessing alternatives to cyclophosphamide
The NORAM (Nonrenal Wegener’s Granulomatosis Treated Alternatively with Methotrexate) study demonstrated that methotrexate was as effective as cyclophosphamide in inducing remission in early systemic AAV, but in the longer term, disease control was less effective in the methotrexate arm.Citation59,Citation60 Therefore, methotrexate induction is usually reserved for patients with localized or early systemic disease without major tissue destruction, although it is used more commonly to maintain remission.
Mycophenolate mofetil (MMF) 2–3 g/day has been compared with intravenous cyclophosphamide for induction therapy in AAV in MYCYC (A randomized clinical trial of mycophenolate mofetil versus cyclophosphamide for remission induction in ANCA-associated vasculitis) and presented in abstract form.Citation61 The primary endpoint was remission whilst adhering to the glucocorticoid regimen, and this occurred in 46/70 of the MMF group compared with 48/70 of the intravenous cyclophosphamide group. This study just failed to demonstrate the noninferiority for MMF, but the secondary endpoint of remission irrespective of steroid compliance was achieved. However, unpublished data would suggest that the relapse rate was higher in the MMF group and further details are awaited.
Targeting B cells with rituximab in combination with corticosteroids has proven to be noninferior to cyclophosphamide and corticosteroids followed by azathioprine for inducing remission in AAV, as demonstrated in the RAVE (Rituximab in ANCA-Associated Vasculitis) and RITUXVAS (Rituximab Versus Cyclophosphamide in ANCA-Associated Vasculitis) studies.Citation62,Citation63 In the RAVE study, rituximab was more effective than cyclophosphamide in achieving remission in the subgroup of patients with relapsing disease.Citation62,Citation64 Rituximab is a chimeric monoclonal antibody binding to CD20, an antigen expressed on the surface of B cells, leading to a reduction in the numbers of circulating B cells for a variable period of 4–12 months. B cells are the precursors of ANC-producing plasma cells, and in addition, PR3-specific affinity-matured B cells were found in nasal biopsy specimens from patients with active GPA.Citation65 Rituximab is not known to reduce fertility or to promote cancer, so this treatment has provided an important additional option for induction therapy of AAV, and may also enable patients with relapsing disease to avoid high cumulative doses of cyclophosphamide.
Reducing corticosteroid exposure during induction treatment
For systemic GPA, high-dose corticosteroids are used as part of the induction regimen, initially at 1 mg/kg up to 60 mg/day. These are weaned down over several weeks to a dose of 10 mg daily after around 3 months or once remission is attained. High-dose corticosteroids are associated with a number of short-term and long-term side effects (). Infections are the commonest cause of death in the first year after the diagnosis of AAV.Citation52 Substitution of rituximab for cyclophosphamide failed to reduce the early adverse event rates in the RAVE and RITUXVAS trials, including infections.Citation62,Citation63 This disappointing result tends to implicate high-dose corticosteroids, used as part of the induction regimen, in the infection rate. The RAVE and RITUXVAS trials both used intravenous methylprednisolone at induction, which may have contributed to the adverse event rate. Minimization of steroid exposure is a key target for future trials. PEXIVAS [An International Randomized Controlled Clinical Trial Assessing Plasma Exchange and Steroid Dosing in the Treatment of Severe Anti-Neutrophil Cytoplasm Antibody (ANCA) Associated Vasculitis] is examining the effect of more rapid weaning of steroids on the outcomes in patients with AAV.Citation66 The CLEAR (C5aR inhibitor on Leukocytes Exploratory ANCA-associated Renal Vasculitis) study is examining whether a C5a receptor antagonist (CCX168) can reduce or eliminate the need for oral corticosteroids in induction therapy for AAV.Citation67
Management of severe and refractory GPA
Plasma exchange is recommended for patients presenting with AAV with severe renal inflammation causing acute renal dysfunction. The rationale is for rapid removal of ANCA, and possibly other inflammatory mediators, before immunomodulatory and anti-inflammatory drugs have had an impact.Citation68 The MEPEX (Methylprednisolone versus Plasma Exchange) trial was designed to assess renal recovery in patients with AAV and serum creatinine >500 μmol/L.Citation69 Although benefit from plasma exchange in terms of renal recovery was achieved, longer-term follow-up demonstrated no difference in long-term mortality.Citation70 This uncertainty led to the design of the PEXIVAS trial, recruiting a larger number of patients (n=500) and including those with pulmonary hemorrhage. Hopefully, this study will answer the question of whether plasma exchange is indicated in severe renal AAV more definitively, and also determine the utility of plasma exchange in pulmonary hemorrhage and lesser degrees of renal impairment.Citation66
Approximately 5% of patients with GPA fail to go into remission after induction therapy, and are termed refractory.Citation71,Citation72 This group of patients is particularly difficult to manage. The German Registry of Autoimmune Diseases published an analysis of the responses of patients with AAV (50/58 with GPA) refractory to rituximab. Forty percent were classified as complete responders, and a further 52.7% demonstrated a partial response, indicating that rituximab should be considered as first-line therapy in patients not responding to cyclophosphamide induction.Citation73 Open-label studies suggest a number of options for patients not responding to cyclophosphamide or rituximab, ie, alemtuzumab (anti-CD52), tumor necrosis factor (TNF) antagonists, mycophenolate mofetil, 15-deoxyspergualin (an antiproliferative agent targeting antigen-stimulated B cells), or intravenous immunoglobulin.Citation72,Citation74–Citation79 Intravenous immunoglobulin can be helpful as an option in patients with active infection or those who have not tolerated immunosuppression, although frequent retreatment may be required.Citation74,Citation75
Predictive tools to determine who can safely stop maintenance immunosuppression
Disease relapse remains a problem in GPA, with a 50% relapse rate over the course of 5 years.Citation3 Minor relapses may be managed with an increase in oral glucocorticoids or optimization of maintenance immunosuppression dosing, but major relapses require repeated induction therapy. The risk of relapse of GPA and PR3-ANCA-positive vasculitis is higher than for other forms of vasculitis such as MPA, and previous relapse is also predictive of future flares.Citation10,Citation43,Citation64 Lung and upper airways involvement in GPA are associated with higher relapse rates.Citation10 Reduced intensity induction therapyCitation42,Citation59 or early withdrawal of immunosuppression or glucocorticoidsCitation80 has been associated with an increased risk of relapse. However, although risk factors of relapse can be defined at a population level, we are unable to predict with any great degree of certainty the outcome for any one particular patient with GPA. Therefore, close clinical monitoring of symptoms, signs, and inflammatory markers is required to detect relapses at an early stage, and it is likely that many patients are immunosuppressed for longer than necessary, due to uncertainty about their risk of relapse. ANCA positivity is a good diagnostic marker for GPA.Citation45 However, a meta-analysis of available studies showed that a rise in ANCA titers during remission was only weakly associated with relapse, and therefore not very useful for predicting the disease course in individual patients.Citation44 It is our practice not to modify treatment based on changes in ANCA status alone, but to monitor patients with rising ANCA titers carefully for clinical signs of relapse, along with monitoring inflammatory markers such as C-reactive protein. There is a need for more reliable biomarkers to predict relapse in AAV patients.
