Abstract
Background
Current studies mostly suggest that hyperhidrosis is caused by relative sympathetic hyperactivity. Sympathetic radiofrequency thermocoagulation is widely used in clinics. Previous studies have demonstrated that surgery at T3 is effective and safe compared with higher levels, so craniofacial hyperhidrosis in our hospital is selected to be treated at T3. However, some patients pursue repeat medical treatment due to an increase in hyperhidrosis at the original site after surgery. Previous studies have demonstrated the significance of Perfusion index (PI) value in the recurrence of palmar hyperhidrosis, but there is no relevant study on craniofacial hyperhidrosis.
Methods
Clinical data from patients with craniofacial hyperhidrosis, who underwent T3 sympathetic radiofrequency thermocoagulation at Jiaxing First Hospital (Jiaxing, China) between January 1, 2018 and December 31, 2021, were analyzed. Recurrence in patients 1 year after surgery was recorded through a case search and telephone follow-up system that registered patient information. Clinical data were analyzed using binary logistic regression analysis to investigate risk factors associated with recurrence in patients with craniofacial hyperhidrosis 1 year after surgery.
Results
Of 83 patients included in the present study, 34 (40%) experienced increased craniofacial sweating 1 year after surgery. Results of univariate logistic regression analysis revealed that computed tomography (CT) scan count, increase in pulse index (PI) at the fingertips, and differences in forehead temperature were potential risk factors for postoperative recurrence in patients with craniofacial hyperhidrosis (p<0.2), and the results were consistent on both sides. Three potential risk factors were included in the multivariate logistic regression analysis and results revealed that the risk for recurrence was reduced by 48% (left side) and 67% (right side) for every 1 unit increase in PI value.
Conclusion
A small increase in PI was an independent risk factor for recurrence of hyperhidrosis in patients with craniofacial hyperhidrosis after undergoing T3 sympathetic radiofrequency thermocoagulation.
Introduction
Hyperhidrosis (HH) is a condition in which sweat glands overproduce more than what is required for physiological regulation, and negatively affects quality of life and social, emotional, and mental health. In a study investigating the epidemiology of HH, Estevan et alCitation1 reported the following distribution of patient complaints: hand hyperhidrosis (48.1%); armpit hyperhidrosis (36.1%); craniofacial hyperhidrosis (9.2%); and foot hyperhidrosis (6.5%). Although the pathogenesis of HH is not fully understood, and is believed to be related to relative hyperfunction of the sympathetic nerves.Citation2,Citation3 The Hyperhidrosis Disease Severity Scale (HDSS) is used to evaluate the severity of focal HH (). An HDSS score > 2 is classified as severe HH.Citation4 Craniofacial HH often conveys negative information, including embarrassment and lack of self-confidence in social communication. Affected patients often experience psychological problems, such as low self-esteem and anxiety, which seriously affect quality of life and, in turn, prompts patients to seek treatment.Citation5,Citation6 Treatment methods for craniofacial HH include local administration of antiperspirant, intradermal botulinum toxin (ie, “Botox”) injection(s), endoscopic thoracic sympathectomy (ETS), and computed tomography (CT)-guided radiofrequency (RF) thermocoagulation of the sympathetic nerves.Citation6,Citation7 ETS is considered to be the most effective treatment for HH; however, the incidence of severe compensatory HH is high; therefore, some patients do not benefit from surgery but experience more pain.Citation8,Citation9 In recent years, CT-guided sympathetic RF thermocoagulation has been applied in the clinic due to its advantages, such as the lack of shoulder effect(s) compared with ETS, minimal invasiveness, and low incidence of compensatory HH.Citation10 However, the recurrence rate of after sympathetic nerve RF thermocoagulation is relatively high, and some patients relapse after treatment and seek medical treatment a second time, thus increasing their psychological and medical burdens.
Table 1 Hyperhidrosis Disease Severity Scale
Clinical data from patients with craniofacial HH treated at the Jiaxing First Hospital (Jiaxing, China) between January 1, 2018 and December 31, 2021, were retrospectively analyzed to explore the risk factors for recurrence of HH. This study complied with the Declaration of Helsinki and proved by the research ethics committee board of The First hospital of Jiaxing ap. The ethical number is LS2018–141. All patient participants submitted written informed consent forms.
