Abstract
Purpose
Although brain metastasis (BM) from gastric cancer (GC) is relatively uncommon, its incidence has been increasing owing to advancements in treatment modalities. Unfortunately, patients diagnosed with BM from gastric cancer have poor life expectancy. Our study aims to establish a predictive model for brain metastasis in advanced gastric cancer patients, thus enabling the timely diagnosis of brain metastasis.
Patients and Methods
The clinicopathological features of a cohort which included 40 GC patients with brain metastasis, 32 of whom from the First Affiliated Hospital of Nanchang University, 2 from Gaoxin Branch of the First Affiliated Hospital of Nanchang University, remaining 6 from Anyang District Hospital, and 80 non-metastatic advanced GC patients from the First Affiliated Hospital of Nanchang University between 2018 and 2022. Data were retrospectively analyzed.
Results
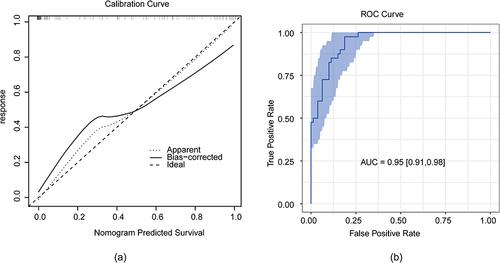
Age, tumor size, differentiation, lymph node grade, tumor location, Lauren classification, liver metastasis, carbohydrate antigen 199 (CA199), lactate dehydrogenase (LDH), and human epidermal growth factor receptor 2 (Her-2) were associated with BM. A nomogram integrated with nine risk factors (tumor size, differentiation, lymph node grade, tumor location, Lauren classification, liver metastasis, CA-199, LDH, and Her-2) showed good performance (Area Under Curve 0.95, 95% CI: 0.91–0.98).
Conclusion
We developed and validated a nomogram that achieved individualized prediction of the possibility of BM from GC. This model enables personalized imaging review schedules for timely brain metastasis detection in advanced gastric cancer patients.
Introduction
Gastric cancer (GC) is one of the most important malignancies in the world.Citation1 Gastric adenocarcinoma is the fifth most common malignancy and the fourth most frequent cause of cancer-associated death worldwide.Citation2 Metastases of GC frequently manifest as peritoneal dissemination in the liver, lymph nodes, and bones. Brain metastasis from gastric cancer is exceedingly rare. According to various researches, the occurrence of BM ranges from 0.7 to 6.6%.Citation2 Up to date, there has been only one large study conducted in America that referred to metastatic brain tumors from primary gastric cancer, which included over 3,000 gastric cancer cases in the M.D. Anderson Cancer Center over a 40-year period. Brain metastases have been reported in only 0.7% of patients.Citation3 However, the incidence is rising with more effective systemic treatments.Citation4 Usually, the onset of BM is associated with poor response to treatment and represents a sign of poor prognosis. Among the 19 patients with brain metastases from gastric cancer, the mean interval time was 2.4 months.Citation3 However, with the abundance of therapeutic options, aggressive treatment may provide patients with a better prognosis, especially younger patients.Citation5 Most reported cases of good survival are in young patients.Citation6–10
In recent decades, brain metastasis imaging has made significant progress--MRI is the cornerstone of diagnosis and evaluation of BMs, contributing to improved diagnostic capabilities. However, the cost of incorporating brain metastasis imaging into routine examinations remains a barrier for many patients. Typically, patients who lack neurological deficits or symptoms in their early stages can be diagnosed with brain metastases after the onset of neurological features. And also, there is no consensus on when to perform screening leads to missing best therapy time.Citation11 Establishing an appropriate imaging review cycle is crucial for the effective management of patients with advanced gastric cancer. Therefore, we are trying to find an economical and efficient method to predict the possibility of brain metastases in gastric cancer. Patients with a higher risk of brain metastases should have appropriately short review intervals. And plus, we emphasize the recognition of patients without clinical symptoms.
Recent studies have demonstrated a correlation between clinicopathological characteristics and brain metastasis in gastric cancer. However, neither case reports nor the available small-sample studies were comprehensive enough in terms of the risk factors they addressed.Citation12–15 Therefore, a systematic analysis of brain dissemination is essential.