McKinney et al have discovered a CD8 T cell messenger RNA signature detectable at diagnosis, which was associated with relapse in patients with AAV and a range of autoimmune conditions.Citation81 This signature was only discernible in isolated leukocyte subsets and not in whole blood. If a clinically applicable test could be devised based on these findings it could have some utility in predicting relapse in GPA. Calprotectin (S100A8/S100A9) is a damage associated molecular pattern produced by neutrophils and monocytes, and is commonly raised in inflammatory conditions. In a subset of 27 patients with early systemic AAV recruited into the NORAM study, levels of calprotectin over 626 ng/mL at one month after treatment was initiated were associated with future relapse, with a sensitivity of 72.6% and a specificity of 92.3%, and similarly higher calprotectin levels at 6 months were also significantly associated with relapse.Citation82 Serum calprotectin levels are now being prospectively assessed as biomarkers for relapse in AAV. Urinary monocyte chemoattractant protein 1 levels were associated with active renal vasculitis; however, it has not yet been determined whether these changes occur earlier than other clinical evidence of renal vasculitis, and therefore whether they have value in predicting relapse.Citation83
Improving the efficacy of maintenance therapy for GPA
Once patients with GPA attain clinical remission, they are switched to a less toxic maintenance regimen. However, relapse commonly occurs, and some patients will have several relapses during their lifetime, leading to accumulation of disease-associated damage and drug-related toxicity. A number of clinical trials have compared the safety and efficacy of remission maintenance agents in AAV (), and trials that are ongoing are listed in .
Table 5 Completed multicenter randomized controlled trials of remission maintenance therapy in AAV
Table 6 Trials of maintenance therapy in AAV in progress or completed and not yet published
Trials comparing maintenance therapies in GPA
The IMPROVE (International Mycophenolate Mofetil Protocol to Reduce Outbreaks of Vasculitides) study found that MMF was less effective at maintaining remission than azathioprine in AAV.Citation84 Therefore, MMF is usually reserved for patients who do not tolerate azathioprine. The WEGENT (Wegener’s Granulomatosis-Entretien/Maintenance) study found no differences in efficacy or safety between azathioprine and methotrexate for maintenance of remission, and so they are considered equivalent,Citation85 although methotrexate cannot be used in patients with significant renal dysfunction. Etanercept was not effective as an add-on maintenance strategy in AAV.Citation86 Leflunomide is an alternative maintenance agent, usually reserved for patients intolerant of other agents, given that the clinical trial suggested more adverse events than methotrexate.Citation87
Eighteen-month follow-up data from the RAVE study demonstrated that patients treated with rituximab and corticosteroids alone had the same relapse rate as patients treated with cyclophosphamide followed by azathioprine maintenance.Citation64 Patients who achieved remission in this study were weaned off oral glucocorticoids. One third of patients relapsed during the study, and therefore neither regimen was very effective at maintaining remission in this setting. Two cohort studies have demonstrated that in relapsing patients, scheduled maintenance therapy with rituximab appears to be an effective option for maintaining remission in AAV, although relapses did occur after discontinuation.Citation88,Citation89 The relative efficacy of azathioprine maintenance compared with scheduled rituximab retreatment has been investigated in MAINRITSAN (Efficacy Study of Two Treatments in the Remission of Vasculitis), a randomized study that has been published in abstract form and showing superior disease control in patients maintained on rituximab.Citation90 In addition, RITAZAREM (the Rituximab Vasculitis Maintenance Study),Citation91 comparing rituximab or azathioprine maintenance is currently ongoing. The cumulative effects of rituximab therapy in AAV are unknown. There have been a few reports of John Cunningham (JC) virus infection leading to progressive multifocal leukoencephalopathy following rituximab, although these cases have involved patients exposed to significant immunosuppression over a period of time.Citation92 The other long-term complication ascribed to rituximab is hypogammaglobulinemia, which again seems to be related to cumulative exposure to immunosuppression.Citation93 There is an obligation therefore to try to avoid excessive immunosuppression, even in patients at risk of relapse, and means to better predict relapse are urgently required.
Evidence to determine duration of maintenance therapy in GPA
The optimal duration of corticosteroid and maintenance immunosuppressant treatment in GPA is not known, and in reality is likely to vary between individual patients. In one retrospective study, withdrawal of corticosteroid treatment at 6 months was associated with reduced infections and no increase in relapse rate compared with those maintained on corticosteroids.Citation94 However, in other studies, early withdrawal of steroids has been associated with increased relapse rates.Citation80 A randomized trial to compare maintenance of patients presenting with PR3-ANCA-positive disease using azathioprine for 2 years or 4 years has been reported in abstract form.Citation95 It was reported that there was no significant improvement in relapse-free survival with the extended course of azathioprine at 48 months, with relapse rates of 52%–74% in the different groups. It is likely that remission maintenance strategies will evolve significantly once the results of current trials are available.