Method
Patients
Inclusion Criteria
Primary HH, with craniofacial HH as the primary complaint;
Location and degree of HH was symmetrical;
Preoperative HDSS score > 2; and
Age, 18 to 60 years.
Exclusion Criteria
Any history of thoracic sympathectomy-related surgery;
Secondary HH, including endocrine system diseases such as hyperthyroidism and tuberculosis; and
Refusal to attend follow up.
Surgical Procedure
Before the procedure, patients were informed about the operative process and risks, and required to sign an informed consent form. Patients were advised to refrain from consuming any food or drink for at least 8 h before the operation.
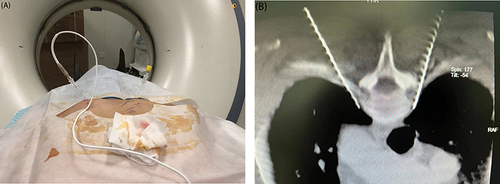
A trocar was inserted into the upper limb vein for subsequent use. The patient was then transferred to the CT operating room and positioned prone on the console. Vital signs, forehead temperature (T), and pulse index (PI) on the left and right sides were monitored throughout the operation, and all patients underwent sympathetic nerve chain treatment at the level of the third thoracic vertebra (ie, “T3”). A CT scan frame was set on the chest CT localization image covering the first to third ribs. Subsequently, an axial scan using a layer thickness of 3 mm was performed to determine the layer encompassing the upper border of the small head of the third rib, which was identified as the optimal puncture site. The puncture target points on both sides were pulled straight to the back skin through the lamina-transverse process gap, and the intersection point with the skin was identified as the puncture entry point. Puncture depth and angle were measured using a CT software ruler.
Following local anesthesia, a 10 cm, No.7, blunt thoracic sympathetic RF puncture needle (with a bare end of 10 mm) was used to puncture the target point according to the designed puncture path until the needle tip was close to the front upper edge of the small head of the third rib (). After determining the location of the needle tip, the needle core was removed and a RF electrode was inserted. Electrophysiological tests were performed on the motor and sensory nerves. RF thermocoagulation was performed at 95°C for 300 s if a current below 1.5 mA elicited no numbness or muscle twitching within the area of spinal nerve innervation. CT revealed no numbness or movement disorder in the lower extremities or pneumothorax in the lung window. The RF electrode was then removed, a small bandage was applied to the puncture point, and the treatment was concluded. T and PI were recorded on the left and right sides before and after surgery, respectively.
Data Collection
Information regarding sex, age, height, weight, disease course, CT scan count, and preoperative resting heart rate (HRr) of the patients were queried and collected from the case search system. The preoperative and postoperative PI and T values on the left and right sides were collected. Preoperative HRr was measured within 1 h of admission, and the patient remained quiet for > 30 min before the measurement. The PI and T of the left and right sides were measured while the patient was lying on the CT bed. Through telephone follow-up, patients were asked about their recurrence and compensatory hyperhidrosis 1 year after surgery in the authors’ hospital. An HDSS score > 2 was defined as recurrence. We still used the HDSS score to assess the severity of compensatory hyperhidrosis, with tolerable compensatory hyperhidrosis defined as an HDSS score of ≤2 and severe compensation defined as an HDSS score of >2.
Statistical Analysis
Statistical analysis was performed using SPSS version 25.0 (IBM Corporation, Armonk, NY, USA). The Shapiro–Wilk test was used to determine whether the measurement data followed a normal distribution, which are expressed as mean ± standard deviation, whereas non-normally distributed data are expressed as median (interquartile range). Candidate variables were screened using univariate analysis, with the p-value adjusted to 0.2. In the single factor analysis, candidate variables (p < 0.2) were included in the multifactor regression model. Finally, binary and multifactor logistic regression analyses were performed to establish a prediction model.
