Abstract
Radium-223 is a first-in-class alpha particle-emitting radiopharmaceutical approved for the treatment of bone metastatic castration-resistant prostate cancer. Radium-223 is administered intravenously with no requirement for complex shielding and specifically targets areas of bone metastasis. In a randomized placebo-controlled Phase III study, treatment with radium-223 was shown to improve overall survival, time to skeletal-related events, and health-related quality of life. Apart from radium-223, the cytotoxic chemotherapy agents docetaxel and cabazitaxel, androgen biosynthesis inhibitor abiraterone acetate, novel anti-androgen enzalutamide, and immunotherapy sipuleucel-T have also been shown to improve survival of men with advanced prostate cancer in Phase III trials. This review will outline current treatment approaches for advanced prostate cancer with a focus on the role of radium-223 in changing treatment paradigms.
Introduction
Radium-223 dichloride (radium-223, Xofigo [previously known as Alpharadin; Bayer AG, Leverkusen, Germany]) is a novel alpha-emitting radionuclide recently approved for the treatment of castration-resistant prostate cancer (CRPC) metastatic to bone. Radium-223 administered intravenously forms a complex with hydroxyapatite, selectively targeting areas of increased bone turnover associated with bone metastasis.Citation1 Beta-emitting radiopharmaceuticals such as strontium-89 and samarium-153 have been used in the past for palliation of bone pain associated with diffuse metastatic disease; however, duration of response is relatively short with no evidence of an impact on survival.Citation2,Citation3
In a randomized Phase III study (ALSYMPCA [ALphradin in SYMptomatic Prostate CAncer Patients]), treatment with radium-223 significantly prolonged survival of patients with bone metastatic CRPC compared to placebo, resulting in approval for use in this setting in the United States in May 2013.Citation4 This review will outline current treatment approaches for advanced prostate cancer with a focus on the role of radium-223 in changing treatment paradigms.
Data for this review were compiled using MEDLINE/PubMed, American Society of Clinical Oncology (ASCO), and European Society of Medical Oncology (ESMO) abstracts published before February 2014. The search terms included “castrate resistant prostate cancer”, “radium-223”, “Alpharadin”, “abiraterone”, “enzalutamide”, “cabazitaxel”, and “sipuleucel-T”. Information regarding ongoing clinical trials was obtained using the United Stated National Institute of Health’s online resource clinicaltrials.gov. Only articles published in English were considered.
Existing and emerging treatment options for CRPC
The treatment of advanced prostate cancer is rapidly evolving; patients are living longer with better quality of life despite a diagnosis of castration-resistant disease.Citation5 Apart from radium-223, the cytotoxic chemotherapy agents docetaxel and cabazitaxel, androgen biosynthesis inhibitor abiraterone acetate, novel anti-androgen enzalutamide, and immunotherapy sipuleucel-T have also been shown to improve survival of men with CRPC in randomized Phase III trials (see ).Citation6–Citation10 summarizes currently available treatment options for asymptomatic CRPC and symptomatic CRPC in the first-, second-, and third-line settings.
Table 1 Systemic treatment options for advanced prostate cancer showing survival benefit in randomized studies
Table 2 Systemic treatment options for patients with metastatic castration-resistant prostate cancer progressing after LHRH and antiandrogen therapy
Cytotoxic chemotherapy
Docetaxel chemotherapy became the standard of care for the treatment of CRPC in 2004 following the publication of two randomized trials showing a survival advantage over mitoxantrone.Citation10,Citation11 Three artificial treatment spaces then emerged in prostate cancer drug development: pre-docetaxel, docetaxel combinations, and post-docetaxel. Despite promising signals in Phase II studies, attempts to combine docetaxel with novel therapeutics have been unsuccessful to date. Negative results have been announced for large Phase III trials combining docetaxel with the endothelin receptor antagonist atrasentan,Citation12 the tyrosine kinase inhibitor dasatinib,Citation13 and the antiangiogenic agents bevacizumab,Citation14 lenalidomide,Citation15 and aflibercept.Citation16
