Abstract
Background
Topical diclofenac sodium 1% gel (DSG) has demonstrated efficacy and tolerability in patients with osteoarthritis (OA) of the knees or hands, including elderly patients and those with an increased risk of gastrointestinal, cardiovascular, and renal adverse events (AEs). Medications known to interact with diclofenac were disallowed in a clinical trial of DSG for knee OA; however, patients were not to be discontinued for intake of disallowed treatment, unless there was a safety issue. This post hoc analysis examined the frequency and type of AEs in patients who received DSG concomitantly with drugs known to have potential interactions with diclofenac.
Materials and methods
This was a post hoc analysis of a randomized controlled trial of DSG for knee OA pain. Patients (n = 254) aged ≥ 35 years with OA in one or both knees, but with clinical OA symptoms in only one knee, administered DSG topically to the target knee four times daily (total dose, 16 g/d) for 12 weeks. Drugs with the potential for major or moderate drug–drug interactions (DDIs) were identified via Drugs.com. AE rates were compared in patients with versus those without ≥1 potential DDI.
Results
At least one AE was experienced by 62.6% (107/171) of patients with ≥1 DDI and by 55.4% (46/83) of patients with no DDIs. Gastrointestinal AEs (upper and lower) were reported in 5.3% (9/171) and 7.2% (6/83), cardiovascular AEs in 4.7% (8/171) and 1.2% (1/83), renal AEs in 1.2% (2/171) and 0%, and hepatic AEs in 0% and 1.2% (1/83) of patients with ≥1 DDI compared with patients with no DDIs, respectively.
Conclusion
Concurrent use of DSG with medications that had potential for major to moderate DDIs had little impact on the frequency of AEs in this population. Further research is needed to consider how factors such as dose, duration, and timing of concomitant drug administration may affect the likelihood of clinically evident AEs resulting from a potential DDI.
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) are an accepted treatment for osteoarthritis (OA).Citation1 However, guidelines for the management of osteoarthritis recommend that NSAIDs be administered at the lowest effective dose for the shortest duration possible because of the dose-related risk of gastrointestinal adverse events (AEs) (including bleeding, perforation, and ulcers) as well as cardiovascular and renal AEs.Citation2,Citation3
Drug–drug interactions (DDIs) should always be avoided; however, many patients with osteoarthritis have comorbid conditions requiring treatment with medications that may have potential for interaction with NSAIDs such as diclofenac. American Geriatrics Society (AGS) guidelines for the management of chronic pain in older patients recommend that NSAIDs be avoided altogether if possible and cite the potential for NSAIDs to have DDIs with selective serotonin reuptake inhibitors, acetylsalicylic acid (ASA), and corticosteroids.Citation4 The potential for NSAIDs to negate the therapeutic effects of antihypertensive medications has been well documented;Citation5 NSAID use also has been associated with acute renal failure in patients with hypertension or heart failure.Citation6
In randomized controlled trials, diclofenac sodium 1% gel (DSG; Voltaren® Gel, Novartis Consumer Health, Parsippany, NJ, USA) demonstrated efficacy similar to that of oral NSAIDs in patients with osteoarthritis of the knees or hands, with an occurrence of systemic AEs similar to placebo.Citation7–Citation9 Subgroup analyses have shown similar efficacy and tolerability in elderly and younger patients and in those with and without an increased risk of cardiovascular and renal AEs.Citation10,Citation11 Pharmacokinetic evidence indicates that diclofenac sodium 1% gel facilitates the guideline recommendation to use the lowest effective dose of NSAID to achieve effective relief. Therapeutic doses of DSG (16 g/day[d]; contains 160 mg of diclofenac sodium) applied to one knee produce peak plasma diclofenac levels approximately 150-fold lower than therapeutic doses of oral diclofenac (150 mg/d).Citation12 Even at a supratherapeutic dose (48 g/d; contains 480 mg diclofenac sodium), peak plasma diclofenac concentrations were approximately 45-fold lower than those observed with a 150-mg/d dose of oral diclofenac.Citation12
It has been reported anecdotally that DSG does not cause AEs when used with warfarin or other drugs known to interact with diclofenac. This suggests that minimizing NSAID dose may mitigate the risk of DDIs that could lead to clinically evident AEs.Citation13 This analysis was conducted to determine whether the concurrent use of DSG with drugs known to have major or moderate risk of DDIs with diclofenac is associated with an increased frequency of AEs.
Methods
Study design
This was a post hoc analysis of a 12-week, randomized, double-blind, vehicle-controlled, parallel-group trial. Efficacy and overall tolerability results have been reported elsewhere.Citation9 For this analysis, patients were categorized as either receiving or not receiving concurrent medications known to have a DDI with diclofenac. Drugs with the potential for DDIs with diclofenac were identified using Drugs.com.Citation14 Only drugs with “major” or “moderate” DDIs with diclofenac according to Drugs.com criteria were considered. The original study received institutional review board approval, and all patients provided written informed consent.