Management of localized GPA
For localized disease, a multidisciplinary approach with close communication between otolaryngologists, ophthalmologists, and physicians is required. Systemic immunosuppression combined with local therapy is often necessary. There is evidence of the efficacy of cotrimoxazole in preventing relapse of upper respiratory tract GPA, possibly due to the elimination of nasal carriage of Staphylococcus aureus.Citation96,Citation97 Localized disease can sometimes be treated with methotrexate, but it is our practice to reserve this treatment for manifestations where the risk of long-term damage is low, based on the results of the NORAM study.Citation60 There have been reports that rituximab is less effective in treating granulomatous upper airways manifestations of GPA than generalized disease,Citation98 but this finding has not been confirmed in all studies.Citation99,Citation100 The time course of response for granulomatous disease differs from vasculitic manifestations, with a more delayed response, and this may have affected the assessment of response to rituximab in studies with less than 6 months of follow-up. Obstructive tracheobronchial disease can be poorly responsive to systemic therapy, and if not effectively treated, can lead to permanent tracheal scarring with respiratory compromise and increased susceptibility to chest infections. In addition to systemic treatment, active tracheobronchial disease can be treated locally with intralesional steroids and endoluminal surgery with intralesional laser and dilatation.Citation101–Citation103 Tracheal endoscopy can be used to monitor treatment response. Nasal and sinus disease can be managed locally with intranasal corticosteroids, and regular saline douching of nasal crusts. Middle ear obstruction may require insertion of grommets. Reconstructive surgery for nasal bridge collapse is an option, but is only recommended in the absence of active nasal vasculitis.
Management of damage and disease/treatment-associated comorbidity
Compared with age-matched and sex-matched controls in the general population, a study of 535 patients with AAV enrolled into four EUVAS trials showed a mortality rate of 2.6.Citation8 Forty-four percent of the deaths during the 5-year follow-up of this study occurred within the first year after induction therapy, and the main causes of death in the first year were infection (48%) and active vasculitis (19%).Citation8 It is not currently clear which component of induction therapy contributes most to the risk of infection. Half of the infections occurring in the first year were observed during the first 2 months of induction treatment, and unspecified septicemia and bacterial infections of the respiratory tract accounted for half of the cases.Citation52 Cumulative adverse events during induction therapy are significantly associated with mortality.Citation52 Elderly patients and those with severe renal impairment are particularly susceptible to treatment- associated complications.Citation55,Citation104 Cotrimoxazole is routinely given to patients receiving cyclophosphamide as prophylaxis against Pneumocystis jirovecii pneumonia.Citation105
After the first year, the major causes of death in the EUVAS cohorts were cardiovascular disease (26%), malignancy (22%), and infection (20%).Citation8 Long-term follow-up data from these trials after 7.3 years of follow-up showed a significant burden of morbidity, with 34.4% of patients having more than five items of damage on the Vasculitis Damage Index at long-term follow-up.Citation48 In patients with GPA, the commonest items of damage were nasal blockage/crusting (44.3%), hypertension (39.5%), hearing loss (32.3%), and a glomerular filtration rate <50 mL per minute (31.7%). Impaired pulmonary function (13.8%) and peripheral neuropathy (22.2%) were also prominent features. Cardiovascular endpoints of angina/coronary artery bypass, stroke, and myocardial infarction were also significantly increased.Citation48,Citation106 In view of this, attention must be drawn to management of cardiovascular risk factors, including smoking, exercise, hypertension, weight management, lipids, and management of diabetes, where present. End-stage renal disease occurs in up to 25% of patients with AAV.Citation8 Dialysis and renal transplantation are options for these patients, and patients with AAV have good outcomes of transplantation when it is performed after disease activity is controlled.Citation107 More difficult to manage is permanent lung scarring due to pulmonary fibrosis and respiratory compromise due to tracheal and bronchial stenosis, which can also predispose to recurrent chest infections.
Damage in GPA is not only related to the disease itself, but also to treatment. Short-term and long-term toxicities associated with treatments commonly used for GPA are listed in . In the EUVAS trials, potential treatment-related damage items were reported for two thirds of patients. Cohorts of GPA patients exposed to high cumulative doses of cyclophosphamide have been shown to be at an increased risk of bladder malignancy (standardized incidence ratio [SIR] 3.6–4.8),Citation49–Citation51 acute myeloid leukemia,Citation50 (SIR 19.6), and nonmelanoma skin cancer (SIR 4.7).Citation50 The risk is known to be dose-dependent, and increase substantially with cumulative doses of cyclophosphamide over 25 g,Citation49,Citation50 but a safe threshold dose for cyclophosphamide has not been established. However, the risks of bladder malignancy, leukemia, and non-melanoma skin cancer in the recent EUVAS trials were lower than in previous cohorts (SIR 2.4, 3.2, and 2.8, respectively), probably due to reduced cyclophosphamide exposure.Citation47 Azathioprine has been associated with nonmelanoma skin cancer in other conditions;Citation108,Citation109 however, in AAV, it is rarely used alone and so its contribution to skin cancer in GPA is difficult to quantify. Recommendations for treatment of AAV, including prophylaxis for the prevention of treatment-associated complications have been produced.Citation105,Citation110
Management of GPA in the future
There is an ongoing need to reduce the toxicity of treatment for GPA, and to increase the efficacy of maintenance therapy in preventing relapse. Currently, a means to restore immunological tolerance to ANCA autoantigens in AAV does not exist. However, the development of biological therapeutics and small molecules targeting specific cell types, cytokines, and immunological pathways has enabled more rational drug targeting in inflammatory diseases. A number of new therapeutics targeting immunological pathways have a rationale for efficacy in AAV, and these are discussed below and shown in . Most have yet to be formally evaluated for this indication. Clinical trials of emerging therapeutics for AAV that are currently ongoing are listed in and .
Figure 1 A selection of biologics and small molecule inhibitors targeting the autoimmune response and effector responses that may be important in GPA.
Abbreviations: ANCA, anti-neutrophil cytoplasm antibody; GPA, granulomatosis with polyangiitis; IL, interleukin; CCX168, C5a receptor antagonist; BlyS, B cell activating factor; TNF, tumor necrosis factor; CD, cluster of differentiation; ANCA, Anti-neutrophil cytoplasm antibody; SYK, spleen tyrosine kinase.
