Abstract
Background
The role of immune checkpoint inhibitors in endometrial cancer is limited. At present, the anti-programmed cell death protein 1 (anti-PD-1) antibody is only used in patients with recurrence or metastasis. CD40 is an important immune checkpoint, which is expressed in tumor cells and immune cells, but its distribution characteristics in endometrial carcinoma have not been explored.
Methods
Sixty-eight cases of primary endometrial carcinoma treated in Peking University People’s Hospital from January 2010 to December 2020 were collected, including 28 cases of poorly differentiated endometrioid adenocarcinoma, 23 cases of serous carcinoma and 17 cases of clear cell carcinoma. The relationship of CD40 expression and PD-L1 expression with their prognosis was analyzed by immunohistochemistry.
Results
We found that CD40 had higher expression in non-endometrioid endometrial carcinoma, which lead to the worse prognosis. The effect of high expression of CD40 on the prognosis of endometrioid adenocarcinoma was not significantly different, and most patients with good prognosis. We found that the proportion of CD40 distribution in tumor cells and immune cells may be associated with this heterogeneity.
Conclusion
The expression of CD40 in different endometrial cancers may indicate the difference prognosis, which may become a potential target for drug treatment of non-endometrioid endometrial carcinoma.
Introduction
Endometrial cancer (EC) is one of the most common cancer in women in the world.Citation1 According to the estrogen exposure and pathological characteristics, EC is classified into Type I endometrioid endometrial adenocarcinoma (EEC), Type II non-endometrioid endometrial adenocarcinoma: endometrial serous carcinoma (ESC), endometrial clear cell carcinoma (ECC), and other non-endometrioid carcinoma. Although ESC represents only 5% of all endometrial carcinomas, ESC accounts for approximately 40% of all endometrial carcinoma-related deaths.Citation2 In addition, endometrial cancer is also divided into different risk levels according to its pathological characteristics. High-risk endometrial cancer includes non-endometrioid (serous, clear, or carcinosarcoma), stage II and III endometrioid adenocarcinoma, and endometrioid stage I, grade 3 with >50% myometrial invasion and accounts for 80% of all endometrial cancer-related deaths.
Immune checkpoint inhibitors have significantly improved the choice of treatment strategies of advanced/recurrent EC. However, Anti-PD-1 regimens are more effective in POLE-mutated patients than in CN-High patients, mostly serous-like patients. Research has found that only 15% to 30% of patients respond to checkpoint inhibitors. Repeated antigen stimulation in the absence of durable antigen presentation and/or appropriate costimulatory signals may lead to T cell exhaustion and/or anergy and tolerance.Citation3 Combination regimens that can decrease tumor microenvironment immunosuppression and increase tumor immunogenicity represent a viable treatment option to broaden the activity of immune checkpoint inhibitors.Citation4 Therefore, novel biomarkers for targeted immunotherapy in endometrial cancer will greatly improve treatment options.
CD40, a tumor necrosis factor (TNF) receptor family member, is expressed in a variety of cell types, including B lymphocytes, macrophages, fibroblasts, endothelial and epithelial cells, and this widespread expression is likely to account for its central role in normal physiology and disease pathogenesis.Citation5 The researchers analyzed the immune microenvironment of endometrial carcinoma using the data of TCGA database, classified it into four types, POLE (DNA polymerase epsilon) mutations, microsatellite instability-high (MSI-H), copy number low (CN-low, wild type p53), and copy number high (CN-high, abnormal p53). The results showed that CD40 was highly expressed in CN-high endometrial carcinoma tissues which lacked inflammatory stroma, mainly serous like.Citation6 This study suggests that CD40 may present a cancer-promoting mechanism in this subtype and may be a potential therapeutic target. In the current study, we investigated the protein expression levels of CD40 and PD-L1 in relation to patient prognosis in different pathological types of high-risk endometrial carcinomas in 68 patients.