This study aimed to estimate the probability of brain metastasis by establishing a nomogram associated with more comprehensive clinicopathological characteristics.
Materials and Methods
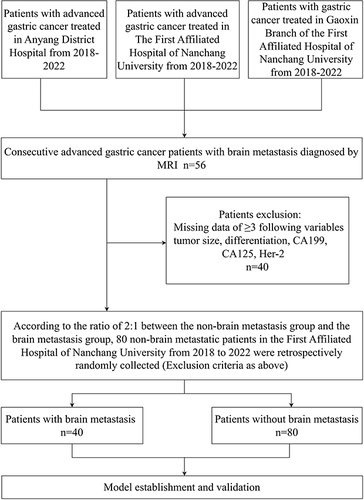
The researchers reviewed patients with gastric cancer in the oncology departments of three hospitals (First Affiliated Hospital of Nanchang University, Gaoxin Branch of the First Affiliated Hospital of Nanchang University, and Anyang District Hospital) between 2018–2022, with a predominance of patients with advanced gastric cancer. Patients with advanced gastric cancer were diagnosed with secondary brain metastases based on magnetic resonance imaging (MRI) findings. According to clinical experience and previous studies, five types of clinical data are closely related to the advanced progression of gastric cancer. Patients with three or more missing clinical data points were excluded from the study. The exclusion criteria were as follows: differentiation, tumor size, CA199, CA125, Her-2. Forty patients were enrolled after screening. Based on the research design, in which the ratio of the experimental group to the control group was 2:1, we randomly selected 80 patients with advanced gastric cancer but without brain metastasis at the Oncology of First Affiliated Hospital of Nanchang University from 2018 to 2022 (excluding the above five types of clinical data). The cohorts of patients with non-distant metastasis (n=80) and brain metastasis (n=40) were used for further analyses. The flow diagram of the study design is shown in .
Figure 1 Flow chart of study design.
Electronic medical records provided the demographic characteristics (age at onset and sex) and other clinical data which was performed by uniformly trained researchers. The oncological features of the first diagnosis of gastric cancer included tumor size, tumor location, differentiation, Lauren classification, tumor stage, and node stage. Laboratory parameters included routine blood tests (platelets, lymphocytes, and neutrophils), blood biochemistry (albumin and LDH), serum tumor markers (AFP, carcinoembryonic antigen, CA199, and CA125), and immunohistochemistry (Her-2 and Ki-67). Other parameters included the number of metastases to organs other than the brain, liver, and lung. These data were obtained from the records of the first visit when gastric cancer had progressed to an advanced stage or after the first diagnosis of brain metastases.
All relative analyses were performed using the SPSS statistical package (version 20.0; SPSS Inc., Chicago, IL, USA) and R studio 4.2.3 (R Studio, Boston, MA, USA). Due to the limited sample size, our study did not include a validation set. The baseline characteristics were summarized using descriptive statistics. Continuous variables were provided as medians, and categorical information was expressed numerically as a percentage. Univariate analysis was used to assess the clinical baseline data. Variables were evaluated using the chi-square test, Fisher’s exact test, and Mann–Whitney U-test, as appropriate. Then univariate logistic hazards regression analysis of characteristics of the metastatic and non-metastatic groups was con-ducted with “autoreg” package. Since this was a small sample study, we relaxed the p-value appropriately, so a two-tailed p-value of less than 0.1 was deemed statistically significant. Nomogram was established with the “regplot” package based on characteristics of significance.Citation16
Receiver operating characteristic (ROC) curves were used to compare the sensitivity and specificity of the brain metastasis nomogram. The Area under the curve (AUC) is an indicator of the ability of the model to discriminate. The value is between 0 and 1, and the closer the AUC is to 1, the better is the diagnosis. The Youden index was used as the cut-off point. It is defined as the maximum vertical distance between the ROC curve and diagonal or chance line.Citation17
Bootstrap resampling was used to evaluate the repeatability of the model with “fbroc” package while a calibration plot was generated to examine the calibration of the nomogram with ‘rms’ package.Citation18 The OS predicted by nomogram with brain metastasis and non-brain metastasis was compared using the Kaplan–Meier (K-M) analysis.