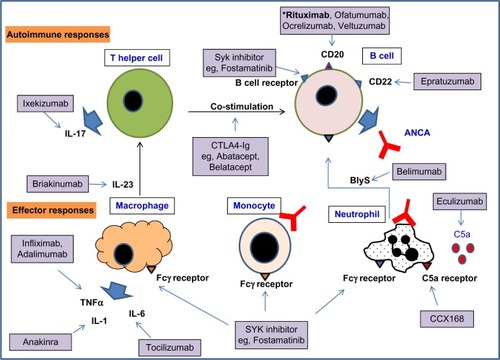
B cells
Rituximab, a chimeric human/mouse anti-CD20 B cell-depleting agent, has proven efficacy as induction therapy in AAV and is being evaluated for maintenance of remission.Citation62,Citation63,Citation91 Fully humanized CD20 monoclonal antibodies have been developed, including ofatumumab, ocrelizumab, and veltuzumab. Ofatumumab has been licensed for treatment of resistant chronic lymphocytic leukemia.Citation111 These agents are not currently licensed for treatment of AAV, but they have been used “off label” in patients who have allergic reactions to rituximab.
B cell activating factor (BlyS) is a cytokine involved in the proliferation and maturation of B cells. ANCA-stimulated neutrophils release BlyS, and BlyS levels have been shown to be elevated in patients with GPA.Citation112–Citation114 Belimumab is a fully humanized monoclonal antibody to soluble BlyS, which is currently being evaluated in Phase II studies as a relapse prevention agent in AAV ().
Epratuzumab is an anti-CD22 monoclonal antibody expressed on follicular B cells in germinal centers. CD22 is a transmembrane sialoglycoprotein involved in negative regulation of signaling via the B cell receptor, and is also involved in B cell migration.Citation115 This agent has not yet been evaluated for AAV indications, but is being investigated in a Phase III study for nonrenal systemic lupus erythematosus.Citation116
T cells
T effector memory cells are present in chronic vasculitic lesions in patients with GPA,Citation24,Citation25 and T cell help is also required for generation of ANCA. Targeting T cell costimulation with CTLA4-Ig, abatacept, showed promising results in a small open-label study.Citation117 Lymphocyte depletion with anti-CD52 (Campath-1H®) caused reductions in circulating lymphocytes, including CD4 T cells and was used in a small study in refractory AAV (total dose 134 mg).Citation76 Whilst effective in these difficult-to-treat patients, the infection rate was high, and so now a second study is ongoing using lower doses of Campath-1H (30 mg or 60 mg) in 24 patients with refractory AAV.Citation118 Raised serum levels of IL-23 and IL-17 and antigen-specific Th17 cells have been demonstrated in AAV,Citation26 implicating the Th17 pathway in pathogenesis. Agents targeting the Th17 axis (inhibitors of IL-17, IL-17 receptor, and IL-12/IL-23) are under development, in particular for resistant psoriasis,Citation119–Citation121 but these agents have not been evaluated for safety or efficacy in AAV.
Complement
Raised levels of alternative pathway complement breakdown products have been detected in the serum of patients with active AAV and in kidney biopsy specimens.Citation31,Citation32 There is also evidence of the importance of the alternative pathway of complement in a mouse model of MPO-positive vasculitis.Citation33 C5a is a potent neutrophil chemoattractant, and C5a receptor signaling increases the expression of activatory Fc gamma receptors on neutrophils.Citation122 These data make C5a and its receptor a potential anti-inflammatory target in AAV. The CLEAR study is evaluating an orally bioavailable C5a receptor antagonist as a steroid-sparing agent in induction therapy in AAV.Citation67
Cytokines and small molecule signaling inhibitors
TNF-α primes neutrophil responses to ANCA, and increases the expression of PR3 on the neutrophil surface.Citation15 In small studies, blockade of TNF-α by monoclonal antibodies has appeared to be effective in having a steroid-sparing effect in AAV.Citation78,Citation123 However, the TNF receptor blocker etanercept failed to show benefit in a larger study.Citation86 Anti-TNF antibodies block both soluble and membrane-bound TNF-α, whilst etanercept blocks only the effects of soluble TNF-α, and this might account for the differing results. However, due to the results with etanercept, TNF-α blockade has not subsequently been evaluated in AAV in a large clinical trial. Blockade of the IL-6 and IL-1β pathways are other potential anti-inflammatory targets that have not yet been evaluated in AAV.
Spleen tyrosine kinase (SYK) is a nonreceptor tyrosine kinase that transmits intracellular signals for the B cell receptor and activatory Fc gamma receptors, amongst other immunoreceptors. SYK inhibitors not only target antibody production, but also downstream effector mechanisms. Fostamatinib, an orally bioavailable inhibitor of SYK, has been found to be highly effective in rat experimental autoimmune glomerulonephritis and nephrotoxic nephritis.Citation124,Citation125 Fostamatinib has been shown to have efficacy in rheumatoid arthritis in a Phase II study,Citation126 but has not been evaluated in AAV.
Conclusion
Management of GPA requires a multidisciplinary approach, aiming not only to treat the disease and prevent relapse, but also to manage long-term cardiovascular risk, organ damage, and side effects of therapy. Treatments have been refined as a result of large multicenter randomized controlled trials in AAV; however, there are unmet needs to resolve granulomatous inflammation more rapidly, to reduce steroid exposure, and to improve the efficacy of remission maintenance strategies in GPA. There are a large number of potentially promising agents for the treatment of AAV in development, and clinical trials will be required to determine whether these can meet unmet needs for patients with GPA.
Disclosure
CDP has received research funding from GlaxoSmithKline. The authors report no other conflicts of interest in this work.