Materials and Method
Endometrial Cancer Patients
Sixty-eight cases of primary endometrial carcinoma treated in Peking University People’s Hospital from January 2010 to December 2020 were collected, including 28 cases of poorly differentiated endometrioid adenocarcinoma, 23 cases of serous carcinoma and 17 cases of clear cell carcinoma. Inclusion criteria: (1) patients all undergo surgery. Surgery included hysterectomy, bilateral adnexectomy, omentectomy, and in most cases lymphadenectomy, and confirmed by pathological examination; (2) None of them received radiotherapy, chemotherapy or targeted therapy before admission; Exclusion criteria: (1) combined with other non female reproductive system malignant tumors; (2) Combined with other gynecological tumors, eg fallopian tube cancer; (3) Previous history of breast cancer; (4) Incomplete clinical data and incomplete relevant examinations; (5) Mixed carcinoma. The clinical pathological factors, body mass index (BMI), diabetes, hypertension, history of tubal ligation, lymphadenectomy and postoperative adjuvant therapy were retrospectively evaluated in 68 cases.
Follow-Up
Overall survival (OS) time of the patients was calculated as the interval from the date of diagnosis to the date of the last clinical control or death from the endometrial cancer-related causes until December 31, 2020. The follow-up time of the relapse-free survival rate (RFS) is defined as the time of the first recurrence or the last follow-up (if there is no recurrence) after hysterectomy. Kaplan–Meier curve was used to reflect the prognosis of patients with endometrial cancer.
Immunohistochemistry
All specimens were fixed with 10% formalin, routinely stained, dehydrated, embedded in paraffin, sectioned, stained with HE and immunohistochemistry. CD40 (Rabbit-anti-human monoclonal antibody, Abcam, ab224639, Clone no. EPR20735, Cambridge, UK) and PD-L1 (Rabbit anti-human monoclonal antibody, Abcam, ab205921, Clone no. 28-8, Cambridge, UK) were primary antibodies. The expressions of PD-L1 and CD40 were observed under inverted microscope. Immunohistochemical staining criteria: The evaluations of PD-L1 and CD40 expression were performed by independent pathologists, who were blinded to the clinicopathologic data, including the therapeutic response and survival time. Tumor Proportion Score (TPS, %) is used which is the percentage of viable cancer cells with partial or complete membrane expression (≥1+) relative to all viable cancer cells present in the entire sample (positive and negative). PD-L1 positivity or overexpression was defined as TPS more than 1% positive expression in tumor cells. The positive expression of CD40 is defined as more than 10% of tumor cells or interstitial cells.
Statistical Analysis
SPSS 22.0 statistical software was used for data processing and statistical analysis. χ2 test or Fisher exact probability method and Ridit analysis were used for the comparison of ordered classification data between groups; Kendall’s tau-b method was used for correlation analysis of grade data. Prism 8 software is used to analyze the Kaplan–Meier survival curve. The P value of survival curve was calculated by log-rank (Mantel-Cox) test. The difference was statistically significant (P<0.05).
Results
Clinical Baseline Data for Three Types of Endometrial Cancer
There were no significant differences among the three types of high-risk endometrial cancers in menopause, complications and BMI, but there were significant differences in age among the three groups (P<0.05). About 82.6% of patients with endometrial serous carcinoma were older than 60 years old ().
Table 1 Clinical Baseline Data for Three Different Pathological Types of Endometrial Cancer
CD40 Expression in Endometrial Cancer and Prognostic Outcomes
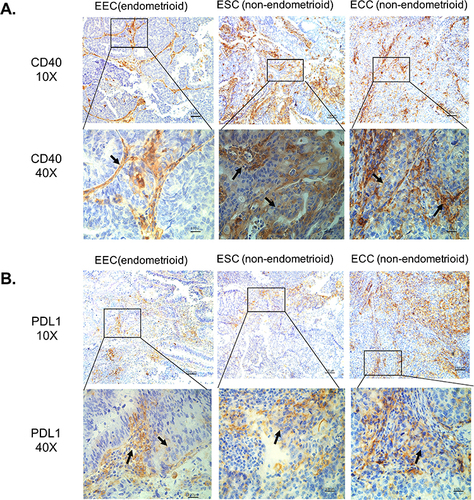
CD40 protein expression in situ were evaluated by IHC in 68 high-risk endometrial cancer samples with different pathological types. CD40 was expressed in 14.3% (4/28) of EEC, and the staining was mainly interstitial immune cells (, ). CD40 was expressed in 69.6% (16/23) of ESC, and the staining was distributed to more tumor cells (, ). CD40 was expressed in 47.1% (8/17) of ECC, and the staining is present both in cancer cells and interstitial immune cells (, ). The above results suggest that the expression of CD40 in non-endometrioid EC is significantly higher than in EEC, which suggests that CD40 may have different roles in the two types of endometrial cancer.
