Abstract
Purpose
To evaluate the effect of vidian neurectomy (VN) on the ocular surface and the possibility of dry eye in the treatment of allergic rhinitis.
Methods
Twelve participants were recruited in this prospective study. Prior to and after 1 and 6 months of VN, an ocular surface disease index (OSDI) questionnaire was obtained, and the Schirmer’s tear test (STT), break-up time (BUT), corneal fluorescence staining (CFS) score, and Keratograph 5M were used to evaluate the ocular surface condition.
Results
Two patients (16.67%) met the dry eye diagnosis criteria one month after surgery; however, their symptoms were relieved after to 3–4 months and none of them met the diagnostic criteria for dry eye after six months. Compared with the baseline values, the STT was significantly reduced (P=0.002), while the tear meniscus height (TMH) (P=0.262), break-up time (BUT) (P=0.916), first keratographic tear film break-up time (NK-BUTfirst) (P=0.791), and average keratographic break-up time (NK-BUTave) (P=0.970) did not change significantly 6 months after surgery. The degree of STT decreased from baseline to 6-month and was related to the basic STT (ρ= 0.837, P=0.001) and sex (ρ= −0.584, P= 0.026) but not to age, OSDI score, BUT, NK-BUTfirst, NK-BUTave or CFS (all P>0.05). Among these factors, STT at baseline was confirmed to be a predictor of a decline in tear secretion after surgery (B = 0.731, P<0.001).
Conclusion
In this 6-month prospective pilot study, decreased tearing was observed after VN, but this decrease did not increase the possibility of dry eyes.
Keywords:
Introduction
The Vidian nerve, formed by the confluence of presynaptic parasympathetic fibers and postsynaptic sympathetic fibers,Citation1 governs the nasal mucosa, palate, and lacrimal gland. By blocking cholinergic innervation, vidian neurectomy (VN) is performed to inhibit excessive activity of the parasympathetic system, which is utilized for treating allergic and chronic rhinitis, with particular efficacy in intractable vasomotor rhinitis.Citation1–3 Although its efficacy in decreasing rhinorrhea has been demonstrated in several studies, 23.8–100%Citation1,Citation4–9 of patients were reported to have dry eye after VN. The occurrence of severe bilateral neurotropic keratopathy associated with dry eye indicates that ocular surface complications after VNCitation10 cannot be ignored. However, to the best of our knowledge, no studies have investigated the effects of VN on the ocular surface, especially on the tear film, and its impact on patients’ lives.
Dry eye is a common disorder of tear secretion on the ocular surface, which disturbs 5–50% of the population and deteriorates their quality of life.Citation11 Dry eye symptoms (DES) have typically been evaluated by patient reporting rather than by using an ocular surface disease index (OSDI) questionnaire and objective testing of tear volume. Therefore, this prospective study aimed to evaluate the effect of VN on the ocular surface and the possibility of DES use in treating allergic rhinitis.
Methods
This prospective study was performed between January 2022 until August 2022 at the Eye and ENT Hospital, Fudan University, Shanghai, China. This study was performed in accordance with the tenets of the Declaration of Helsinki and approved by the Medical Ethics Committee of the Eye and ENT Hospital, Fudan University, Shanghai, China (EENTIRB-2019097-1). Written informed consent was obtained from all participants and their parents after the study was explained.
Participants
All Participants underwent VN using endoscopic endonasal approaches, with the sphenoid process of the palatine bone as a landmark, as described by Zhao et al. No eye drops were used after the surgery.
Participants were included in the study if they met the following inclusion criteria: (1) aged between 18 and 60 years, (2) had allergic rhinitis requiring vidian neurectomy, and (3) agreed to be enrolled in the study by both participants and their parents via informed consent.
Participants were excluded if they had (1) a history of systemic or ocular disease that required treatment, including keratitis, ocular allergic disease, any other ocular surface disease, glaucoma, active or chronic uveitis, or previous ocular surgery or injury; (2) a history of dry eye or meeting the diagnostic criteria for dry eye; (3) pathological involvement of the pterygopalatine fossa; and (4) direct tumor infiltration into the vidian canal.