The study was conducted in accordance with the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University (IIT [2023] Proceedings No. 300 and 26th October 2023), Gaoxin Branch of the First Affiliated Hospital of Nanchang University ([2023] No.30 and 17th November 2023), and Anyang District Hospital (AD2023-09-01 and 24th April 2023). The research project did not involve personal privacy or commercial interests, and the requirement for informed consent was waived. The identifiable patient information was maintained with confidentiality.
Results
Patient Baseline Data
We retrospectively identified 120 patients with advanced gastric cancer (40 with and 80 without brain metastases). The median age of the patients with brain metastases from gastric cancer was 59 years. Meanwhile, 55.4 percentage (31/56) of patients had isolated brain metastases and 51.8 percentage (29/56) of patients had no neurological symptoms prior to MRI diagnosis. The clinicopathological characteristics of the cancer were summarized in . According to the Lauren classification, there were 63 patients with intestinal disease, 48 patients with diffuse disease, and 9 patients with mixed disease. Moreover, 65 patients had lower 1/3 gastric, 23 had middle 1/3 gastric, and 15 had upper 1/3 gastric. There were 36 patients had liver metastases and 42 had lung metastases. There were 19 patients with her2 amplification, 101 patients with her2 negative.
Table 1 Univariate Analysis of Clinicopathological Characteristics
Risk Factors
Univariate analysis was conducted using a logistic regression model to identify the P-value and odds ratio of the risk factors for brain metastasis in patients with advanced gastric cancer. Age, tumor size, differentiation, lymph node status, tumor location, Lauren classification, liver metastasis, CA199, LDH, Her-2 were identified as risk factors for brain metastasis. ()
Table 2 Univariate Logistic Hazardsregression Analysis
Predictive Model Construction
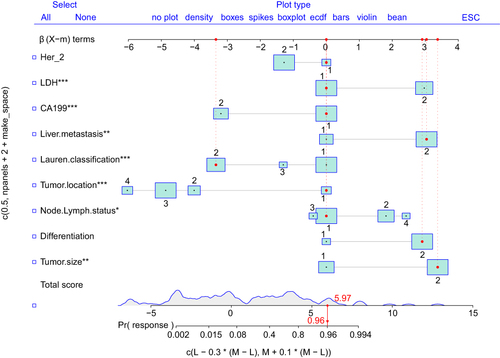
Univariate logistic hazard regression analysis identified risk factors in the training cohort and incorporated these nine variables to establish the nomogram model prediction, including size, differentiation, lymph node grade, tumor location, Lauren classification, liver metastasis, CA-199, LDH, and Her-2. Each variable is represented by a horizontal line, and the patient’s information is marked on the coordinates. The regression coefficient of each predictive variable corresponded to a score in the range of 0–100. The higher the weight of the variable, the higher is the score. Furthermore, the total score of the five variables was obtained by adding the corresponding scores and possibility of metastasis obtained for ET-LUAD ().
Predictive Model Validation
The prediction model of the line graph was verified using the ROC curves. The area under the curve was 0.95 ().
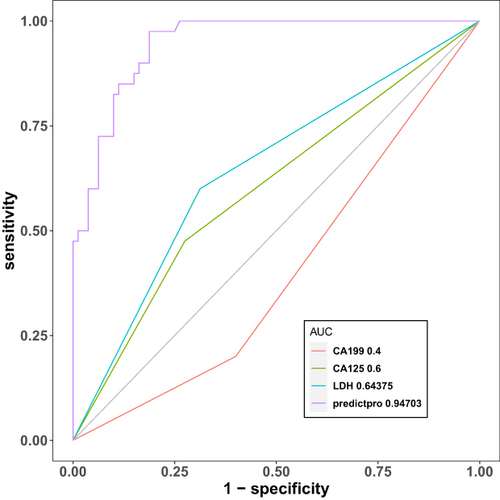
Figure 3 Validation of the brain metastasis nomogram using ROC and cut-off value determined by ROC. (a) The y-axis indicates the specificity of the risk prediction, while the x-axis indicates the sensitivity. (b) When 1.28 was set as the cut-off value determined by ROC analysis and Youden index, nomogram had the best sensitivity and specificity. (c) Regression coefficients corresponding to each variable in the multivariate regression analysis.