Table 2 Positive Proportion of PD-L1 and CD40 in Immunohistochemistry
Figure 1 In situ protein expression of CD40 and PD-L1 in different types of endometrial carcinoma. (A) CD40 expression in different types of endometrial carcinoma. (B) PD-L1 expression in different types of endometrial carcinoma. The black tip points to the positive CD40 expression.
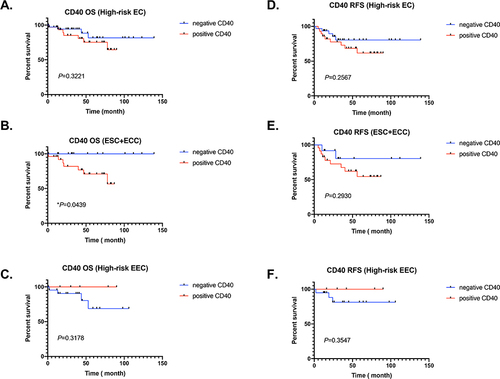
We first analyzed the impact of CD40 expression level on the prognosis of high-risk endometrial cancer patients. We found that high level of CD40 has no significant effect on the OS and RFS of high-risk endometrial cancer ( and ). Interestingly, we have some new findings when we stratified the pathological subtypes of these endometrial cancer patients. Positive expression of CD40 was associated with worse overall survival (OS) of non-endometrioid endometrial cancer including ESC and ECC (P < 0.05) (). Unfortunately, the data shows that there is no significant difference between high and low expression of CD40 in the prognosis of RFS in ESC and ECC patients, which may be due to the limitation of the number of cases (). On the contrary, it was observed that the high expression of CD40 in type I EEC showed no significant difference in OS and RFS, and most patients with good prognosis ( and ).
Figure 2 Heterogeneity of CD40 expression in different types of endometrial cancer affects opposite prognostic outcomes. (A–C) CD40 expression and OS of high-risk EC patients, non-endometrioid endometrial carcinoma (ESC+EEC) and high-risk endometrioid endometrial carcinoma (EEC). The red line represents positive expression, and the blue line represents negative expression. (D–F) CD40 expression and RSF of high-risk EC patients, non-endometrioid endometrial carcinoma (ESC+EEC) and high-risk endometrioid endometrial carcinoma (EEC). The red line represents positive expression, and the blue line represents negative expression.
PD-L1 Expression in Endometrial Cancer and Prognostic Outcomes
The PD-L1 expression was detected by IHC in the same 68 endometrial cancer samples. PD-L1 was expressed in 17.9% (5/28) of EEC, 17.4% (4/23) of ESC, and 35.3% (6/17) of ECC, and the staining is present both in cancer cells and interstitial immune cells (, ).