Results
Follow-Up
Between January 1, 2018 and December 31, 2021, 99 patients with craniofacial HH underwent T3 sympathetic RF thermocoagulation at Jiaxing First Hospital. Among these, 11 patients had a history of T3 sympathetic nerve surgery, 1 was secondary to HH after trauma, and 4 refused follow-up, resulting in 16 patients being excluded. As such, a total of 83 cases were ultimately included. One year after surgery, 34 (40%) patients experienced recurrence of HH, as follows: bilateral (n=19); left-sided (n=10); and right-sided (n=5).24 and 29 patients experienced right- and left-sided recurrence, respectively.
Variable Selection and Description
Among the 83 patients included, 70 were male and 13 were female, and the sex ratio was unbalanced; as such, sex was not used as a candidate variable. The disease course was divided into three groups, with 10 years and 20 years as categorical variables. Body mass index (BMI) was calculated according to patient height and weight and was used as a candidate variable. The rising multiples of the left- and right-hand PI values were calculated according to the preoperative and postoperative PI values, namely PIm (L) and PIm (R). The difference between the left and right hands was calculated according to the preoperative and postoperative palm temperatures (T), namely, Td (L) and Td (R). Other candidate variables included CT scan count and preoperative HRr ( and ).
Table 2 Baseline Characteristics- Categorical Data
Table 3 Baseline Characteristics-Quantitative Data
Binary Logistic Regression
Univariate logistic analysis was performed according to recurrence on the left and right sides to filter out meaningless factors ( and ). In the univariate analysis, candidate variables with p < 0.2 were included in the multivariate logistic regression analysis. Results of the univariate analysis on both sides were consistent, and the CT scan count, PIm, and Td were included in the multifactor analysis. Results of the multifactor analysis revealed that PI value was an independent risk factor for recurrence 1 year after surgery, and the risk for recurrence was reduced by 48% (left side) and 67% (right side) for every 1 unit increase in PI value ().
Table 4 Univariate Logistic Regression Analysis (L)
Table 5 Univariate Logistic Regression Analysis (R)
Table 6 Multiple Logistic Regression Analysis
Discussion
Our hospital began studying and implementing sympathetic nerve RF ablation in 2017, and has been applied to a variety of diseases including HH, trigeminal neuralgia, and herpes zoster neuralgia.Citation11,Citation12 The sympathetic ganglion is longitudinally arranged on both sides of the spinal cord, and the craniofacial region innervates the nerves, mostly from the first to fifth thoracic medullary segments. The second to fifth thoracic medullary segments are separated to supply the upper limbs.Citation13 T3 was selected as the site of sympathetic RF thermocoagulation for craniofacial HH at our hospital. Although T1-2 innervation of the head and face is more dominant, high-level nerve injury complications are more likely to lead to compensatory HH and Horner syndrome.Citation14 In a study involving 193 patients with HH, who underwent sympathetic nerve block at the T2, 3, and 4 segments, LinCitation15 reported that operation Y at the T3 segment was sufficient.
In the present study, data from patients with craniofacial HH treated at our hospital over the past 18–21 years were collected, and recurrence of HH at 1 year after surgery was investigated by telephone follow-up. In previous studies, the recurrence of HH was usually defined as the return of the HDSS score to a similar level before surgery or the HDSS score of 4. However, an HDSS score of 3 affects the activities of daily living and, in this study, 2 patients had an HDSS score of 3 before surgery. Similarly, an HDSS score of was sufficient to negatively affect patients and prompt them to undergo surgery. Therefore, we believe that it is more reasonable to define a relapse as an HDSS score > 2.
This study included suspected risk factors based on clinical experience and previous studies, including age, BMI, CT scan count, HRr, PI increase times, and increases in head temperature. Although the occurrence of HH is typically symmetrical, studies have shown bilateral sympathetic nerve differences,Citation16,Citation17 and there have also been reports of cases of primary HH occurring only on 1 side.Citation18 During follow-up, we also found that many patients experienced different degrees of re-sweating after surgery on both sides. Therefore, the PI and forehead temperature measured on both sides were studied and analyzed for recurrence on this side.
Binary logistic regression analysis was used. Univariate logistic regression analysis was performed for all suspected risk factors. The results revealed that p-values for both Td and PIm were < 0.05, and the difference was statistically significant. Finally, three factors, CT scan count, PIm, and Td, were included in the multivariate logistic regression analysis. Multivariate analysis of PIm and CT scan counts was consistent with the univariate analysis. However, temperature differences yielded varying results, suggesting that they were not independent risk factors for recurrence. We believe this may be due to a strong correlation between increases in T and increases in PI; therefore, although Td did not exhibit a statistical difference in multivariate analysis, it is still reasonable to use the difference in T and the increase in PI as reference factors during the procedure. The results of both analyses were generally consistent.