Drug development in the pre- and post-docetaxel settings has been more successful (). Cabazitaxel is a novel taxane cytotoxic chemotherapy shown to improve survival in men with CRPC post-docetaxel compared to second-line mitoxantrone.Citation6 Treatment with cabazitaxel is associated with significant myelosuppression with relatively high rates of febrile neutropenia reported in the Phase III TROPIC study. Data from expanded-access programs have shown that, with experience and appropriate use of growth factor support, toxicity is manageable with good quality of life outcomes.Citation17
Targeting androgen receptor signaling
The androgen receptor (AR) signaling pathway remains a key driver of disease progression in CRPC.Citation18,Citation19 The peripheral conversion of circulating adrenal androgens and de novo intratumoral androgen synthesis are mechanisms leading to continued AR signaling; however, activation of this pathway may also be ligand-independent.Citation20–Citation22 Preclinical data suggest that, in addition to direct cytotoxic effects, taxanes such as docetaxel and cabazitaxel may also act via inhibition of AR nuclear translocation.Citation23
Abiraterone acetate (Zytiga; Janssen Biotech Inc, Horsham, PA, USA) is an oral inhibitor of CYP17A1, a key enzyme in the testosterone biosynthesis pathway. The use of single-agent abiraterone leads to a rebound increase in luteinizing hormone, hence the development of abiraterone for use in combination with medical or surgical castration.Citation24 The addition of low-dose glucocorticoid resulted in normalization of mineralocorticoid levels and an improvement in blood pressure control in early-phase studies, leading investigators to recommend that abiraterone should be used with prednisone in further clinical trials. Abiraterone 1,000 mg daily plus prednisone has been approved for use in the pre- and post-docetaxel setting for CRPC following two Phase III studies demonstrating superiority over prednisone plus placebo ().Citation7,Citation25
Enzalutamide (Xtandi; Astellas Pharma Inc., Tokyo, Japan) is an oral androgen receptor antagonist that binds to the androgen receptor more avidly than first generation anti-androgens.Citation26 The Phase III AFFIRM study, which randomized patients with metastatic CRPC who had progressed after docetaxel chemotherapy to enzalutamide 160 mg daily versus placebo, showed a significant survival benefit associated with enzalutamide treatment.Citation8 Enzalutamide has recently been shown to improve both radiographic progression-free survival and overall survival (OS) versus placebo prechemotherapy in CRPC, with approval in this setting anticipated in 2014.Citation27 A potential advantage of enzalutamide over androgen biosynthesis inhibitors such as abiraterone is the fact that concurrent steroids are not required; however, approximately 30% of patients in each arm of the AFFIRM study received concurrent corticosteroid treatment.Citation8
Immunotherapy
The approval of sipuleucel-T (Provenge; Dendreon, Seattle, WA, USA) for the treatment of prostate cancer in April 2010 saw the first antigen-specific immunotherapy to be approved for cancer treatment. Preclinical studies demonstrated that dendritic cells loaded with an antigen–cytokine fusion protein consisting of prostatic acid phosphatase (PAP) and granulocyte-macrophage colony-stimulating factor (GM-CSF) induced strong cellular immune responses in vivo to tumors and tissues expressing PAP.Citation28 Based on these preclinical observations, sipuleucel-T was developed for clinical use, consisting of autologous dendritic cells loaded with the human PAP–GM-CSF fusion protein.
Sipuleucel-T is individually manufactured for each patient, which involves harvesting peripheral-blood mononuclear cells and their ex vivo incubation with the chimeric protein linking GM-CSF to PAP. Three intravenous infusions are given over a 4-week period. In a Phase III trial involving 512 patients with minimally symptomatic CRPC, median OS was 25.8 months in the group treated with sipuleucel-T compared with 21.7 months in the placebo group (unadjusted hazard ratio [HR] for death in the sipuleucel-T group 0.77; 95% confidence interval [CI] 0.61–0.97, P=0.02).Citation9
Bone-targeted therapy
Bone metastases are a major cause of prostate cancer-specific morbidity and mortality. The treatment and prevention of skeletal related events (SREs) in prostate cancer has the potential to impact both symptoms and survival in advanced disease. Bisphosphonates such as zoledronic acid and the novel receptor activation of nuclear factor kappa-B (RANK) ligand inhibitor denosumab (Xgeva; Amgen, Thomas Oaks, CA, USA) are commonly used in combination with other forms of systemic therapy for CRPC.
