Abstract
Objective
This retrospective study examines risk factors and electromyographic (EMG) characteristics associated with acquired weakness in critically ill patients and assesses their impact on patient prognosis.
Methods
Ninety-seven critically ill patients, ventilated for over 48 hours, were included. Patient data, encompassing general condition, medical history, Medical Research Council (MRC) scores, serum markers (c-reactive protein, calcitonin gene, albumin, brain natriuretic peptide, urea nitrogen, creatinine), EMG characteristics, respiratory treatment modalities, and parameters, were recorded. Mechanical ventilation duration, ICU stay duration, hospitalization duration, and patient prognosis were documented. Based on MRC scores, patients were categorized into the ICU-acquired weakness (ICU-AW) group (MRC <48 points) and the non-ICU-AW group (MRC ≥48 points).
Results
The study comprised 47 ICU-AW and 50 non-ICU-AW patients. Significant differences (p <0.05) were observed in age, MRC scores, albumin levels, c-reactive protein, calcitonin gene, brain natriuretic peptide, urea nitrogen, creatinine, mechanical ventilation duration, ICU stay duration, and hospitalization duration between groups. In the ICU-AW group, nerve conduction examinations revealed slow conduction velocity, reduced wave amplitude, and in severe cases, a complete loss of motor and sensory potentials. Multivariate logistic analysis identified low serum albumin levels and MRC scores as potential ICU-AW risk factors.
Conclusion
This study suggests that low serum albumin levels and MRC scores may contribute to ICU-AW risk. The ICU-AW group exhibited varied peripheral nerve damage and slow conduction velocities on EMG. Additionally, severe systemic inflammatory responses, renal function, brain natriuretic peptide levels, prolonged mechanical ventilation, and peripheral nerve damage may be associated with ICU-AW. Follow-up studies are essential for further understanding these complex interactions.
Introduction
Annually, millions of patients are admitted to intensive care units (ICUs),Citation1 making the enhanced recovery of ICU survivors a primary focus for both clinicians and researchers.Citation2,Citation3 Since the first case of quadriplegia secondary to mechanical ventilation was reported in 1977,Citation4 understanding of ICU-acquired weakness (ICU-AW) has gradually deepened. ICU-AW is defined as clinically detected weakness in critically ill patients with no plausible cause other than critical illness, with sequelae that may persist for months or even years after ICU discharge.Citation5–7 These sequelae include post-intensive care syndrome, which primarily manifests as persistent physical, mental, and cognitive impairments after discharge from the ICU.Citation8 ICU-AW is a common acute neuromuscular complication.Citation9 The reported incidence of ICU-AW varies widely, ranging from 7% to 76% of ICU patients, largely due to inconsistent diagnostic criteria, with an estimated average of approximately 40% in the general ICU population.Citation10 Patients with ICU-AW experience longer ICU stays and higher mortality rates compared to ICU patients who do not develop this condition.Citation11 Currently, there are no targeted therapies available to prevent or reverse the progression of ICU-AW, highlighting a critical and unmet need in the treatment of this condition.
ICU-AW has been classified into three main categories according to electrophysiological methods: critical illness polyneuropathy (CIP), critical illness myopathy (CIM), and critical illness neuromyopathy (CINM).Citation12 ICU-AW is usually generalized symmetrical and affects the limbs (often more proximally than distally) and respiratory muscles, whereas the facial and ocular muscles are not impaired,Citation13 with reduced muscle tone. Most diaphragms are affected, leading to prolonged mechanical ventilation time and difficulty weaning off the ventilator.Citation13
Given the complexity of ICU-AW, standard definitions and lists of histopathological features cannot be easily applied in clinical practice, and simple bedside examinations are difficult to diagnose. For example, it is widely accepted that quantitative assessment of peripheral muscle strength is the most important clinical basis for diagnosing ICU-AW, with the most commonly used assessment tool being the Medical Research Council (MRC) sum score.Citation14 However, this process requires patients to be awake and cooperative, making diagnosis difficult for those in sedated or delirious states.