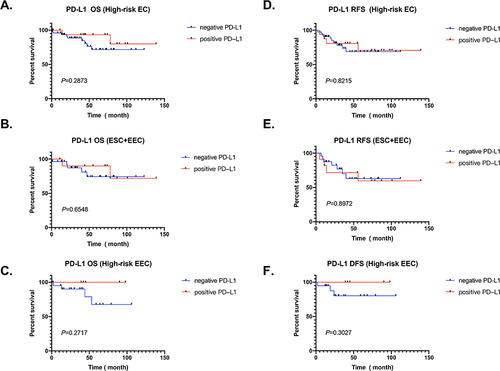
Figure A and D data show that PD-L1 has no significant impact on OS and RFS in 68 high-risk endometrial cancer patients ( and ). We conducted further stratified analysis and found that the expression level of PD-L1 still has no relevant influence on the OS and RFS of non-endometrioid ESC and ECC patients ( and ). Encouragingly, EEC patients with high expression of PD-L1 showed a better prognosis trend in OS and DFS, although it was not statistically significant ( and ).
Figure 3 PD-L1 expression in different types of endometrial cancer and prognostic outcomes. (A–C) PD-L1 expression and OS of high-risk EC patients, non-endometrioid endometrial carcinoma (ESC+EEC) and high-risk endometrioid endometrial carcinoma (EEC). The red line represents positive expression, and the blue line represents negative expression. (D–F) PD-L1 expression and RSF of high-risk EC patients, non-endometrioid endometrial carcinoma (ESC+EEC) and high-risk endometrioid endometrial carcinoma (EEC). The red line represents positive expression, and the blue line represents negative expression.
Discussion
In this study, we demonstrate that the relationship of CD40 and PD-L1 with prognostic outcomes of high-risk endometrial cancer patients. The higher expression level of CD40 positively correlates with the worse prognosis of non-endometrioid EC patients. EEC patients with high expression of PD-L1 showed a better prognosis trend, although there was no statistical significance.
Immune checkpoint inhibitors targeting the PD-1/PD-L1 pathway have significantly increased survival in randomized trials.Citation7 It prevents the activation of T-cells by blocking antibodies to activate T cell and inhibit adaptive immune resistance.Citation8 However, the latest KEY-NOTE-028 clinical trial reported only a 48% response rate with pembrolizumab in an all-comers EC population.Citation9 The objective response rate of patients with recurrent endometrial cancer to pembrolizumab and lenvatinib was only 23.8%.Citation10 One theory of this resistance was existed in tumor T-cell infiltration, impaired T cell function and lack of tumor-infiltrating lymphocytes in the tumor microenvironment (TME). The best EC molecular type candidates to respond to anti-PD-1/PD-L1 treatment are POLE-mutated MSI EC and MS-stable/TMB-high tumors.Citation11,Citation12 The majority of patients with recurrent/metastatic disease will be in the serous-like or CN-high, with lower number of mutations and less important or absent inflammatory stroma. Restore T cell functionality and infiltration and elimination of immune suppression is one way for therapy.
Our research result is partially consistent with that of Zong et al, which mainly shows that the high expression of PD-L1 in tumor cells is related to favorable prognosis of high-risk endometrial cancer.Citation13 Recently, a meta-analysis study showed that high expression of PD-L1 in immune cells had a significant association with worse OS of EC patients.Citation14 These studies suggest that the expression of PD-L1 in immune cells and tumor cells has different effects on the prognosis of patients with endometrial cancer.
CD40 is a costimulatory receptor molecule of the tumor necrosis factor (TNF) receptor superfamily. Activation of CD40 by its ligand, CD40L (also called CD154), enables DCs to mature into professional APCs, often referred to as licensed DCs, and provides necessary signals for T cell activation as well as numerous other signals. Agonistic CD40 antibodies have been shown to effectively inhibit tumor growth and prolong survival in several tumor models.Citation15 Recent research showed that the combination of CD40 agonists has a good response rate in some cancer patients with resist to Anti-PD-1/PD-L1.Citation16,Citation17
Our data showed that positive CD40 expression associated with the worse prognosis of non-endometrioid EC patients. We observed that many CD40 are expressed not only in immune cells but also in a large number of tumor cells. The up-regulated expression of CD40 in cancer cells of non-endometrioid EC patients may promote the malignant progression of cancer, immune escape, and lead to poor prognosis. The research on CD40 mainly focuses on tumor-related immune cells, such as macrophages, B cells and dendritic cells, boosting antitumor immunity. But its research on tumor cells is limited.Citation18,Citation19 A small number of literature have explored the dual role of CD40 pathway in the activation of macrophages in the survival and invasion of human endometrial carcinoma cells.Citation20,Citation21 The effect of CD40/CD40L signal pathway on the proliferation and apoptosis of tumor cells is inconsistent in different tumor types, such as malignant B cells, breast cancer, and renal cell carcinoma.Citation22 Studies showed that the expression of CD40 on melanoma cells is indispensable for its better response to immune checkpoint.Citation19,Citation23 CD40-CD40L is a complex signal pathway, which is involved in multiple interactions between tumor cells and immune cells in the microenvironment.