RANK signaling is a potent stimulus for osteoclast proliferation and bone resorption. Denosumab is a fully humanized monoclonal antibody targeting RANK-ligand that has recently been shown to be superior to zoledronic acid in preventing or delaying SREs in patients with bone metastases from CRPC.Citation29 A large double-blind Phase III noninferiority study randomized 1,904 patients to denosumab 120 mg subcutaneously monthly or zoledronic acid 4 mg intravenously monthly. The primary endpoint was time to SRE as defined by pathological fracture, radiotherapy to bone, surgery to bone, or spinal cord compression. Denosumab was shown to be superior to zoledronic acid for prevention of SRE (median time to SRE 20.7 months versus 17.1 months; HR =0.82; P=0.008).Citation29 A Phase III placebo-controlled study also demonstrated that denosumab significantly improved bone metastasis-free survival in men with CRPC (29.5 versus 25.2 months; HR =0.85; 95% CI: 0.73–0.98; P=0.028); however there was no improvement in OS.Citation30
Radium-223: mechanism of action
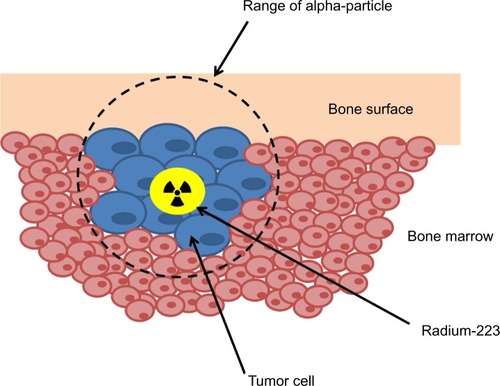
Radium-223 is an alpha particle-emitting radionuclide that delivers tumor cell-damaging radiation directly to sites of bone metastasis. Radium forms complexes with the bone mineral hydroxyapatite as a natural calcium mimetic, preferentially targeting areas of increased bone turnover associated with metastatic disease.Citation31 Radium-223 is administered intravenously in chloride salt solution, decaying to stable lead-207 in a six-stage process. Four alpha particles are emitted per decay, accounting for approximately 95% of the total radiation energy emitted.Citation32
Alpha particles (composed of two protons and two neutrons) have more than 7,000 times the mass of beta particles, resulting in high linear energy transfer and significantly more biological damage over a very short range (). The relatively long range of beta-emitting radiopharmaceuticals may cause significant bone marrow suppression and limit repeated administration.Citation33 The track length of the alpha particle is 0.10 mm (five to ten cell diameters) compared with 0.6 mm for samarium-153 and 2.4 mm for strontium-89, limiting damage to normal tissues.Citation1 Preclinical studies performed in rats demonstrated a significant bone marrow-sparing advantage with radium-223 compared with strontium-89.Citation34 In an experimental bone metastasis model in nude rats, radium-223 was found to be selectively concentrated in bone compared with soft tissues with a dose-dependent increase in symptom-free survival observed.Citation35
Radium-223 is rapidly cleared from the blood following intravenous injection. In a Phase I pharmacokinetic and biodistribution study, 60% of injected radioactivity was sequestered in bone metastasis within 4 hours and the highest absorbed radiation doses were to the osteogenic cells, red bone marrow, and intestinal wall. Radium-223 is not metabolized by the body and excretion is predominately fecal; renal excretion is less than 5%.Citation36,Citation37
Raduim-223 is administered using conventional nuclear medicine equipment on an outpatient basis with few radiation protection limitations recommended post-therapy, since the activity administered is considerably lower than the levels administered in standard diagnostic nuclear medicine studies. In patients treated in the Phase I study, dose rates from patients were typically less than 2 μSv/h/MBq on contact and averaged 0.02 μSv h−1 MBq−1 at 1 minute immediately following administration.Citation38
Radium-223: efficacy and safety
Early-phase clinical development of radium-223 has been reviewed in detail.Citation39 A randomized Phase II study of radium-223 in patients with CRPC and symptomatic bone metastasis showed a significant improvement in serum alkaline phosphatase (ALP) and delayed time to prostate specific antigen progression, and an improved median overall survival at 2 years.Citation40,Citation41 The subsequent Phase III ALYSMPCA study randomized 921 patients in a 2:1 ratio to receive six 4-weekly intravenous injections of radium-223 (50 kBq/kg) or placebo. Eligible patients had progressive symptomatic CRPC with two or more bone metastasis on bone scintigraphy scan and no evidence of visceral disease. Patients randomized within this study had either received or were deemed unfit to receive docetaxel chemotherapy. Recruitment took place from June 2008 to February 2011 and the study was stopped in June 2011 on the recommendation of an independent data monitoring committee after a planned interim analysis showed a significant improvement in overall survival in the patients who received radium-223 compared with placebo.