Electromyography (EMG) is the gold standard for diagnosing ICU-AW.Citation15 A review reported the electromyographic characteristics of ICU-AW patients. Nerve conduction studies reveal reduced amplitudes of compound muscle action potentials (CMAPs) and sensory nerve action potentials (SNAPs), with CMAPs sometimes showing prolonged durations. Direct muscle stimulation responses may be diminished or absent. Needle electromyography can show nonspecific abnormal electrical activity, as well as reduced and prolonged motor unit potentials (MUPs).Citation16
However, EMG and nerve conduction studies are challenging to perform in many critically ill patients; the former requires the patient to be awake and able to contract muscles voluntarily; but, as altered mental status is common in ICU patients, they often cannot cooperate fully with the examination, while nerve conduction studies can be confounded by issues such as tissue edema.Citation16 Outside of the research setting, the diagnosis of acquired weakness in the intensive care unit based on formal electrophysiological criteria remains controversial.Citation16
In recent years, various imaging techniques have been widely applied in the diagnosis of ICU-AW. Evidence suggests that, compared to relying solely on clinical scores, imaging methods such as ultrasound, CT, and MRI can more accurately grade the severity of ICU-AW.Citation17,Citation18 However, these imaging techniques also have several limitations. Although ultrasound can quickly and repeatedly assess muscle quantity and quality at the bedside, it may underestimate muscle and protein loss.Citation18 Additionally, the effectiveness of ultrasound heavily depends on the operator’s experience, lacks standardized diagnostic criteria, and its clinical relevance is yet to be determined.Citation19 CT scans involve high doses of ionizing radiation. MRI, while having significant advantages in soft tissue imaging and being able to detect muscle cross-sectional area reduction and fat infiltration in advanced malnutrition patients, is costly and lacks portability, making its routine use in the ICU challenging.Citation17
Severe patients in the ICU represent a diverse population with varying clinical presentations, underlying diseases, and therapeutic interventions. Risk factors for ICU-AW may differ among different patient populations.Citation13,Citation20,Citation21 Conducting risk factor prediction studies can help take a more targeted approach, considering specific patient characteristics and identifying risk factors in particular subgroups. Some studiesCitation20–28 have indicated that higher disease scores, sepsis, inflammation, hyperglycemia, neuromuscular blocking agents (NMBA), acute kidney injury (AKI), multiorgan failure, longer duration of mechanical ventilation and ICU stay, and the use of certain antibiotics, such as aminoglycosides and vancomycin, are potential risk factors. When the duration of mechanical ventilation exceeds five days, the incidence of ICU-AW significantly increases.Citation29 Renal replacement therapy can lead to skeletal muscle dysfunction through various pathophysiological mechanisms, including alterations in amino acid and protein metabolism, inflammatory signaling, and the adverse removal of micronutrients. Patients with AKI and those receiving renal replacement therapy are more likely to develop ICU-AW.Citation27
While current research has identified several risk factors associated with ICU-AW, its pathophysiological mechanisms remain incompletely understood.Citation16 Partly due to the challenges of clinical research and ethical issues, it is difficult to conduct prospective, randomized controlled studies; for example, muscle biopsy, electromyography, and nerve conduction studies are challenging to perform in many critically ill patients.Citation30 Therefore, few studies have been conducted to analyze the severity of the ICU-AW patient’s condition, laboratory biochemical findings, muscle strength, electromyography, length of mechanical ventilation treatment, and prognosis in a comprehensive manner.Citation30
This retrospective clinical study aims to investigate the risk factors and electromyographic characteristics of acquired weakness in critically ill patients. It also assesses the impact of ICU-AW on the duration of mechanical ventilation, ICU stay, and patient outcomes. The goal is to identify ICU-AW risk factors, understand its underlying mechanisms, and help healthcare professionals recognize high-risk patients early in clinical practice.
Materials and Methods
A retrospective analysis study was performed to analyze critically ill patients admitted to the department of critical care medicine of the first hospital of Zhejiang University school of medicine between January 2016 and December 2021 who were mechanically ventilated for >48 hours. According to the MRC score,Citation14,Citation31 the patients were divided into an ICU-AW group (ICU-AW group, MRC <48 points) and a non-ICU-AW group (MRC≥48 points).
Inclusion Criteria
(1) Age over 18 years; (2) Patients who have been mechanically ventilated for >48 hours and who have undergone MRC scoring.
Exclusion Criteria
(1) Patients with previous generalized neuromuscular disease or with abnormal muscle strength (less than grade five in any limb); (2) Pregnancy; (3) Patients with a Glasgow score of less than fifteen; (4) Patients with pre-existing mental illness; (5) Patients with previous limb trauma resulting in limb muscle atrophy; (6) Age over 90 years.