Participants
Patients treated in the original study included ambulatory men and women aged ≥ 35 years with mild to moderate OA in one or both knees (Kellgren-Lawrence grades 1–3), diagnosed ≥ 6 months before screening, and with a self-reported history of moderate to severe pain in one knee only. Immediately before randomization, patients had to have pain on movement scored as ≥50 on a 100-mm Visual Analog Scale (VAS) and a Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)Citation15 pain score ≥ 9 on a 20-point Likert scale.
Patients were excluded primarily for having other inflammatory conditions that might confound efficacy results or require systemic anti-inflammatory therapy. These included significant pain in the contralateral knee or a history or present evidence of secondary OA, rheumatoid arthritis, fibromyalgia, or other chronic inflammatory disease.Citation9 In addition, patients were excluded if they had evidence of an active peptic ulcer or history of gastrointestinal bleeding, and if they had a clinically significant medical disease that might compromise their welfare or confound study results. These included a recent history (≤12 months [m]) or current signs of severe uncontrolled renal, hepatic, hematological, endocrine, cardiovascular, and neurological disease.
Intervention and assessment
Following a 7-day washout of analgesics, eligible patients were randomized in a 1:1 ratio to receive DSG or its vehicle (identical in composition except for the absence of diclofenac sodium). Patients applied 4 g of DSG or vehicle to only the target knee 4 times daily for 12 weeks (equivalent to 5 standard 100-g tubes per month). Any other medications taken starting within 30 days before the screening visit and continuing through the end of the study were recorded.
All treatment-emergent AEs were reported and rated according to severity and relationship to treatment. All AEs occurring during the study were graded by investigators according to severity and likelihood of relatedness to study medication, and coded in the Medical Dictionary for Regulatory Activities (v 7.0, MedDRA).Citation16 Cardiovascular AEs combined the MedDRA codes for cardiac AEs and vascular AEs. Renal AEs combined the MedDRA codes for renal AEs and urinary AEs and also included elevated creatinine levels, which MedDRA classifies as an abnormal laboratory investigation. Hepatic AEs were limited to clinically meaningful elevations in alanine aminotransferase, aspartate aminotransferase, and gamma-glutamyltransferase (≥3 times upper limit of normal).
No formal statistical analysis was performed.
Results
Patients
A total of 254 patients were randomly assigned to receive DSG (). Demographic and clinical characteristics for the study population are presented in . Of the active-treatment patients, 171 were also receiving ≥1 concurrent medication with a potential DDI with diclofenac (). The most frequently administered drugs were antihypertensive medications, antidepressants, and anti-inflammatories. As shown in , most patients randomized to DSG (209 of 254; 82%) completed the 12-week treatment period. Only 13 patients (5.1%) discontinued owing to AEs, including four patients (1.6%) who discontinued because of application-site conditions and four who discontinued because of musculoskeletal or connective tissue disorders (arthralgia, back pain, neck pain). No patient discontinued DSG because of a gastrointestinal AE or laboratory abnormality.
Figure 1 Disposition of patients.
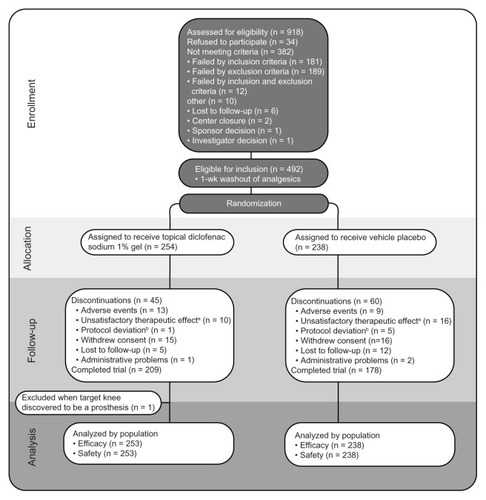
Table 1 Baseline demographic characteristics by treatment group
Table 2 Diclofenac drug interactions identified among clinical trial patients treated with topical diclofenac sodium 1% gel by drug class and risk
Tolerability
The occurrence of treatment-emergent AEs was slightly higher (62.6%) in patients taking medications with potential DDIs compared with patients who were not taking such medications (55.4%) (). Most of these AEs were not considered related to treatment with DSG. The only treatment-related AEs reported in ≥3% of patients in either group were application-site dermatitis (2.9% with potential DDIs versus 7.2% without DDIs) and pruritus (0.6% with potential DDIs vs 3.6% without DDIs).