There are some limitations to this study. Firstly, the total number of cases is relatively small, and larger sample size studies are needed to further validate the expression of CD40 and PD-L1 in different pathological subtypes of endometrial cancer. Secondly, this study did not independently score stromal cells and tumor cells due to the limitations of the number of cases. Thirdly, the expression of CD40 and PD-L1 may be highly correlated with the new molecular typing of endometrial cancer, and we need further analysis in the future. In conclusion, this study found that the expression of CD40 in different endometrial cancers may indicate the difference prognosis and may become a target for drug treatment of non-endometrioid endometrial carcinoma.
Ethical Approval
This study was approved by the ethical committee of the Peking University People’s Hospital. The paraffin section samples used for immunohistochemistry were discarded samples after surgery, and clinical data were also exported from the hospital’s medical record system, with relevant personal information such as patient names removed. All patients have signed relevant informed consent forms before the surgery, which can be used for research purposes. This study was conducted in accordance with the Helsinki Declaration.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
We thank Dr. Chen Xi for improving and following up on the clinical data of some cases.
Additional information
Funding
References
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi:10.3322/caac.21708
- McGunigal M, Liu J, Kalir T, Chadha M, Gupta V. Survival differences among uterine papillary serous, clear cell and grade 3 endometrioid adenocarcinoma endometrial cancers: a national cancer database analysis. Int J Gynecol Cancer. 2017;27(1):85–92. doi:10.1097/IGC.0000000000000844
- Ma HS, Poudel B, Torres ER, et al. A CD40 agonist and PD-1 antagonist antibody reprogram the microenvironment of Nonimmunogenic tumors to allow T-cell-mediated anticancer activity. Cancer Immunol Res. 2019;7(3):428–442.
- Mutlu L, Harold J, Tymon-Rosario J, Santin AD. Immune checkpoint inhibitors for recurrent endometrial cancer. Expert Rev Anticancer Ther. 2022;22(3):249–258. doi:10.1080/14737140.2022.2044311
- Baxendale AJ, Dawson CW, Stewart SE, et al. Constitutive activation of the CD40 pathway promotes cell transformation and neoplastic growth. Oncogene. 2005;24(53):7913–7923. doi:10.1038/sj.onc.1208929
- Piulats JM, Guerra E, Gil-Martín M, et al. Molecular approaches for classifying endometrial carcinoma. Gynecol Oncol. 2017;145(1):200–207. doi:10.1016/j.ygyno.2016.12.015
- Yoon HH, Jin Z, Kour O, et al. Association of PD-L1 expression and other variables with benefit from immune checkpoint inhibition in advanced gastroesophageal cancer: systematic review and meta-analysis of 17 Phase 3 randomized clinical trials. JAMA Oncol. 2022;8(10):1456–1465. doi:10.1001/jamaoncol.2022.3707
- Yamaguchi H, Hsu JM, Yang WH, Hung MC. Mechanisms regulating PD-L1 expression in cancers and associated opportunities for novel small-molecule therapeutics. Nat Rev Clin Oncol. 2022;19(5):287–305.