Data Collection
In this study, we collected patients general information, medical history, acute physiology and chronic health status II (APACHE II) and sequential organ failure (SOFA) scores within 24 hours of admission to ICU, arm circumference, leg circumference, limb muscle strength, MRC scores during treatment, serum c-reactive protein (CRP), calcitoninogen (PCT), serum creatine kinase (CK), serum albumin (ALB), brain natriuretic peptide (BNP), renal function, respiratory treatment methods and treatment parameters. We recorded the time of mechanical ventilation, treatment in ICU, hospital stay, and prognosis.
EMG examination When applicable, electrical stimulation was performed to evaluate nerve conduction velocity and integrity. Square wave pulses were utilized, with voltage (100–400 mV), current (25–100 mA), and duration (0.05–1.0 ms) appropriately selected. The stimulus intensity was gradually escalated until the potential waveform plateaued, then increased by 20% to ensure supramaximal stimulation. These procedures were followed to measure the sensory or motor branches of the peroneal, tibial, median, ulnar, sural, superficial peroneal, superficial ulnar, and phrenic nerves.
Statistical Analysis
SPSS 20.0 was applied for statistical analysis. Continuous variables were first tested for normal distribution. Normally distributed variables were described as mean ± standard deviation using the t-test; non-normally distributed variables were described as median (quartiles) using the Mann-Whitney test. A Chi-Square test was applied to the count data to analyze differences and effects between variables. For linear continuous variables, Pearson correlation was chosen for inter-variate correlation analysis. A binary logistic regression analysis was performed to explore the risk factors for ICU-AW, using the occurrence of ICU-AW as the dependent variable and the factors of clinical significance identified in the univariate analysis as the independent variables.
Results
Comparison of Demographic Characteristics and Baseline Indicators Between the Two Groups of Patients
The differences between the ICU-AW and non-ICU-AW groups were not statistically significant in terms of sex and body mass index (BMI; p =0.970 and p =0.738, respectively), and the mean age of the ICU-AW group was significantly greater than that of the non-ICU-AW group (p =0.010). Both the APACHE II and SOFA scores were higher in the ICU-AW group than in the non-ICU-AW group (p <0.05). In terms of underlying diseases, patients in the ICU-AW group had a combination of hypertension, tumor, coronary heart disease, diabetes, chronic obstructive pulmonary disease (COPD), and chronic kidney disease in 21, 3, 8, 14, 12 and 4 cases, respectively, with 20 of them suffering from multiple chronic diseases. The non-ICU-AW group patients had hypertension, tumor, coronary heart disease, diabetes, COPD, and chronic kidney disease in 15, 13, 4, 11, 1 and 6 cases, respectively, with 10 cases suffering from multiple chronic diseases, as shown in .
Table 1 Demographic and Clinical Characteristics of the Patients at Baseline
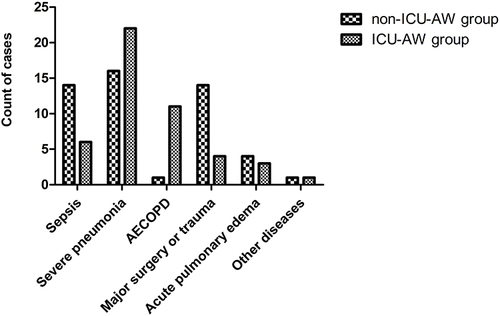
Reasons for Admission to the ICU
The causes of ICU admission in the ICU-AW group were severe pneumonia in 22 cases (46.81%), acute exacerbation of chronic obstructive pulmonary disease in 11 cases (23.40%), acute pulmonary edema in 3 cases (6.38%), sepsis in 6 cases (12.77%), major surgery and trauma in 4 cases (8.51%) and unexplained respiratory failure in 1 case (2.13%). The causes of ICU admission in the non-ICU-AW group were severe pneumonia in 16 cases (32.00%), acute exacerbation of chronic obstructive pulmonary disease in 1 case (2.00%), acute pulmonary edema in 4 cases (8.00%), sepsis in 14 cases (28.00%), major surgery and trauma in 14 cases (28.00%) and respiratory failure from other diseases in 1 case (2.00%) ().
Laboratory results
The serum ALB level in the ICU-AW group was lower than that in the non-ICU-AW group, and the levels of PCT, CRP, BUN, Cr, and BNP were all higher in the ICU-AW group than in the non-ICU-AW group. The differences were all statistically significant (p <0.01). The CK levels were higher in the ICU-AW group than in the non-ICU-AW group, but the difference was not statistically significant (p >0.05), .