References
- SeoPStoneJHThe antineutrophil cytoplasmic antibody-associated vasculitidesAm J Med2004117395015210387
- JennetteJCFalkRJBaconPA2012 Revised International Chapel Hill Consensus Conference nomenclature of vasculitidesArthritis Rheum20136511123045170
- HoffmanGSKerrGSLeavittRYWegener granulomatosis: an analysis of 158 patientsAnn Intern Med19921164884981739240
- FalkRJHoganSCareyTSJennetteJCClinical course of anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and systemic vasculitis. The Glomerular Disease Collaborative NetworkAnn Intern Med19901136566632221646
- NilesJLBottingerEPSaurinaGRThe syndrome of lung hemorrhage and nephritis is usually an ANCA-associated conditionArch Intern Med19961564404458607730
- WattsRALaneSEBenthamGScottDGEpidemiology of systemic vasculitis: a ten-year study in the United KingdomArthritis Rheum20004341441910693883
- BoothADAlmondMKBurnsAOutcome of ANCA-associated renal vasculitis: a 5-year retrospective studyAm J Kidney Dis20034177678412666064
- FlossmannOBerdenAde GrootKLong-term patient survival in ANCA-associated vasculitisAnn Rheum Dis20117048849421109517
- MiloslavskyESpecksUMerkelPClinical outcomes of remission induction therapy for severe ANCA-associated vasculitisArthritis Rheum2013652441244923754238
- PagnouxCHoganSLChinHPredictors of treatment resistance and relapse in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis: comparison of two independent cohortsArthritis Rheum2008582908291818759282
- JennetteJCFalkRJHuPXiaoHPathogenesis of antineutrophil cytoplasmic autoantibody-associated small-vessel vasculitisAnnu Rev Pathol2013813916023347350
- TarziRMCookHTPuseyCDCrescentic glomerulonephritis: new aspects of pathogenesisSemin Nephrol20113136136821839369
- LudemannJUtechtBGrossWLAnti-cytoplasmic antibodies in Wegener’s granulomatosis are directed against proteinase 3Adv Exp Med Biol19912971411501722626
- FalkRJJennetteJCAnti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritisN Engl J Med1988318165116572453802
- CsernokEErnstMSchmittWBaintonDFGrossWLActivated neutrophils express proteinase 3 on their plasma membrane in vitro and in vivoClin Exp Immunol1994952442508306499
- HarperLCockwellPAduDSavageCONeutrophil priming and apoptosis in anti-neutrophil cytoplasmic autoantibody-associated vasculitisKidney Int2001591729173811318943
- HattarKvan BurckSBickenbachAAnti-proteinase 3 antibodies (c-ANCA) prime CD14-dependent leukocyte activationJ Leukoc Biol200578992100016006536
- SavageCOPottingerBEGaskinGPuseyCDPearsonJDAutoantibodies developing to myeloperoxidase and proteinase 3 in systemic vasculitis stimulate neutrophil cytotoxicity toward cultured endothelial cellsAm J Pathol19921413353421323218
- RadfordDJSavageCONashGBTreatment of rolling neutrophils with antineutrophil cytoplasmic antibodies causes conversion to firm integrin-mediated adhesionArthritis Rheum2000431337134510857792
- BansalPJTobinMCNeonatal microscopic polyangiitis secondary to transfer of maternal myeloperoxidase-antineutrophil cytoplasmic antibody resulting in neonatal pulmonary hemorrhage and renal involvementAnn Allergy Asthma Immunol20049339840115521377
- LittleMASmythCLYadavRAntineutrophil cytoplasm antibodies directed against myeloperoxidase augment leukocyte-microvascular interactions in vivoBlood20051062050205815933057
- XiaoHHeeringaPHuPAntineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in miceJ Clin Invest200211095596312370273
- LittleMAAl-AniBRenSAnti-proteinase 3 anti-neutrophil cytoplasm autoantibodies recapitulate systemic vasculitis in mice with a humanized immune systemPLoS One20127e2862622247758
- AbdulahadWHKallenbergCGLimburgPCStegemanCAUrinary CD4+ effector memory T cells refect renal disease activity in antineutrophil cytoplasmic antibody-associated vasculitisArthritis Rheum2009602830283819714581
- AbdulahadWHStegemanCALimburgPCKallenbergCGCD4-positive effector memory T cells participate in disease expression in ANCA-associated vasculitisAnn N Y Acad Sci20071107223117804529
- NogueiraEHamourSSawantDSerum IL-17 and IL-23 levels and autoantigen-specific Th17 cells are elevated in patients with ANCA-associated vasculitisNephrol Dial Transplant2010252209221720100727
- FreeMEBunchDOMcGregorJANCA-associated vasculitis patients have defective Treg function exacerbated by presence of a suppression-resistant effector populationArthritis Rheum2013651922193323553415
- MorganMDDayCJPiperKPPatients with Wegener’s granulomatosis demonstrate a relative deficiency and functional impairment of T-regulatory cellsImmunology2010130647320113371
- WildeBThewissenMDamoiseauxJRegulatory B cells in ANCA-associated vasculitisAnn Rheum Dis2013721416141923666929
- BunchDOMcGregorJGKhandoobhaiNBDecreased CD5(+) B cells in active ANCA vasculitis and relapse after rituximabClin J Am Soc Nephrol2013838239123293123
- GouSJYuanJChenMYuFZhaoMHCirculating complement activation in patients with anti-neutrophil cytoplasmic antibody-associated vasculitisKidney Int20138312913722913983
- GouSJYuanJWangCZhaoMHChenMAlternative complement pathway activation products in urine and kidneys of patients with ANCA-associated GNClin J Am Soc Nephrol201381884189124115193
- XiaoHDairaghiDJPowersJPC5a receptor (CD88) blockade protects against MPO-ANCA GNJ Am Soc Nephrol20142522523124179165
- LyonsPARaynerTFTrivediSGenetically distinct subsets within ANCA-associated vasculitisN Engl J Med201236721422322808956
- FalkRJJennetteJCANCA disease: where is this field heading?J Am Soc Nephrol20102174575220395376
- Martinez Del PeroMWalshMLuqmaniRLong-term damage to the ENT system in Wegener’s granulomatosisEur Arch Otorhinolaryngol201126873373921085976
- HaworthSJSavageCOCarrDHughesJMReesAJPulmonary haemorrhage complicating Wegener’s granulomatosis and microscopic polyarteritisBr Med J (Clin Res Ed)198529017751778
- HolleJUGrossWLHoll-UlrichKProspective long-term follow-up of patients with localised Wegener’s granulomatosis: does it occur as persistent disease stage?Ann Rheum Dis2010691934119920511614
- StoneJHWegener’s Granulomatosis Etanercept Trial Research GroupLimited versus severe Wegener’s granulomatosis: baseline data on patients in the Wegener’s granulomatosis etanercept trialArthritis Rheum2003482299230912905485
- HoganSLFalkRJChinHPredictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitisAnn Intern Med200514362163116263884
- ArulkumaranNPeriselnerisNGaskinGInterstitial lung disease and ANCA-associated vasculitis: a retrospective observational cohort studyRheumatology (Oxford)2011502035204321873269
- MukhtyarCFlossmannOHellmichBOutcomes from studies of antineutrophil cytoplasm antibody associated vasculitis: a systematic review by the European League Against Rheumatism systemic vasculitis task forceAnn Rheum Dis2008671004101017911225
- WalshMFlossmannOBerdenARisk factors for relapse of antineutrophil cytoplasmic antibody-associated vasculitisArthritis Rheum20126454254821953279
- TomassonGGraysonPCMahrADLavalleyMMerkelPAValue of ANCA measurements during remission to predict a relapse of ANCA-associated vasculitis – a meta-analysisRheumatology (Oxford)20125110010922039267
- TervaertJWvan der WoudeFJFauciASAssociation between active Wegener’s granulomatosis and anticytoplasmic antibodiesArch Intern Med1989149246124652684074
- FauciASWolffSMWegener’s granulomatosis: studies in eighteen patients and a review of the literatureMedicine (Baltimore)1973525356614748591
- HeijlCHarperLFlossmannOIncidence of malignancy in patients treated for antineutrophil cytoplasm antibody-associated vasculitis: follow-up data from European Vasculitis Study Group clinical trialsAnn Rheum Dis2011701415142121616914
- RobsonJDollHSuppiahRDamage in the ANCA-associated vasculitides: long-term data from the European Vasculitis Study group (EUVAS) therapeutic trialsAnn Rheum Dis11152013 [Epub ahead of print.]