The two arms of the study were well-balanced in terms of patient characteristics with a median age of 71 years. In both the group of patients treated with radium-223 and those treated with placebo, 57% had received prior docetaxel chemotherapy. In an updated analysis, median overall survival was 14.9 months in the radium-223 group and 11.3 months in the placebo group (HR 0.70; 95% CI 0.58–0.83; P<0.001). The survival benefit associated with radium-223 was consistent across all subgroups irrespective of factors including baseline ALP level, bisphosphonate use, or prior docetaxel treatment.Citation4
Key secondary endpoints of the study included time to first SRE, time to ALP progression, and time to prostate-specific antigen progression. Time to first SRE was significantly prolonged in the group receiving radium-223 (15.6 months versus 9.8 months; HR 0.66; 95% CI 0.52–0.82; P<0.001). In particular, radium-223 was effective in significantly reducing the rate of spinal cord compression (3% versus 6%), pathological fracture (4% versus 7%), and need for external beam radiation therapy (23% versus 27%) compared to placebo.Citation42
Radium-223 was generally well tolerated with fewer adverse events occurring in patients treated with radium-223 compared with placebo (all adverse events 93% versus 96%; grade 3 or 4 adverse events 56% versus 62%). The investigators considered there to be no clinically meaningful differences in the frequency of hematological adverse events between the two groups; however, it should be noted that 6% of patients treated with radium-223 developed grade 3 or 4 thrombocytopenia compared with 2% of patients in the placebo group. Only one grade 5 hematologic adverse event was considered possibly related to radium-233 treatment; a patient passed away after developing thrombocytopenia, pneumonia, and hypoxia. Radium-223 was not associated with significant myelosuppression; grade 3 or 4 neutropenia occurred in 3% of patients treated with radium-223 compared with 1% of patients in the placebo group. Recently reported follow-up data at 1.5 years showed no increased incidence of second primary cancers, aplastic anemia, or myelodysplasia associated with radium-223 therapy in this cohort.Citation43 Of the nonhematologic adverse events potentially related to radium-223, diarrhea was more commonly observed in the experimental group; however, this was generally low-grade and manageable (25% in patients treated with radium-223 compared to 15% in the placebo group).Citation4
Implications for enhanced patient care, improved quality of life
Data from the ALSYMPCA study suggest that, in addition to prolonging survival, treatment with radium-223 is also associated with an improvement in pain and health-related quality of life. Fewer patients in the radium-223 group required opiate medication for pain relief (36% versus 50%) and fewer patients reported pain as an adverse event (50% versus 62%). In the subgroup of patients not requiring opiates at baseline, median time to opiate use was significantly prolonged in the radium-223 group compared with placebo (HR 0.62; 96% CI 0.46–0.85). Treatment with radium-223 significantly prolonged median time to external-beam radiation therapy for bone pain (HR 0.67; 95% CI 0.53–0.85).Citation44
A significantly higher percentage of patients who received radium-223 compared to placebo had a meaningful improvement in quality of life as measured by the FACT-P (functional assessment of cancer therapy – prostate) questionnaire (defined as an increase ≥ 10 points) during study drug administration (25% versus 16%; P=0.02).Citation4
Conclusion, place in therapy
Radium-223 is a first-in-class alpha particle-emitting radiopharmaceutical shown to prolong overall survival and improve health-related quality of life in bone metastatic CRPC. In the randomized Phase III ALSYMPCA study, radium-223 was evaluated in a cohort of patients who had progressed following docetaxel chemotherapy or who were considered unfit for docetaxel treatment. In this study, 57% of patients had received prior docetaxel chemotherapy, and it should be noted that the mechanism of action of radium-223 with specific targeting of bone metastatic disease does not preclude its use when any other systemic treatment has failed.Citation45 The key question that will determine the place of radium-223 in the sequencing of therapy for CRPC will be the safety of combining this treatment with other survival-prolonging drugs such as abiraterone, enzalutamide, and cabazitaxel. Despite the fact that clinically significant myelosuppression was not observed in the patients treated in the ALSYMPCA study, heavily pretreated patients with poor bone marrow reserve may be at risk of significant toxicity associated with radium-223 as a single agent or in combination with other systemic therapies. Several combination studies are ongoing in prostate cancer and other tumor types (); however, this strategy is not currently recommended outside clinical trials. Another question is the use of radium-223 in patients with both bone and visceral metastasis; if the combination of radium-223 with other systemic therapies proves to be safe and effective, this may become a future standard of care.