Table 3 Total and most frequent AEs among clinical trial patients treated with topical diclofenac sodium 1% gel by presence of drug interaction
The frequency of cardiovascular AEs was somewhat higher in patients with DDIs than in patients without DDIs (4.7% vs 1.2%, respectively) (). However, of the eight patients with cardiovascular AEs in the DDI group, six had a medical history of hypertension. Of the 2 DDI-group patients without a history of cardiovascular disease, one experienced tachycardia and the other hypertension. A single fatal cardiovascular AE (ventricular fibrillation) was reported in a 76-year-old man with multiple medical problems (including hypercholesterolemia and hypothyroidism treated with levothyroxine) but without a diclofenac DDI. This event and all other cardiovascular AEs were considered unrelated to treatment.
Table 4 Gastrointestinal, cardiovascular, renal, and hepatic AEs among clinical trial patients treated with topical diclofenac sodium 1% gel by presence of drug interaction
The frequencies of treatment-emergent gastrointestinal (upper and lower), renal and hepatic AEs were similar regardless of the presence of diclofenac DDIs (). Only two gastrointestinal AEs, single occurrences of nausea (DDI group) and dyspepsia (DDI group), were considered potentially treatment related. Only two patients experienced renal AEs, both of whom had a potential DDI. Renal AEs consisted of blood creatinine increase and blood urea increase in one patient taking atenolol, and pollakiuria in one other patient taking furosemide. According to Drugs.com,Citation14 these renal AEs are typically associated with the interaction of diclofenac and atenolol or diclofenac and furosemide; however, neither AE was considered treatment related. One hepatic AE (increased gamma glutamyl transferase) was reported in a patient without a potential DDI.
Discussion
This post hoc analysis was undertaken to consider the impact of potential DDIs of diclofenac with concomitant medications on the frequency of AEs. Patients who used topical diclofenac 1% gel concomitantly with medications known to have potential DDIs with diclofenac experienced a similar low frequency of AEs compared with patients who used topical diclofenac 1% gel in the absence of potential DDIs. Possibly, the low systemic distribution of diclofenac associated with topical administration may have mitigated the risk of a clinically evident AE resulting from a potential DDI.
The incidence of cardiovascular AEs in this study was slightly higher among patients in the DDI group than in the non-DDI group. However, several medications with the potential to interact with diclofenac are prescribed for cardiovascular disease or for conditions associated with risk of cardiovascular disease ( – anticoagulants, antihypertensives, digitalis alkaloids, glucose-lowering drugs); therefore, patients taking these medications may have had a high risk of cardiovascular events based on the medical condition for which the medications were taken. Indeed, six of eight patients in the DDI group who experienced a cardiovascular AE in this study had a medical history of cardiovascular disease.
Osteoarthritis treatment guidelines recommend that NSAIDs be administered at the lowest effective dose to reduce the risk of dose-related AEs.Citation2,Citation3 Clinical trial evidence suggests that topical administration is an effective strategy to obtain effective OA pain relief with minimal systemic exposure to NSAIDs. This post hoc analysis is based on a safety and efficacy study in which DSG was associated with a mean 43% reduction in WOMAC Index pain score,Citation15 a 44% reduction in pain on movement (100-mm VAS), and an occurrence of systemic AEs similar to the placebo control treatment.Citation9 Similarly, numerous other studies of topical NSAIDs have reported effective relief of OA pain with low rates of dose-related gastrointestinal, cardiovascular, renal, and other systemic AEs; the frequency of these AEs has typically been higher in studies of oral NSAIDs.Citation7–Citation9,Citation17,Citation18
A limitation of this study is that the sample size was not large enough to allow for an analysis of AEs in patients with DDIs based on age and other demographic variables. However, a pooled analysis of 3 trials in which 721 patients with OA of the knees were treated with DSG found no difference in efficacy and similarly low AE rates in patients aged < 65 years versus those aged ≥ 65 years.Citation11 A second limitation of the present analysis is that the dose of DSG administered (16 g/d), although appropriate to treat a single target knee, as required in the study, was only half the maximum dose recommended in the product label (32 mg/d) for use on multiple joints.Citation19 However, in other trials, no gastrointestinal bleeding or treatment-related cardiovascular, renal, or serious AEs were reported in patients applying 16 g/d to one or both knees (up to 32 g/d total) for 12 weeksCitation8 or 16 g/d to both knees and 8 g/d to both hands (48 g/d total) for 1 week.Citation12 As in this trial, treatment-related gastrointestinal AEs were uncommon and did not result in treatment discontinuation.Citation8,Citation12 Nonetheless, additional studies would be required in patients receiving DSG 32 g/d for an extended period of time to confirm the tolerability of DSG when administered with drugs known to interact with diclofenac.