- KnightAAsklingJGranathFSparenPEkbomAUrinary bladder cancer in Wegener’s granulomatosis: risks and relation to cyclophosphamideAnn Rheum Dis2004631307131115130900
- FaurschouMSorensenIJMellemkjaerLMalignancies in Wegener’s granulomatosis: incidence and relation to cyclophosphamide therapy in a cohort of 293 patientsJ Rheumatol20083510010517937462
- Le GuennoGMahrAPagnouxCIncidence and predictors of urotoxic adverse events in cyclophosphamide-treated patients with systemic necrotizing vasculitidesArthritis Rheum2011631435144521337321
- LittleMANightingalePVerburghCAEarly mortality in systemic vasculitis: relative contribution of adverse events and active vasculitisAnn Rheum Dis2010691036104319574233
- HolleJUGrossWLLatzaUImproved outcome in 445 patients with Wegener’s granulomatosis in a German vasculitis center over four decadesArthritis Rheum20116325726620862686
- HilhorstMWildeBvan PaassenPImproved outcome in anti-neutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis: a 30-year follow-up studyNephrol Dial Transplant20132837337923223225
- TarziRMPuseyCDVasculitis: risks and rewards of treating elderly patients with vasculitisNat Rev Nephrol2011725325521423250
- JayneDRasmussenNAndrassyKA randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodiesN Engl J Med2003349364412840090
- de GrootKHarperLJayneDRPulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trialAnn Intern Med200915067068019451574
- HarperLMorganMDWalshMPulse versus daily oral cyclophosphamide for induction of remission in ANCA-associated vasculitis: long-term follow-upAnn Rheum Dis20127195596022128076
- De GrootKRasmussenNBaconPARandomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitisArthritis Rheum2005522461246916052573
- FaurschouMWestmanKRasmussenNBrief report: long-term outcome of a randomized clinical trial comparing methotrexate to cyclophosphamide for remission induction in early systemic antineutrophil cytoplasmic antibody-associated vasculitisArthritis Rheum2012643472347722614882
- JonesRBHarperLBallarinJA randomised trial of mycophenolate mofetil versus cyclophosphamide for remission induction of ANCA associated vasculitisPresse Med201342678 French
- StoneJHMerkelPASpieraRRituximab versus cyclophosphamide for ANCA-associated vasculitisN Engl J Med201036322123220647199
- JonesRBTervaertJWHauserTRituximab versus cyclophosphamide in ANCA-associated renal vasculitisN Engl J Med201036321122020647198
- SpecksUMerkelPASeoPEfficacy of remission-induction regimens for ANCA-associated vasculitisN Engl J Med201336941742723902481
- VoswinkelJMuellerAKraemerJAB lymphocyte maturation in Wegener’s granulomatosis: a comparative analysis of VH genes from endonasal lesionsAnn Rheum Dis20066585986416291812
- WalshMMerkelPAPehCAPlasma exchange and glucocorticoid dosing in the treatment of anti-neutrophil cytoplasm antibody associated vasculitis (PEXIVAS): protocol for a randomized controlled trialTrials2013147323497590
- ChemoCentryxA Study to Evaluate the Safety and Efficacy of CCX168 in Subjects With ANCA-Associated Renal Vasculitis Available from: http://www.clinicaltrials.gov/ct2/show/NCT01363388?term=NCT01363388&rank=1. NLM identifier: NCT01363388Accessed December 11, 2013
- CasianAJayneDPlasma exchange in the treatment of Wegener’s granulomatosis, microscopic polyangiitis, Churg-Strauss syndrome and renal limited vasculitisCurr Opin Rheumatol201123121721124082
- JayneDRGaskinGRasmussenNRandomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitisJ Am Soc Nephrol2007182180218817582159
- WalshMCasianAFlossmannOLong-term follow-up of patients with severe ANCA-associated vasculitis comparing plasma exchange to intravenous methylprednisolone treatment is unclearKidney Int20138439740223615499
- HellmichBFlossmannOGrossWLEULAR recommendations for conducting clinical studies and/or clinical trials in systemic vasculitis: focus on anti-neutrophil cytoplasm antibody-associated vasculitisAnn Rheum Dis20076660561717170053
- RutgersAKallenbergCGRefractory disease in antineutrophil cytoplasmic antibodies associated vasculitisCurr Opin Rheumatol20122424525122410545
- RollPOstermeierEHaubitzMEfficacy and safety of rituximab treatment in patients with antineutrophil cytoplasmic antibody-associated vasculitides: results from a German registry (GRAID)J Rheumatol2012392153215622984269
- JayneDRChapelHAduDIntravenous immunoglobulin for ANCA-associated systemic vasculitis with persistent disease activityQJM20009343343910874052
- MartinezVCohenPPagnouxCIntravenous immunoglobulins for relapses of systemic vasculitides associated with antineutrophil cytoplasmic autoantibodies: results of a multicenter, prospective, open-label study of twenty-two patientsArthritis Rheum20085830831718163506
- WalshMChaudhryAJayneDLong-term follow-up of relapsing/refractory anti-neutrophil cytoplasm antibody associated vasculitis treated with the lymphocyte depleting antibody alemtuzumab (CAMPATH-1H)Ann Rheum Dis2008671322132718055469
- BirckRWarnatzKLorenzHM15-Deoxyspergualin in patients with refractory ANCA-associated systemic vasculitis: a six-month open-label trial to evaluate safety and efficacyJ Am Soc Nephrol20031444044712538745