Table 3 Radium-223 clinical trials
At present, radium-223 can be considered for all patients with symptomatic bone-only metastatic disease,Citation46 particularly those with poor performance status who are unfit for cytotoxic chemotherapy.
Disclosure
Dr Mukherji reports travel support and honoraria from Janssen and Sanofi, and Dr Shamseddine reports honoraria from Sanofi. Other authors have no conflicts of interest to report.
References
- BrulandØSNilssonSFisherDRLarsenRHHigh-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: adjuvant or alternative to conventional modalities?Clin Cancer Res20061220 Pt 26250s6257s17062709
- FinlayIGMasonMDShelleyMRadioisotopes for the palliation of metastatic bone cancer: a systematic reviewLancet Oncol20056639240015925817
- JamesNDPirrieSBartonDClinical outcomes in patients with castrate-refractory prostate cancer (CRPC) metastatic to bone randomized in the factorial TRAPEZE trial to docetaxel (D) with strontium-89 (Sr89), zoledronic acid (ZA), neither, or both (ISRCTN 12808747)J Clin Oncol201331Suppl 18LBA5000
- ParkerCNilssonSHeinrichDAlpha emitter radium-223 and survival in metastatic prostate cancerN Engl J Med2013369321322323863050
- OmlinAPezaroCMukherjiDImproved survival in a cohort of trial participants with metastatic castration-resistant prostate cancer demonstrates the need for updated prognostic nomogramsEur Urol20136430030623313031
- de BonoJSOudardSOzgurogluMPrednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trialLancet201037697471147115420888992
- de BonoJSLogothetisCJMolinaAAbiraterone and increased survival in metastatic prostate cancerN Engl J Med2011364211995200521612468
- ScherHIFizaziKSaadFIncreased Survival with Enzalutamide in Prostate Cancer after ChemotherapyN Engl J Med2012367131187119722894553
- KantoffPWHiganoCSShoreNDSipuleucel-T immunotherapy for castration-resistant prostate cancerN Engl J Med2010363541142220818862
- TannockIFde WitRBerryWRDocetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancerN Engl J Med2004351151502151215470213
- PetrylakDPTangenCMHussainMHDocetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancerN Engl J Med2004351151513152015470214
- QuinnDITangenCMHussainMSWOG S0421: Phase III study of docetaxel (D) and atrasentan (A) versus docetaxel and placebo (P) for men with advanced castrate resistant prostate cancer (CRPC)J Clin Oncol302012Suppl; abstr 4511 2012
- AraujoJCTrudelGCSaadFOverall survival (OS) and safety of dasatinib/docetaxel versus docetaxel in patients with metastatic castration-resistant prostate cancer (mCRPC): Results from the randomized phase III READY trialJ Clin Oncol2013Suppl 6; abstr LBA8 2013
- KellyWKHalabiSCarducciMRandomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401J Clin Oncol201230131534154022454414
- PetrylakDPFizaziKSternbergCA Phase 3 Study to Evaluate the Efficacy and Safety of Docetaxel and Prednisone (DP) With or Without Lenalidomide (LEN) in Patients with Castrate-Resistant Prostate Cancer (CRPC): The MAINSAIL TrialesMO Conference 2012 LBA242012
- TannockIFizaziKIvanovSAflibercept versus placebo in combination with docetaxel/prednisone for first-line treatment of men with metastatic castration-resistant prostate cancer (mCRPC): Results from the multinational phase III trial (VENICE)J Clin Oncol312013Suppl 6; abstr 13 2013
- Bahl AMSMalikSBirtleACabazitaxel for metastatic castration-resistant prostate cancer (mCRPC): Interim safety and quality-of-life (QOL) data from the UK early access program (NCT01254279)J Clin Oncol201230Suppl 5; abstr 44
- AttardGCooperCSde BonoJSSteroid hormone receptors in prostate cancer: a hard habit to break?Cancer Cell200916645846219962664
- NelsonPSMolecular states underlying androgen receptor activation: a framework for therapeutics targeting androgen signaling in prostate cancerJ Clin Oncol201230664464622184375
- MontgomeryRBMostaghelEAVessellaRMaintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growthCancer Res200868114447445418519708
- TitusMASchellMJLihFBTomerKBMohlerJLTestosterone and dihydrotestosterone tissue levels in recurrent prostate cancerClin Cancer Res200511134653465716000557