Patients who met 3 of these 4 were diagnosed as dry eye: OSDI score ≥13, Schirmer’s tear test (STT) without anesthesia ≤10 mm/5min, tear film break-up time (BUT) ≤10s, corneal fluorescein staining (CFS) score ≥1.Citation12
Surgical Procedure of VN
Under general anesthesia, cotton tablets soaked with 0.1% epinephrine were used to astringent the nasal mucosa. According to the patient’s nasal condition, bilateral inferior turbinate was moved out appropriately, and the tail of the middle turbinate was removed. The posterior orifice of the palatovaginal canal can be identified at the indentation of the mucosal surface above the posterior naris. The plane of the posterior orifice of palatovaginal canal can use to locate the lower margin of the sphenoid process of the palatine bone. Then, plasma was used to resect the surface mucosa of the palatine sphenoid process upward and laterally to expose the palatine sphenoid process bone until the anterior orifice of the palatine sheath canal. A nucleus pulposus forceps was used to remove the bone part of the sphenoid process of the palatine bone, expose the contents of the lower palatine sheath canal and the bone ridge at the root of the pterygoid process. Above the plane of the bone ridge, the palatine sheath artery and nerve were ablated close to the root of the pterygoid process. After the vidian nerve was exposed, continued resect laterally can expose the outer orifice of the vidian canal. The isolated vidian nerve was cut off completely, and unipolar electrocoagulation was used for hemostasis.
Ophthalmologic Examination
After recruitment, participants underwent a complete ophthalmic examination. All participants were examined by the same experienced examiner before surgery and at 1 and 6 months after surgery. At each visit, the following ophthalmic examinations were performed in order: OSDI questionnaire was used to assess the participants’ ocular surface symptoms and severity of dry eye, slit-lamp examination (type YZ5E, 66 Vision Tech. Co., China) including BUT and CFS score evaluation, Keratograph 5M Topographer inspection (Oculus Optikgerate GmbH, Wetzlar, Germany), and STT.
Evaluation of OSDI
OSDI questionnaire consisted of three sections (a total of 12 questions), and used to evaluate the severity of ocular discomfort symptoms, visual functions related life quality and environmental triggers in the recent week. OSDI scores = (sum of scores for questions answered × 25)/(number of answered questions). OSDI < 13 indicated the patients were normal, and OSDI score 13~22, 23–32 and 33 to 100 respectively indicated the patients have mild, moderate and severe dry eye-related symptoms.
Slit-Lamp Examination
All the patients underwent a slit-lamp examination at each follow-up visit. BUT and CFS were assessed as previously addressed.Citation13 BUT less than 10s was considered as the cut-off value for dry eye diagnose. CFS score were conducted using the sodium fluorescein strip and scored from 0 to 3 in the five areas of cornea (upper, lower, nasal, temporal and optical diameters).Citation14
Measurement of STT
Schirmer paper strips (Jingming new technology development Co., LTD, China) were placed in the 1/3 of the external low conjunctival sac of patients without topic anesthesia for STT. The patients were asked to close their eye for 5 min, then length of wetting by tears were measured and recorded. 10 mm/5min was considered as the cut-off value of STT for dry eye diagnose.
Assessment of Ocular Surface Parameters by Keratograph 5M
The clinical parameters of tear films were measured based on the placido rings (Keratograph 5M). Keratograph 5M inspection included noninvasive tear meniscus height (TMH), noninvasive keratographic tear film break-up time (NK-BUT, first keratographic break-up time, [NK-BUTfirst] and average keratographic break-up time, [NK-BUTave]). The duration between the blink and the first tear film break-up was recorded as the NK-BUTfirst, and the mean value of the NK-BUTfirst in different zones of cornea was analyzed as NK-BUTave. The height from the lower lid margin measured with the caliper tool in the customized software was TMH. TMH less than 0.2mm is the cut-off value for DED diagnose.Citation15
Statistical Analysis
Statistical analyses were performed using a commercially available statistical software package (SPSS for Mac version 22.0). Categorical variables (frequencies and constituent ratios) were analyzed using Fisher’s exact test. Continuous variables were expressed as medians and ranges. Statistical comparisons were performed using the paired-sample Wilcoxon test. Correlations were determined using Spearman correlation analysis. Statistical significance was set at P-value < 0.05.
Results
A total of 12 patients (8 men and 4 women) aged 22–59 (median 41.5) years old were enrolled in the study from January 2022 to August 2022.
The ocular surface symptoms of the participants were evaluated using OSDI scores. Six patients (50%) had occasional mild symptoms, including photophobia, foreign body sensation, aching pain, blurring, or discomfort in a dry atmosphere. Two patients (16.67%) met the dry eye diagnosis criteria 1 month after surgery; however, their symptoms were relieved after 3–4 months and neither patient met the diagnostic criteria for dry eye after 6 months.
Compared with baseline values, STT was significantly reduced at both 1 month (P=0.001) and 6 months (P=0.002) after surgery, whereas the CFS score (P=1.000), TMH (P=0.262), BUT (P=0.916), NK-BUTfirst (P=0.791), and NK-BUTave (P=0.970) did not change significantly after 6 months of surgery ().