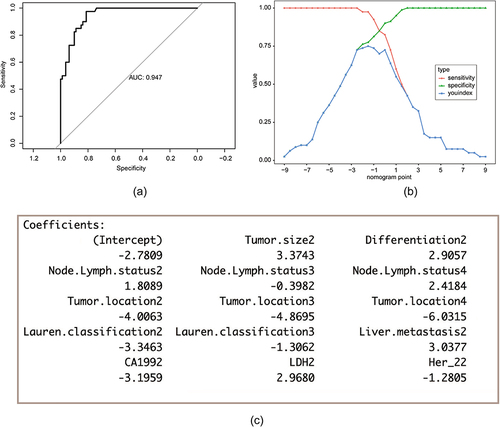
The nomogram model score, corresponding to the maximum Youden index was 1.28. This score was used as the cut-off score. Patients with a score higher than 1.28 were considered to be at risk for brain metastases (). The total score shown in was not the final score of the logistic regression model. There was a constant term difference of −2.78 between it and the actual model score.
The various regression coefficients of the real model were presented in ().
Comparing the AUCs obtained from the nomogram prediction model with those obtained from a single parameter, the model had an obvious advantage ().
Figure 4 Sensitivity and specificity for predicting brain metastasis of nomogram and other single variables was compared by the area under curve (AUC).
The model was calibrated by using a calibration curve. The calibration chart shows that the column chart performs better than an ideal prediction model. It could be seen that the probability of brain metastases predicted by nomogram was in good agreement with the actual probability (). The repeatability of the model was evaluated through bootstrap resampling of 1000 bootstrap samples for internal validation ().
Figure 5 Calibration and repeatability validation of model. (a) Calibration curve for nomogram-predicted and actual probability of having brain metastasis. (b) Boootstrap resampling to realize the confidence interval of the brain metastasis prediction nomogram.
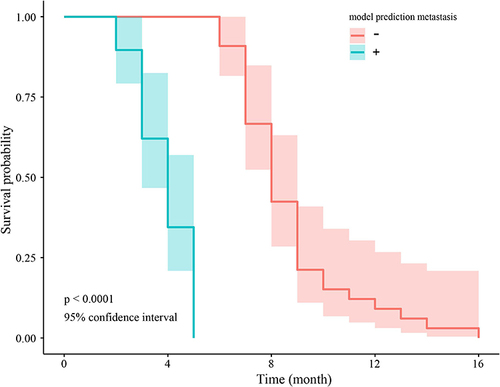
The result showed that OS was remarkably lower in patients predicted as non-brain metastasis compared with those with possibility of brain metastasis (HR=0.50, p < 0.001). ()
Discussion
We established a nomogram that demonstrated strong performance in evaluating the risk of brain metastasis (AUC 0.95), exhibiting favorable repeatability and accuracy. According to this model, patients with a model score above 1.28 are at a high risk of brain metastases.
Existing literature on brain metastasis in gastric cancer primarily consists of case reports. Yiran et al reported that T4 stage was associated with worse survival in patients with brain metastasis.Citation15 Ohdaira et al reported that patients who underwent gastrectomy had abnormally elevated AFP levels after brain metastases.Citation13 However, these two indicators, while significant in the case reports, did not significant in our retrospective study. There have also been some small-sample retrospective Asian and European studies. A study in Italy revealed that Her-2 positive was associated with CNS metastases and recurrence.Citation12 A SEER-based study indicated that the proximal region of the stomach posed a higher risk of metastasis than the distal region. Patients with GC with lung or liver metastases have a higher risk of bone and brain metastasis than those without metastasis.Citation14 This can be explained by the path of metastasis through the portal veins from the stomach to the liver, proceeding to the lungs and then to the brain. We comprehensively integrated clinicopathological characteristics to establish a predictive model and applied it to the field of clinical management of brain metastases from gastric cancer.