- LockeJAGunsESLubikAAAndrogen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancerCancer Res200868156407641518676866
- DarshanMSLoftusMSThadani-MuleroMTaxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancerCancer Res201171186019602921799031
- O’DonnellAJudsonIDowsettMHormonal impact of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancerBr J Cancer200490122317232515150570
- RyanCJSmithMRde BonoJSAbiraterone in Metastatic Prostate Cancer without Previous ChemotherapyN Engl J Med201336813814823228172
- TranCOukSCleggNJDevelopment of a second-generation antiandrogen for treatment of advanced prostate cancerScience2009324592878779019359544
- BeerTMAASternbergCNHiganoCSEnzalutamide in men with chemotherapy-naive metastatic prostate cancer (mCRPC): Results of phase III PREVAIL studyJ Clin Oncol201432Suppl 4 LBA1
- SmallEJFratesiPReeseDMImmunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cellsJ Clin Oncol200018233894390311099318
- FizaziKCarducciMSmithMDenosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind studyLancet2011377976881382221353695
- SmithMRSaadFColemanRDenosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trialLancet20123799810394622093187
- NilssonSLarsenRHFossaSDFirst clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastasesClin Cancer Res200511124451445915958630
- McDevittMRSgourosGFinnRDRadioimmunotherapy with alpha-emitting nuclidesEur J Nucl Med1998259134113519724387
- SartorOReidRHBushnellDLQuickDPEllPJSafety and efficacy of repeat administration of samarium Sm-153 lexidronam to patients with metastatic bone painCancer2007109363764317167764
- HenriksenGFisherDRRoeskeJCBrulandØSLarsenRHTargeting of osseous sites with alpha-emitting 223Ra: comparison with the beta-emitter 89Sr in miceJ Nucl Med200344225225912571218
- HenriksenGBreistølKBrulandØSFodstadØLarsenRHSignificant antitumor effect from bone-seeking, alpha-particle-emitting (223)Ra demonstrated in an experimental skeletal metastases modelCancer Res200262113120312512036923
- HindorfCChittendenSAksnesAKParkerCFluxGDQuantitative imaging of 223Ra-chloride (Alpharadin) for targeted alpha-emitting radionuclide therapy of bone metastasesNucl Med Commun201233772673222513884
- HafeezSParkerCRadium-223 for the treatment of prostate cancerExpert Opin Investig Drugs2013223379387
- DauerLTWilliamsonMJHummJRadiation Safety Considerations for the Use of 223RaCl2 DE in Men with Castration-resistant Prostate CancerHealth Phys2014106449450424562070
- HarrisonMRWongTZArmstrongAJGeorgeDJRadium-223 chloride: a potential new treatment for castration-resistant prostate cancer patients with metastatic bone diseaseCancer Manag Res2013511423326203
- NilssonSFranzenLParkerCBone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II studyLancet Oncol20078758759417544845
- NilssonSFranzenLParkerCTwo-year survival follow-up of the randomized, double-blind, placebo-controlled phase II study of radium-223 chloride in patients with castration-resistant prostate cancer and bone metastasesClin Genitourin Cancer2013111202623021204
- ParkerCNSHeinrichDO’SullivanJMUpdated analysis of the phase III, double-blind, randomized, multinational study of radium-223 chloride in castration-resistant prostate cancer (CRPC) patients with bone metastases (ALSYMPCA)J Clin Oncol201230SupplLBA4512
- NilssonSVNSartorAOBottomleyD1.5-year post-treatment follow-up of radium-223 dichloride (Ra-223) in patients with castration-resistant prostate cancer (CRPC) and bone metastases from the phase 3 ALSYMPCA studyJ Clin Oncol201432Suppl 4; abstr 9
- NilssonSSABrulandOSFangFAksnesAKCiscoPParkerCPain analyses from the phase III ransomized ALSYMPCA study with radium-223 dichloride (radium-223) in castration-resistant prostate cancer (CRPC) patients with bone metastasisJ Clin Oncol201331Suppl 15
- WissingMDvan LeeuwenFWvan der PluijmGGelderblomHRadium-223 chloride: Extending life in prostate cancer patients by treating bone metastasesClin Cancer Res201319215822582724052017
- MohlerJLKantoffPWArmstrongAJProstate cancer, version 1.2014J Natl Compr Canc Netw201311121471147924335682