Conclusion
Topical application of DSG for knee osteoarthritis was associated with only a small increase in AEs when used concomitantly with medications known to have major or moderate interactions with diclofenac. Although the combination of drugs with known potential for adverse interactions should always be avoided, these results suggest that reducing NSAID dose by a strategy such as topical administration may mitigate the risk of a clinically evident AE following from a potential drug interaction. Clinicians may cautiously consider topical DSG to treat osteoarthritis pain in the knees of patients receiving multiple medications.
Acknowledgments
The original study and present post hoc analysis were both supported by Novartis Consumer Healthcare, (Parsippany, NJ, USA) the post hoc analysis also was supported by Endo Pharmaceuticals Inc, (Malvern, PA, USA). Editorial support (medical writing, literature search, document retrieval, and copyediting) for the preparation of this manuscript was provided by Jeffrey Coleman and Robert Gatley of Complete Healthcare Communications, Inc (Chadds Ford, PA, USA), with financial support from Endo Pharmaceuticals Inc.
Disclosure
MG is an employee of Novartis Consumer Healthcare and MW is an employee of Endo Pharmaceuticals. JP and LA received financial support as clinical trial investigators and presented research at scientific congresses with reimbursement of associated expenses by Endo Pharmaceuticals Inc. The authors report no other conflicts of interest in this work.
References
- AltmanRDBarthelHRTopical therapies for osteoarthritisDrugs201171101259127921770475
- ZhangWDohertyMLeebBFEULAR evidence based recommendations for the management of hand osteoarthritis: report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT)Ann Rheum Dis200766337738817046965
- ZhangWMoskowitzRWNukiGOARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelinesOsteoarthritis Cartilage200816213716218279766
- American Geriatrics SocietyPharmacological management of persistent pain in older personsJ Am Geriatr Soc20095781331134619573219
- SnowdenSNelsonRThe effects of nonsteroidal anti-inflammatory drugs on blood pressure in hypertensive patientsCardiol Rev201119418419121646872
- HuertaCCastellsagueJVaras-LorenzoCGarcia RodriguezLANonsteroidal anti-inflammatory drugs and risk of ARF in the general populationAm J Kidney Dis200545353153915754275
- AltmanRDDreiserRLFisherCLDiclofenac sodium gel in patients with primary hand osteoarthritis: a randomized, double-blind, placebo-controlled trialJ Rheumatol20093691991199919648310
- BarafHSGoldMSClarkMBAltmanRDSafety and efficacy of topical diclofenac sodium 1% gel in knee osteoarthritis: a randomized controlled trialPhys Sportsmed2010382192820631460
- BarthelHRHaselwoodDLongleyS3rdGoldMSAltmanRDRandomized controlled trial of diclofenac sodium gel in knee osteoarthritisSemin Arthritis Rheum200939320321219932833
- BarafHSGoldMSPetruschkeRAWiemanMSTolerability of topical diclofenac sodium 1% gel for osteoarthritis in seniors and patients with comorbiditiesAm J Geriatr Pharmacother2012101476022264852
- BarafHSGlothFMBarthelHRGoldMSAltmanRDSafety and efficacy of topical diclofenac sodium gel for knee osteoarthritis in elderly and younger patients: pooled data from three randomized, double-blind, parallel-group, placebo-controlled, multicentre trialsDrugs Aging2011281274021174485
- KienzlerJGoldMNollevauxFSystemic bioavailability of topical diclofenac sodium gel 1% versus oral diclofenac sodium in healthy volunteersJ Clin Pharmacol New Drugs20105015061
- BarkinRLReducing cardiovascular risks of nonsteroidal anti-inflammatory drugs by using topical formulationsAm J Cardiol20091049131519840584
- Diclofenac drug interactions Available at: http://www.drugs.com/drug-interactions/diclofenac.htmlAccessed February 23, 2012
- BellamyNBuchananWWGoldsmithCHCampbellJStittLWValidation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or kneeJ Rheumatol19881512183318403068365
- Medical Dictionary for Regulatory Activities, version 7.0 Available at: http://www.meddramsso.com/Accessed March 20, 2013
- SimonLSGriersonLMNaseerZBookmanAAZev ShainhouseJEfficacy and safety of topical diclofenac containing dimethyl sulfoxide (DMSO) compared with those of topical placebo, DMSO vehicle and oral diclofenac for knee osteoarthritisPain2009143323824519380203
- TugwellPSWellsGAShainhouseJZEquivalence study of a topical diclofenac solution (Pennsaid) compared with oral diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trialJ Rheumatol200431102002201215468367
- Voltaren®Gel 1% (diclofenac sodium topical gel) [package insert]Novartis Consumer Health, IncParsippany, NJ2009 Available at: http://www.voltarengel.com/common/pdf/Voltaren-PI-10-19.pdfAccessed March 12, 2013