The roles of these risk factors in tumor brain metastasis are summarized. First, there was high lymph node status. Up to 68% of lung cancer patients with mediastinal lymph node metastases eventually progress to brain metastases.Citation19 We posit that this might be related to the anatomy of the mediastinal lymph node, which means that tumor cells are more likely to migrate to the thoracic duct and feed into blood circulation, increasing the possibility of brain metastasis. However, compared to the control group, the lymph node status of most patients with brain metastasis in our study was N1. Therefore, we speculated that it only represented disease progression and was not directly relevant to brain metastasis. Second, there was tumor heterogeneity. In our study, the proportion of patients with poor differentiation was higher among those with brain metastases, and the level of Ki-67, a potential indicator of tumor heterogeneity in advanced gastric cancer, was also slightly higher, although not statistically significant. A related explanation involves the theory of BTICs and CSCs. Poorly differentiated tumor cells are more likely to mutate to neuroinvasive stem cells, which have a higher capacity for neuroinvasion.Citation20 Third, tumor location must be considered. As mentioned above, while previous studies have established the prognostic significance of tumor location in brain metastases from gastric cancer, in the case of the sample we studied, some of the patients with brain metastases were selected from Anyang, which has the highest incidence of gastroesophageal junction cancer in China; therefore, there might be a sampling error.
Regarding immunohistochemical markers, Palmieri et al first suggested that Her-2 overexpression increased metastatic breast cancer in the brain.Citation21 Studies on the association between Her-2 expression and brain metastasis have predominantly focused on breast cancer; however, no consensus has been reached regarding brain metastasis from gastric cancer. As a routine examination for patients with gastric cancer, Her-2 positive is associated with poor patient survival.Citation22 Our data selected in China proved the significance of Her-2 in brain metastasis of gastric cancer. A systematic review revealed that for the higher proportion of intestinal classification in the brain metastasis group, for which we were unable to find evidence, we hypothesized that it was an indicator concomitantly elevated with Her-2.Citation23
The serum tumor marker levels were analyzed. Previous studies had primarily focused on the diagnostic significance of lactate dehydrogenase (LDH) in melanocytoma and breast cancer, demonstrating that elevated LDH levels were associated with an increased risk of brain metastasis.Citation24,Citation25 As a glycolytic enzyme, elevated LDH levels are also significant in the development of brain metastasis in gastric cancer, which might indicate an active state in the tumor tissue. The other one, CA199. Brain metastases from GI tumors are less well-studied; thus, the relationship between CA199, a GI highly sensitive serum tumor marker, and the occurrence of brain metastases is poorly understood. In a diagnostic study of brain metastasis from lung cancer, elevated CA199 level were considered an independent risk factor.Citation26 However, our model revealed that decreased CA199 level implied an increased risk of brain metastasis. However, the reasons underlying this phenomenon require further investigation.
The treatment approach for patients with brain metastasis of gastric cancer has evolved beyond the conventional radiotherapy and surgical resection. Chemotherapy, targeted therapy, and immunotherapy, as shown in case-reports and small-sample research have already been used clinically. The availability of progressively diverse treatment modalities may extend patient survival and improve their quality of life. Nevertheless, there is currently no consensus regarding the detection of brain metastases from gastric cancer. Therefore, we anticipate that the predictive models we have developed will help clinicians to some extent. By combining our brain metastasis risk prediction model with magnetic resonance imaging (MRI), we can recommend individualized examination cycles for each patient, thereby reducing underdiagnosis and delayed diagnosis. Consequently, an improved diagnostic and treatment framework has the potential to improve patient prognoses.
Our model was based on a not big sample of three large hospitals in northern and southern China but it is the largest real population research till now concerning risk evaluation in brain metastasis with gastric cancer patients.Citation27–30 As for external evaluation, on one hand, we searched PubMed, WOS databases to try to find models that are consistent with our research objectives for comparative validation. But unfortunately, it was not available. There was only one model of brain metastasis with tumor size, T stage, N stage, and age as variables. However, we were unable to obtain specific scores for each of the variables and also the nomogram cut-off score, so we failed to compare it with our model.Citation30 On the other hand, our study was not carried out in a larger multi-center setting as ethical review would have been time-consuming. However, given its good predictive performance and application of relatively novel variables, we hope that it can be applied in large hospitals in more regions of China in the future, even worldwide, and that it is a further validation of the model.