Figure 1 Comparisons of the clinical values among the baseline, 1 month and 6-month follow-up. Compared with the values at baseline, STT reduced significantly both at one-month (Baseline vs 1-month: 8.50 vs 1.00; P=0.001) and six-month follow-up (Baseline vs 6-month: 8.50 vs 3.00, P=0.002, (A), while values of BUT (Baseline vs 1 month, P=0.847; Baseline vs 6-month: P=0.916, (B), TMH (Baseline vs 1 month, P=0.848; Baseline vs 6-month: P=0.262, (C), CFS score (Baseline vs 1 month, P=1.000; Baseline vs 6-month: P=1.000, (D), NK-BUTfirst (Baseline vs 1 month, P=0.965; Baseline vs 6-month: P=0.791, (E) and NK-BUTave (Baseline vs 1 month, P=0.365; Baseline vs 6-month: P=0.970, (F) did not change significantly after both 1 month and 6 months of surgery. ***P≤0.001, **P<0.01.
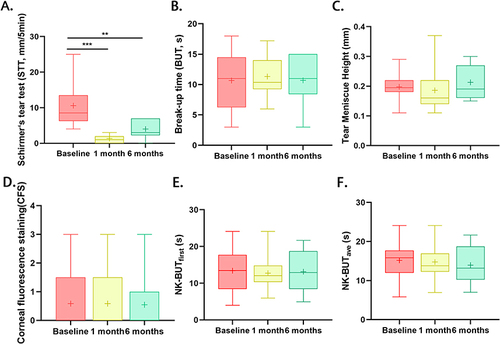
The STT showed a decline of 7.5 mm (88.24%) after 1 month, and 5.5 mm (64.71%) after 6 month post-operatively. The decrease in STT from baseline to 6-month was related to basic STT (ρ = 0.837, P = 0.001) and sex (ρ =−0.584, P = 0.026), but not to age (P = 0.277), basic OSDI score (P=0.692), BUT (P=0.707), NK-BUTfirst (P=0.678), NK-BUTave (P=0.710), and CFS (P=0.514) (). Subsequent multivariate analysis of these factors indicated that basic STT (B = 0.731, P<0.001) was the only factor correlated with the decline in tear secretion after surgery ().
Table 1 Correlation Between the Decline of Tear Secretion and Other Variables
Table 2 Multivariate Analysis Between the Decline of Tear Secretion and Other Variables
Discussion
Our Results showed that tear secretion decreased after VN, and although tear secretion did not recover 6 months post-operatively, none of the participants complained of dry eye or met the diagnostic criteria for DES.
The Vidian nerve is composed of preganglionic parasympathetic neurons that provide parasympathetic innervation to the lacrimal gland and nasal cavity after entering the posterior medial aspect of the pterygopalatine fossa.Citation1 Clinically, loss of parasympathetic input from the vidian nerve leads to a reduction in rhinorrhea and lacrimation. The decrease in tear volume after VN at one month post-operatively was consistent with that reported in the literature. The most common complication after VN in all previous studies was temporary postoperative dry eye in 23.8–100% of patients.Citation5–9,Citation16 In this study, the STT showed a decline of 7.5 mm (88.24%) after 1 month, and 5.5 mm (64.71%) after 6 months post-operatively.
Interestingly, only two patients (16.67%) met the dry eye diagnosis criteria 1 month after surgery, their symptoms were relieved after 3–4 months and none of them met the diagnostic criteria for dry eye after 6 months. Although the STT showed no significant difference between 1 month and 6 months post operatively, tear secretion seemed to recover. It remains unclear whether this is secondary to reinnervation or to increased baseline rates of tear production. A single study reported temporary dry eye in 98.85% of patients undergoing vidian nerve sacrifice; however, only 3.5% of patients had persistent symptoms for more than 6 months.Citation17 Given that the presynaptic vidian nerve had been severed and showed no signs of reinnervation (recurrent nasal allergy), another hypothesis suggested that some compensatory effect of the sphenopalatine ganglion (SPG) instead of the vidian nerve reinnervation might promote the recovery of the lacrimation function. Su et al explained this phenomenon as an effect of synaptic plasticity observed in the brain.Citation17,Citation18 After denervation from the vidian nerve in the SPG, cell bodies begin to receive signal input from the trigeminal afferent nerves of the lacrimal gland, which maintains the electrical bioactivity of the postganglionic parasympathetic fibers. Therefore, adequate tear volume and recovery from DES without recurrent nasal allergy symptoms were observed after VN. Moreover, a modified endoscopic VN approach based on anatomic and CT studies was performed in the present study, which provides the accurate location of the vidian nerve and protects the vessels and nerves of the sphenopalatine foramen as much as possible. Thus, maintaining an intact compensatory effect of the SPG is promising.