- O’Malley DM, Bariani GM, Cassier PA, et al. Pembrolizumab in patients with microsatellite instability-high advanced endometrial cancer: results from the KEYNOTE-158 study. J Clin Oncol. 2022;40(7):752–761. doi:10.1200/JCO.21.01874
- Kim J, Noh JJ, Lee TK, et al. Real-world experience of pembrolizumab and lenvatinib in recurrent endometrial cancer: a multicenter study in Korea. Gynecol Oncol. 2022;165(2):369–375. doi:10.1016/j.ygyno.2022.02.020
- Jones NL, Xiu J, Rocconi RP, Herzog TJ, Winer IS. Immune checkpoint expression, microsatellite instability, and mutational burden: identifying immune biomarker phenotypes in uterine cancer. Gynecol Oncol. 2020;156(2):393–399. doi:10.1016/j.ygyno.2019.11.035
- Goodman AM, Sokol ES, Frampton GM, Lippman SM, Kurzrock R. Microsatellite-stable tumors with high mutational burden benefit from immunotherapy. Cancer Immunol Res. 2019;7(10):1570–1573. doi:10.1158/2326-6066.CIR-19-0149
- Zong L, Sun Z, Mo S, et al. PD-L1 expression in tumor cells is associated with a favorable prognosis in patients with high-risk endometrial cancer. Gynecol Oncol. 2021;162(3):631–637. doi:10.1016/j.ygyno.2021.07.009
- Mamat Yusof MN, Chew KT, Kampan N, et al. PD-L1 expression in endometrial cancer and its association with clinicopathological features: a systematic review and meta-analysis. Cancers. 2022;14(16):3911. doi:10.3390/cancers14163911
- Vonderheide RH. CD40 Agonist Antibodies in Cancer Immunotherapy. Annu Rev Med. 2020;71:47–58. doi:10.1146/annurev-med-062518-045435
- Weiss SA, Djureinovic D, Jessel S, et al. A Phase I study of APX005M and cabiralizumab with or without nivolumab in patients with melanoma, kidney cancer, or non-small cell lung cancer resistant to anti-PD-1/PD-L1. Clin Cancer Res. 2021;27(17):4757–4767. doi:10.1158/1078-0432.CCR-21-0903
- Diggs LP, Ruf B, Ma C, et al. CD40-mediated immune cell activation enhances response to anti-PD-1 in murine intrahepatic cholangiocarcinoma. J Hepatol. 2021;74(5):1145–1154. doi:10.1016/j.jhep.2020.11.037
- Garris CS, Wong JL, Ravetch JV, Knorr DA. Dendritic cell targeting with Fc-enhanced CD40 antibody agonists induces durable antitumor immunity in humanized mouse models of bladder cancer. Sci Transl Med. 2021;13(594):eabd1346. doi:10.1126/scitranslmed.abd1346
- Yan C, Richmond A. Hiding in the dark: pan-cancer characterization of expression and clinical relevance of CD40 to immune checkpoint blockade therapy. Mol Cancer. 2021;20(1):146. doi:10.1186/s12943-021-01442-3
- Dumas G, Dufresne M, Asselin É, Girouard J, Carrier C, Reyes-Moreno C. CD40 pathway activation reveals dual function for macrophages in human endometrial cancer cell survival and invasion. Cancer Immunol Immunother. 2013;62(2):273–283. doi:10.1007/s00262-012-1333-2
- Jia J, Wang Z, Li X, Wang Z, Wang X. Morphological characteristics and co-stimulatory molecule (CD80, CD86, CD40) expression in tumor infiltrating dendritic cells in human endometrioid adenocarcinoma. Eur J Obstet Gynecol Reprod Biol. 2012;160(2):223–227. doi:10.1016/j.ejogrb.2011.11.020
- Tang T, Cheng X, Truong B, Sun L, Yang X, Wang H. Molecular basis and therapeutic implications of CD40/CD40L immune checkpoint. Pharmacol Ther. 2021;219:107709.
- Yan C, Saleh N, Yang J, et al. Novel induction of CD40 expression by tumor cells with RAS/RAF/PI3K pathway inhibition augments response to checkpoint blockade. Mol Cancer. 2021;20(1):85.