Conclusion
We developed and validated a nomogram to predict BM risk of brain metastasis. Patients with advanced gastric cancer can then receive individualized imaging review cycles and timely detection of brain metastases. However, further validation is required in broader regions.
Abbreviations
CA199, carbohydrate antigen 199; LDH, lactate dehydrogenase; Her-2, human epidermal growth factor receptor 2; CA125, carbohydrate antigen 125; MRI, magnetic resonance imaging; ROC curve, receiver operating characteristic curve; AFP, elevated alpha fetoprotein; CEA, carcinoembryonic antigen; OR, Odds ratio; 95% CI, 95% confidence interval.
Ethics Committee Approval
This study was approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University, Gaoxin Branch of the First Affiliated Hospital of Nanchang University and Anyang District Hospital. A waiver of informed consent was granted by the Ethics Committee as the study meets exemption based on design and objectives. The research project did not involve personal privacy or commercial interests. The identifiable patient information was maintained with confidentiality.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
We would like to thank Medical Academic Affairs of the First Affiliated Hospital of Nanchang University, Gaoxin Branch of the First Affiliated Hospital of Nanchang University, and Anyang District Hospital.
Data Sharing Statement
The data are not available to the general public due to the regulations of our institution, but they are available to researchers on reasonable request by emailing Li Zhang ([email protected])
Additional information
Funding
References
- López MJ, Carbajal J, Alfaro AL. et al. Characteristics of gastric cancer around the world. Crit Rev Oncol Hematol. 2023;181:103841. doi:10.1016/j.critrevonc.2022.103841
- Megid TBC, Baskurt Z, Ma LX, et al. Leptomeningeal carcinomatosis and brain metastases in gastroesophageal carcinoma: a real-world analysis of clinical and pathologic characteristics and outcomes. J Neuro-oncol. 2024;1:1.
- York JE, Stringer J, Ajani JA, Wildrick DM, Gokaslan ZL. Gastric cancer and metastasis to the brain. Ann Surg Oncol. 1999;6(8):771–776. doi:10.1007/s10434-999-0771-3
- Go PH, Klaassen Z, Meadows MC, Chamberlain RS. Gastrointestinal cancer and brain metastasis: a rare and ominous sign. Cancer. 2011;117(16):3630–3640. doi:10.1002/cncr.25940
- Nomura T, Yoshikawa T, Kato H, et al. Early gastric cancer manifested as brain metastasis: report of a case. Surg Today. 1997;27(4):334–336. doi:10.1007/BF00941808
- Hizawa K, Nagata Y, Taniguchi M, Nakamori M, Matsumoto T, Iida M. [Case of gastric cancer with isolated brain metastasis successfully managed by gamma knife radiotherapy and chemotherapy]. Japanese J Gastro-Enter. 2008;105(8):1200–1204.
- Yang Y, Pei X, Yang M. Combination of apatinib and continuous nutritional support for a gastric cancer patient with brain metastasis prolongs survival. J Clin Pharm Therapeutics. 2018;43(5):726–729. doi:10.1111/jcpt.12708
- Kojima Y, Watanabe T, Sanada T, et al. [A case report of brain metastasis due to an advanced gastric cancer]. Gan No Rinsho. 1988;34(12):1731–1734.
- Yamamoto H, Tanabe S, Ishida Y, et al. [nivolumab and gamma knife radiosurgery for a gastric cancer patient with brain metastasis-a case report]. Gan to Kagaku Ryoho Can Chemo. 2021;48(7):1.
- Wang Q, Shen Z, Ge M, et al. Unexpected curative effect of PD-1 inhibitor in gastric cancer with brain metastasis: a case report. Front Oncol. 2023;13(1042417):1.
- Derks S, van der Veldt AAM, Smits M, van der Veldt AAM. Brain metastases: the role of clinical imaging. Br J Radiol. 2022;95(1130):20210944. doi:10.1259/bjr.20210944
- Lai MY, Guan WL, Yang J, et al. The relationship between brain metastasis and HER2 expression status in gastric cancer. Clin Transl Oncol. 2023. doi:10.1007/s12094-023-03306-2
- Ohdaira H, Murai R, Hanyu N, Abe M, Yanaga K. Gastric cancer producing AFP/HCG which had a rapidly progressive course with metastasis to the brain discovered postoperatively. Japanese J Gastro-Enter. 2007;104(5):666–670.