VN has a potential impact on tear secretion, implying that a comprehensive preoperative evaluation is essential. The benefits of surgery must be weighed if dry eye symptoms are present or are diagnosed. This study had several limitations. First, this is a pilot study with relatively small subjects, and more patients are needed for further evaluation, considering the safety and efficacy of VN. Second, we observed the participants for 6 months, and further observations are needed to determine the long-term effects of the VN. Further research is needed on the mechanism of recovery of tear secretion function.
Conclusions
The results from this 6-month prospective study showed that decreased tearing was observed after VN, but this decrease did not increase the possibility of dry eyes. Further studies are needed to determine the long-term effects and possible mechanisms of tear secretions after VN.
Disclosure
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Data Sharing Statement
All data supporting the findings of this study are available within the paper.
Additional information
Funding
References
- Su WF, Liu SC, Hsu WC, Chen YC. Randomized, double-blind, controlled study to evaluate the effect of vidian nerve cauterization on lacrimation. Am J Rhinol Allergy. 2014;28(3):255–259. doi:10.2500/ajra.2014.28.4029
- Halderman A, Sindwani R. Surgical management of vasomotor rhinitis: a systematic review. Am J Rhinol Allergy. 2015;29(2):128–134. doi:10.2500/ajra.2015.29.4141
- Marshak T, Yun WK, Hazout C, Sacks R, Harvey RJ. A systematic review of the evidence base for vidian neurectomy in managing rhinitis. J Laryngol Otol. 2016;130(4):S7–S28. doi:10.1017/S0022215116008008
- Liu SC, Wang HW, Su WF. Endoscopic vidian neurectomy: the value of preoperative computed tomographic guidance. Arch Otolaryngol Head Neck Surg. 2010;136(6):595–602. doi:10.1001/archoto.2010.72
- Jang TY, Kim YH, Shin SH. Long-term effectiveness and safety of endoscopic vidian neurectomy for the treatment of intractable rhinitis. Clin Exp Otorhinolaryngol. 2010;3(4):212–216. doi:10.3342/ceo.2010.3.4.212
- Lee JC, Kao CH, Hsu CH, Lin YS. Endoscopic transsphenoidal vidian neurectomy. Eur Arch Otorhinolaryngol. 2011;268(6):851–856. doi:10.1007/s00405-010-1482-x
- Tan G, Ma Y, Li H, Li W, Wang J. Long-term results of bilateral endoscopic vidian neurectomy in the management of moderate to severe persistent allergic rhinitis. Arch Otolaryngol Head Neck Surg. 2012;138(5):492–497. doi:10.1001/archoto.2012.284
- Ma Y, Tan G, Zhao Z, Li W, Huang L, Liu G. Therapeutic effectiveness of endoscopic vidian neurectomy for the treatment of vasomotor rhinitis. Acta Otolaryngol. 2014;134(3):260–267. doi:10.3109/00016489.2013.831478
- Robinson SR, Wormald PJ. Endoscopic vidian neurectomy. Am J Rhinol. 2006;20(2):197–202. doi:10.1177/194589240602000216
- Lin PY, Cheng CY, Wu CC, et al. Bilateral neurotrophic keratopathy complicating Vidian neurectomy. Am J Ophthalmol. 2001;132(1):106–108. doi:10.1016/s0002-9394(00)00958-2
- Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017;15(3):334–365. doi:10.1016/j.jtos.2017.05.003
- Messmer EM, Von Lindenfels V, Garbe A, Kampik A. Matrix metalloproteinase 9 testing in dry eye disease using a commercially available point-of-care immunoassay. Ophthalmology. 2016;123(11):2300–2308. doi:10.1016/j.ophtha.2016.07.028
- Le Q, Ge L, Li M, et al. Comparison on the vision-related quality of life between outpatients and general population with dry eye syndrome. Acta Ophthalmol. 2014;92(2):e124–32. doi:10.1111/aos.12204
- Yin Y, Gong L. Reversibility of gland dropout and significance of eyelid hygiene treatment in meibomian gland dysfunction. Cornea. 2017;36(3):332–337. doi:10.1097/ico.0000000000001042
- Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15(3):539–574. doi:10.1016/j.jtos.2017.05.001
- Su WF, Liu SC, Chiu FS, Lee CH. Antegrade transsphenoidal vidian neurectomy: short-term surgical outcome analysis. Am J Rhinol Allergy. 2011;25(6):e217–20. doi:10.2500/ajra.2011.25.3704
- Ulupinar E, Yucel F, Erol K. Lesion-induced synaptic plasticity in the somatosensory cortex of prenatally stressed rats. Neurotoxicol Teratol. 2011;33(5):548–557. doi:10.1016/j.ntt.2011.07.009
- Bologna M, Agostino R, Gregori B, Belvisi D, Manfredi M, Berardelli A. Metaplasticity of the human trigeminal blink reflex. Eur J Neurosci. 2010;32(10):1707–1714. doi:10.1111/j.1460-9568.2010.07446.x