- BoothAHarperLHammadTProspective study of TNFalpha blockade with infliximab in anti-neutrophil cytoplasmic antibody-associated systemic vasculitisJ Am Soc Nephrol20041571772114978174
- JoyMSHoganSLJennetteJCFalkRJNachmanPHA pilot study using mycophenolate mofetil in relapsing or resistant ANCA small vessel vasculitisNephrol Dial Transplant2005202725273216188901
- WalshMMerkelPAMahrAJayneDEffects of duration of glucocorticoid therapy on relapse rate in antineutrophil cytoplasmic antibody-associated vasculitis: a meta-analysisArthritis Care Res (Hoboken)2010621166117320235186
- McKinneyEFLyonsPACarrEJA CD8+ T cell transcription signature predicts prognosis in autoimmune diseaseNat Med20101658659120400961
- PepperRJHamourSChaveleKMLeukocyte and serum S100A8/S100A9 expression reflects disease activity in ANCA-associated vasculitis and glomerulonephritisKidney Int2013831150115823423260
- TamFWSandersJSGeorgeAUrinary monocyte chemoattractant protein-1 (MCP-1) is a marker of active renal vasculitisNephrol Dial Transplant2004192761276815353578
- HiemstraTFWalshMMahrAMycophenolate mofetil vs azathioprine for remission maintenance in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized controlled trialJAMA20103042381238821060104
- PagnouxCMahrAHamidouMAAzathioprine or methotrexate maintenance for ANCA-associated vasculitisN Engl J Med20083592790280319109574
- [No authors listed]The Wegener’s granulomatosis etanercept (WGET) research group. Etanercept plus standard therapy for Wegener’s granulomatosisN Engl J Med200535235136115673801
- MetzlerCMiehleNMangerKElevated relapse rate under oral methotrexate versus leflunomide for maintenance of remission in Wegener’s granulomatosisRheumatology (Oxford)2007461087109117519271
- SmithRMJonesRBGuerryMJRituximab for remission maintenance in relapsing antineutrophil cytoplasmic antibody-associated vasculitisArthritis Rheum2012643760376922729997
- Cartin-CebaRGolbinJMKeoghKARituximab for remission induction and maintenance in refractory granulomatosis with polyangiitis (Wegener’s): ten-year experience at a single centerArthritis Rheum2012643770377822730028
- GuillevinLPagnouxCKarrasARituximab versus for maintenance in ANCA-associated vasculitis. A prospective study in 117 patientsPresse Med201342679
- Cambridge University Hospitals NHS Foundation TrustRituximab Vasculitis Maintenance Study (RITAZAREM) Available from: http://www.clinicaltrials.gov/ct2/show/NCT01697267?term=RITAZAREM&rank=1. NLM identifier: NCT01697267Accessed July 9, 2013
- MolloyESCalabreseLHProgressive multifocal leukoencephalopathy associated with immunosuppressive therapy in rheumatic diseases: evolving role of biologic therapiesArthritis Rheum2012643043305122422012
- BesadaEKoldingsnesWNossentJCLong-term efficacy and safety of pre-emptive maintenance therapy with rituximab in granulomatosis with polyangiitis: results from a single centreRheumatology (Oxford)2013522041204723934313
- McGregorJGHoganSLHuYGlucocorticoids and relapse and infection rates in anti-neutrophil cytoplasmic antibody diseaseClin J Am Soc Nephrol2012724024722134625
- De JoodeAAESandersJSCohen TervaertJWStegemanCRandomized clinical trial of extended versus standard azathioprine maintenance therapy in newly diagnosed PR3-ANCA positive vasculitis patients at high risk for disease relapsePresse Med201342680 French
- ZycinskaKWardynKAZielonkaTMKrupaRLukasWCo-trimoxazole and prevention of relapses of PR3-ANCA positive vasculitis with pulmonary involvementEur J Med Res200914Suppl 426526720156769
- StegemanCATervaertJWde JongPEKallenbergCGTrimethoprim-sulfamethoxazole (co-trimoxazole) for the prevention of relapses of Wegener’s granulomatosis. Dutch Co-Trimoxazole Wegener Study GroupN Engl J Med199633516208637536
- HolleJUDubrauCHerlynKRituximab for refractory granulomatosis with polyangiitis (Wegener’s granulomatosis): comparison of efficacy in granulomatous versus vasculitic manifestationsAnn Rheum Dis20127132733322021864
- TaylorSRSalamaADJoshiLPuseyCDLightmanSLRituximab is effective in the treatment of refractory ophthalmic Wegener’s granulomatosisArthritis Rheum2009601540154719404964
- JoshiLLightmanSLSalamaADRituximab in refractory ophthalmic Wegener’s granulomatosis: PR3 titers may predict relapse, but repeat treatment can be effectiveOphthalmology20111182498250321907416
- LangfordCASnellerMCHallahanCWClinical features and therapeutic management of subglottic stenosis in patients with Wegener’s granulomatosisArthritis Rheum199639175417608843868
- HoffmanGSThomas-GolbanovCKChanJAkstLMEliacharITreatment of subglottic stenosis, due to Wegener’s granulomatosis, with intralesional corticosteroids and dilationJ Rheumatol2003301017102112734898
- NouraeiSAObholzerRIndPWResults of endoscopic surgery and intralesional steroid therapy for airway compromise due to tracheobronchial Wegener’s granulomatosisThorax200863495217573443
- HarperLSavageCOANCA-associated renal vasculitis at the end of the twentieth century – a disease of older patientsRheumatology (Oxford)20054449550115613403