The PI reflects the pulse intensity at the monitoring site. The oximeter emits a specific wavelength of infrared light. A constant amount of light is absorbed by the skin, subcutaneous tissue, and non-arterial blood, whereas a variable amount of light is absorbed by pulsating arterial blood. A constant amount of light is divided by a variable amount of light to obtain the pulse intensity. This detection method is simple and easy, and has been widely used in the monitoring of sympathetic nerve procedures for HH, which can help surgeons gauge the success of an operation.Citation19,Citation20 Previous studies have shown that PI at the fingertips is a risk factor for the recurrence in patients with plamar hyperhidrosis after thoracic sympathetic radiofrequency thermocoagulation.Citation21
Due to the difficulty in measuring the PI of the head, the terminal PI of the fingers is also used to monitor patients with craniofacial HH. From an anatomical perspective, T3 of the palm is dominant, and an increase in the finger perfusion index can assist in judging the success of the operation. We hypothesized that PI at the fingertip would also be associated with postoperative recurrence, as was the case in the hand sweating study, and the final analysis confirmed this.
During follow-up, complaints were distributed as follows: compensatory HH (n=7); tolerable compensatory HH (n=6); and severe compensatory HH of the chest and back, which was unbearable and required frequent dressing everyday (n=1). No complications, such as Horner syndrome or intercostal neuralgia, occurred in any of the patients.
Overall, T3 RF thermocoagulation for craniofacial HH benefited more than one-half (60%) of patients 1 year after surgery from the disturbances to daily life caused by HH. In addition, this surgical method is less invasive and safer, and the incidence of severe compensatory HH is low. By minimizing the number of PI elevations during surgery, more patients can benefit in the long term.
Conclusion
An increase in PI value was an independent risk factor for recurrence in patients with craniofacial HHs 1 year after T3 thoracic sympathetic RF thermocoagulation. The PI value can be increased as much as possible to reduce the postoperative recurrence rate. There may be a correlation between increases in head temperature and increases in hand perfusion index, which can also be used as a reference for improving the operative effect.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors have no conflicts to declare for this work.
Additional information
Funding
References
- Estevan FA, Wolosker MB, Wolosker N, Puech-Leão P. Epidemiologic analysis of prevalence of the hyperhidrosis. An Bras Dermatol. 2017;92(5):630–634. doi:10.1590/abd1806-4841.20175551
- Shih CJ, Wu JJ, Lin MT. Autonomic dysfunction in palmar hyperhidrosis. J Auton Nerv Syst. 1983;8(1):33–43. doi:10.1016/0165-1838(83)90021-8
- Sato K, Kang WH, Saga K, et al. Biology of sweat glands and their disorders. II. Disorders of sweat gland function. J Am Acad Dermatol. 1989;20(5):713–726. doi:10.1016/S0190-9622(89)70081-5
- Solish N, Bertucci V, Dansereau A, et al. Canadian hyperhidrosis advisory committee. a comprehensive approach to the recognition, diagnosis, and severity-based treatment of focal hyperhidrosis: recommendations of the Canadian hyperhidrosis advisory committee. Dermatol Surg. 2007;33(8):908–923. PMID: 17661933. doi:10.1111/j.1524-4725.2007.33192.x
- Parashar K, Adlam T, Potts G. The impact of hyperhidrosis on quality of life: a review of the literature. Am J Clin Dermatol. 2023;24(2):187–198. PMID: 36622502; PMCID: PMC9838291. doi:10.1007/s40257-022-00743-7
- Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: a comprehensive review: etiology and clinical work-up. J Am Acad Dermatol. 2019;81(3):657–666. doi:10.1016/j.jaad.2018.12.071
- Pariser DM. Incorporating diagnosis and treatment of hyperhidrosis into clinical practice. Dermatol Clin. 2014;32(4):565–574. PMID: 25152351. doi:10.1016/j.det.2014.06.010
- Chen J, Liu Y, Yang J, et al. Endoscopic thoracic sympathicotomy for primary palmar hyperhidrosis: a retrospective multicenter study in China. Surgery. 2019;166(6):1092–1098. PMID: 31378477. doi:10.1016/j.surg.2019.05.039
- Lin TS, Kuo SJ, Chou MC. Uniportal endoscopic thoracic sympathectomy for treatment of palmar and axillary hyperhidrosis: analysis of 2000 cases. Neurosurgery. 2002;51(5 Suppl):S84–7. PMID: 12234434. doi:10.1097/00006123-200211002-00012
- Hasimoto FN, Cataneo DC, Hasimoto EN, Ximenes AMG, Cataneo AJM. Radiofrequency in the treatment of primary hyperhidrosis: systematic review and meta-analysis. Clin Auton Res. 2020;30(2):111–120. PMID: 31552511. doi:10.1007/s10286-019-00640-w
- Wang T, Xu S, He Q, et al. Efficacy and safety of radiofrequency thermocoagulation with different puncture methods for treatment of v1 trigeminal neuralgia: a prospective study. Pain Physician. 2021;24(2):145–152. PMID: 33740347.