- Qiu MZ, Shi SM, Chen ZH, et al. Frequency and clinicopathological features of metastasis to liver, lung, bone, and brain from gastric cancer: a SEER -based study. Cancer Med. 2018;7(8):3662–3672. doi:10.1002/cam4.1661
- Zhang Y, Lin Y, Duan J, Xu K, Mao M, Wang X. A population-based analysis of distant metastasis in stage iv gastric cancer. Med Sci Monit. 2020;26:e923867. doi:10.12659/MSM.923867
- Knewitz D, Almerey T, Gabriel E. A narrative review of prognostic indices in the evaluation of gastrointestinal cancers. J Gastrointest Oncol. 2023;14(4):1849–1855. doi:10.21037/jgo-23-159
- Akobeng AK. Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr. 2007;96(5):644–647. doi:10.1111/j.1651-2227.2006.00178.x
- Min Y, Huang Y, Wei M, et al. Preoperatively predicting the central lymph node metastasis for papillary thyroid cancer patients with hashimoto’s thyroiditis. Front Endocrinol. 2021;12(713475). doi:10.3389/fendo.2021.713475
- Ebben JD, You M. Brain metastasis in lung cancer: building a molecular and systems-level understanding to improve outcomes. Int J Biochem Cell Biol. 2016;78:288–296. doi:10.1016/j.biocel.2016.07.025
- Singh M, Manoranjan B, Mahendram S, McFarlane N, Venugopal C, Singh SK. Brain metastasis-initiating cells: survival of the fittest. Int J Mol Sci. 2014;15(5):9117–9133. doi:10.3390/ijms15059117
- Palmieri D, Bronder JL, Herring JM, et al. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 2007;67(9):4190–4198. doi:10.1158/0008-5472.CAN-06-3316
- Palmieri D, Chambers AF, Felding-Habermann B, Huang S, Steeg PS. The biology of metastasis to a sanctuary site. Clin Cancer Res. 2007;13(6):1656–1662. doi:10.1158/1078-0432.CCR-06-2659
- Lei YY, Huang JY, Zhao QR, et al. The clinicopathological parameters and prognostic significance of HER2 expression in gastric cancer patients: a meta-analysis of literature. World J Surg Oncol. 2017;15(1):68. doi:10.1186/s12957-017-1132-5
- Dong T, Liu Z, Xuan Q, Wang Z, Ma W, Zhang Q. Tumor LDH-A expression and serum LDH status are two metabolic predictors for triple negative breast cancer brain metastasis. Sci Rep. 2017;7:1.
- Bedikian AY, Wei C, Detry M, et al. Predictive factors for the development of brain metastasis in advanced unresectable metastatic melanoma. American J Clini On-Can Clini Tria. 2011;34(6):603–610.
- Ren XL, Zhang YX, Lyu Y, et al. Lactate dehydrogenase and serum tumor markers for predicting metastatic status in geriatric patients with lung adenocarcinoma. Cancer Biomarkers. 2019;26(2):139–150. doi:10.3233/CBM-190201
- Zhu Y, Fang X, Wang L, Zhang T, Yu D. A predictive nomogram for early death of metastatic gastric cancer: a retrospective study in the SEER database and China. J Cancer. 2020;11(18):5527–5535. doi:10.7150/jca.46563
- Zhang Y, Yu C. Development and validation of a surveillance, epidemiology, and end results (SEER)-based prognostic nomogram for predicting survival in elderly patients with gastric cancer after surgery. J Gastroin Onco. 2021;12(2):278–296. doi:10.21037/jgo-20-536
- Liu B, Li K, Ma R, Zhang Q. Two web-based dynamic prediction models for the diagnosis and prognosis of gastric cancer with bone metastases: evidence from the SEER database. Front Endocrinol. 2023;14:1.
- Lin Z, Wang R, Zhou Y, et al. Prediction of distant metastasis and survival prediction of gastric cancer patients with metastasis to the liver, lung, bone, and brain: research based on the SEER database. Ann Translat Med. 2022;10(1):1.