- MukhtyarCGuillevinLCidMCEULAR recommendations for the management of primary small and medium vessel vasculitisAnn Rheum Dis20096831031718413444
- FaurschouMMellemkjaerLSorensenIJIncreased morbidity from ischemic heart disease in patients with Wegener’s granulomatosisArthritis Rheum2009601187119219333952
- MoranSLittleMARenal transplantation in antineutrophil cytoplasmic antibody-associated vasculitisCurr Opin Rheumatol201426374124257368
- AstenPBarrettJSymmonsDRisk of developing certain malignancies is related to duration of immunosuppressive drug exposure in patients with rheumatic diseasesJ Rheumatol1999261705171410451066
- SetshediMEpsteinDWinterTAUse of thiopurines in the treatment of inflammatory bowel disease is associated with an increased risk of non-melanoma skin cancer in an at-risk population: a cohort studyJ Gastroenterol Hepatol20122738538921793904
- LapraikCWattsRBaconPBSR and BHPR guidelines for the management of adults with ANCA associated vasculitisRheumatology (Oxford)2007461615161617804455
- BarthMJCzuczmanMSOfatumumab: a novel, fully human anti-CD20 monoclonal antibody for the treatment of chronic lymphocytic leukemiaFuture Oncol201391829183924295413
- HoldenNJWilliamsJMMorganMDANCA-stimulated neutrophils release BLyS and promote B cell survival: a clinically relevant cellular processAnn Rheum Dis2011702229223321859691
- KrumbholzMSpecksUWickMBAFF is elevated in serum of patients with Wegener’s granulomatosisJ Autoimmun20052529830216242914
- BaderLKoldingsnesWNossentJB-lymphocyte activating factor levels are increased in patients with Wegener’s granulomatosis and inversely correlated with ANCA titerClin Rheumatol2010291031103520582728
- DaridonCBlassfeldDReiterKEpratuzumab targeting of CD22 affects adhesion molecule expression and migration of B-cells in systemic lupus erythematosusArthritis Res Ther201012R20421050432
- WallaceDJKalunianKPetriMAEfficacy and safety of epratuzumab in patients with moderate/severe active systemic lupus erythematosus: results from EMBLEM, a phase IIb, randomised, double-blind, placebo-controlled, multicentre studyAnn Rheum Dis20147318319023313811
- LangfordCAMonachPASpecksUAn open-label trial of abatacept (CTLA4-IG) in non-severe relapsing granulomatosis with polyangiitis (Wegener’s)Ann Rheum Dis1292013 [Epub ahead of print.]
- Cambridge University Hospitals NHS Foundation TrustAlemtuzumab for ANCA Associated Refractory Vasculitis (ALEVIATE) Available from: http://www.clinicaltrials.gov/ct2/show/NCT01405807?term=NCT01405807&rank=1. NLM identifier: NCT01405807Accessed July 28, 2011
- ReichKLangleyRGPappKAA 52-week trial comparing briakinumab with methotrexate in patients with psoriasisN Engl J Med20113651586159622029980
- LeonardiCMathesonRZachariaeCAnti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasisN Engl J Med20123661190119922455413
- PappKALeonardiCMenteABrodalumab, an anti-interleukin-17-receptor antibody for psoriasisN Engl J Med20123661181118922455412
- KarstenCMKohlJThe immunoglobulin, IgG Fc receptor and complement triangle in autoimmune diseasesImmunobiology20122171067107922964232
- LaurinoSChaudhryABoothAConteGJayneDProspective study of TNFalpha blockade with adalimumab in ANCA-associated systemic vasculitis with renal involvementNephrol Dial Transplant2010253307331420368305
- SmithJMcDaidJPBhangalGA spleen tyrosine kinase inhibitor reduces the severity of established glomerulonephritisJ Am Soc Nephrol20102123123619959716
- McAdooSPReynoldsJSmithJSpleen tyrosine kinase (SYK) inhibition in experimental autoimmune glomerulonephritis (EAG)Presse Med201342653 French
- WeinblattMEKavanaughAGenoveseMCAn oral spleen tyrosine kinase (Syk) inhibitor for rheumatoid arthritisN Engl J Med20103631303131220879879
- LamprechtPVoswinkelJLilienthalTEffectiveness of TNFalpha blockade with infliximab in refractory Wegener’s granulomatosisRheumatology (Oxford)2002411303130712422004
- VenhoffNEffelsbergNMSalzerUImpact of rituximab on immunoglobulin concentrations and B cell numbers after cyclophosphamide treatment in patients with ANCA-associated vasculitidesPLoS One20127e3762622629432
- SaravananVKellyCDrug-related pulmonary problems in patients with rheumatoid arthritisRheumatology (Oxford)20064578778916527879
- Nordic Pharma SASClinical Study Comparing the New Immunosuppressive Drug Gusperimus With the Conventional Treatment in Wegener’s Granulomatosis (SPARROW) Available from: http://www.clinicaltrials.gov/ct2/show/NCT01446211?term=NCT01446211&rank=1. NLM identifier: NCT01446211Accessed January 19, 2012
- University Medical Centre GroningenPrevention of Relapses in Proteinase 3 (PR3)-Anti-neutrophil Cytoplasmic Antibodies (ANCA)-Associated Vasculitis Available from: http://www.clinicaltrials.gov/ct2/show/NCT00128895?term=NCT00128895&rank=1. NLM identifier: NCT00128895Accessed November 1, 2011
- Human Genome Sciences Inc., a GSK CompanyBelimumab in Remission of VASculitis (BREVAS) Available from: http://www.clinicaltrials.gov/ct2/show/NCT01663623?term=NCT01663623&rank=1. NLM identifier: NCT01663623Accessed February 6, 2014