- Zhang Z, Xia Z, Luo G, Yao M. Analysis of efficacy and factors associated with recurrence after radiofrequency thermocoagulation in patients with postherpetic neuralgia: a long-term retrospective and clinical follow-up study. Pain Ther. 2022;11(3):971–985. PMID: 35778672; PMCID: PMC9314488. doi:10.1007/s40122-022-00412-x
- Vannucci F, Araújo JA. Thoracic sympathectomy for hyperhidrosis: from surgical indications to clinical results. J Thorac Dis. 2017;9(Suppl 3):S178–S192. PMID: 28446983; PMCID: PMC5392541. doi:10.21037/jtd.2017.04.04
- Cerfolio RJ, De Campos JR, Bryant AS, et al. The society of thoracic surgeons expert consensus for the surgical treatment of hyperhidrosis. Ann Thorac Surg. 2011;91(5):1642–1648. PMID: 21524489. doi:10.1016/j.athoracsur.2011.01.105
- Lin CC, Telaranta T. Lin-Telaranta classification: the importance of different procedures for different indications in sympathetic surgery. Ann Chir Gynaecol. 2001;90(3):161–166.
- Ray BS, Hinsey JC, Geohegan WA. Observations On The Distribution Of The Sympathetic Nerves To The Pupil And Upper Extremity As Determined By Stimulation Of The Anterior Roots In Man. Ann Surg. 1943;118(4):647–655. PMID: 17858298; PMCID: PMC1617782. doi:10.1097/00000658-194310000-00013
- Cho HM, Lee DY, Sung SW. Anatomical variations of rami communicantes in the upper thoracic sympathetic trunk. Eur J Cardiothorac Surg. 2005;27(2):320–324. PMID: 15691689. doi:10.1016/j.ejcts.2004.10.057
- Kopelman D, Hashmonai M, Assalia A, Bahous H. Primary palmar hyperhidrosis presenting with unilateral symptoms: a report of two cases and review of the literature. Cardiovasc Surg. 1998;6(1):94–96. PMID: 9546853. doi:10.1016/s0967-2109(97)00095-1
- Klodell CT, Lobato EB, Willert JL, Gravenstein N. Oximetry-derived perfusion index for intraoperative identification of successful thoracic sympathectomy. Ann Thorac Surg. 2005;80(2):467–470. PMID: 16039187. doi:10.1016/j.athoracsur.2005.02.075
- Jeng EI, Gravenstein N, Klodell CT. Perfusion Index: an indicator of success during endoscopic thoracic sympathectomy for hyperhidrosis. Ann Thorac Surg. 2017;104(2):426–430. PMID: 28527965. doi:10.1016/j.athoracsur.2017.02.023
- Kuang J, Luo G, Tao J, et al. Risk factors affecting the outcomes of ct-guided radiofrequency thermocoagulation of thoracic sympathetic nerve in the treatment of primary palm hyperhidrosis. Pain Physician. 2022;25(8):E1219–E1228. PMID: 36375194.