Table 2 APACHE II and SOFA Scores, Limb Circumference, MRC-Scores, Laboratory Results and Prognosis
Electromyographic Features and MRC Score
The ICU-AW group exhibited significantly lower arm and leg circumferences compared to the non-ICU-AW group, with differences that were statistically significant (p<0.01) (). Additionally, the ICU-AW group had lower MRC scores than the non-ICU-AW group (). EMG was completed in 29 patients in the ICU-AW group, along with nerve conduction examinations. The nerve conduction examination in the ICU-AW group showed slow conduction velocity, reduced wave amplitude, and, in severe cases, loss of motor and sensory potentials, as shown in .
Table 3 Electromyographic Features (n=29)
Primary Outcome
In this study, the ICU-AW group had significantly longer durations of mechanical ventilation, ICU stay, and hospital stay compared to the non-ICU-AW group, with statistical significance (p < 0.01) (). Additionally, the ICU-AW group exhibited poorer clinical outcomes and a higher mortality rate compared to the non-ICU-AW group (p < 0.01) ().
Correlation Analysis
This study further analyzed whether there was a correlation between ICU-AW and the duration of mechanical ventilation, length of stay in the ICU, length of hospitalization, and prognosis. Ultimately, statistical analysis revealed significant differences between ICU-AW and time on mechanical ventilation, length of stay in the ICU, length of hospitalization, and prognosis (p =0.000, p =0.000, p =0.005, and p =0.002, respectively). Moreover, the duration of mechanical ventilation, ICU stay, total hospitalization period and prognosis all showed a positive correlation (R =0.548, R =0.656, R =0.283, and R =0.306, respectively), as shown in .
Table 4 Analysis of the Correlation Between ICU Acquired Weakness and Duration of Mechanical Ventilation, Duration of ICU Monitoring Treatment, Length of Stay and Prognosis
ICU-AW Risk Factor Analysis
In the clinic, the condition of critically ill patients is complex. Therefore, risk factors were further explored to facilitate the early detection and diagnosis of ICU-AW. A binary logistic regression analysis was performed using the occurrence of ICU-AW as the dependent variable and the statistically significant factors in the univariate analysis as the independent variables. The hypothesis was first tested by combining the significant variables from the one-way analysis of variance and performing a test for covariance, excluding independent variables with severe covariance (VIF ≥10). Finally, binary logistic regression analysis was performed with age, MRC score, admission APACHE II, admission SOFA, serum ALB, PCT, CRP, serum urea nitrogen, serum creatinine, BNP, duration of mechanical ventilation, length of ICU stay, and total length of stay as independent variables. The analysis showed that low MRC scores and low serum ALB levels were risk factors for ICU-AW and that CRP was a protective factor (p =0.000, p =0.001, and p =0.004, respectively), as shown in . Due to the numerous confounding factors associated with serum CRP in clinical practice, it was considered not clinically meaningful. Eventually, the lower MRC score and serum ALB level were identified as risk factors for ICU-AW.
Table 5 Logistic Regression Analysis of Risk Factors for ICU-Acquired Weakness
Discussion
In this study, a retrospective survey was conducted to comprehensively analyze and study the severity of illness, relevant biochemical findings, muscle strength, electromyography, duration of mechanical ventilation, and clinical prognosis of critically ill patients requiring mechanical ventilation in the ICU. Here, we compared the clinical data of patients in the ICU-AW group with those in the non-ICU-AW group and found that the APACHE II score and SOFA score on the first day of ICU admission were higher in the ICU-AW group than in the non-ICU-AW group, and the difference was statistically significant, suggesting that the severity of illness was positively correlated with the occurrence of ICU-AW. The APACHE II and SOFA scores are the most common scoring systems used in the ICU to measure the severity of illness, with higher scores indicating a more critical condition.Citation32 The high APACHE II and SOFA scores in the ICU-AW group suggest a serious condition with a more severe degree of hypercatabolism in the body, which may lead to a greater degree of skeletal muscle breakdown and more muscle loss.Citation33 Numerous studies have revealed that sepsis-induced ICU-AW is characterized by a significant reduction in muscle mass and muscle quality.Citation34 In critically ill patients, muscle biopsies frequently display pathological features such as inflammation, necrosis, fat infiltration, and fibrosis.Citation35 CK levels were mildly elevated in the ICU-AW group, with some cases showing significant increases due to trauma with skeletal myolysis, indicating muscle inflammation or necrosis. However, there was no significant difference in CK levels between the two groups. This lack of significant difference may be due to the slow rate of muscle breakdown or the relatively small sample size of our study. In addition, patients with high APACHE II and SOFA scores require mechanical ventilation for longer periods, higher doses of sedative drugs, longer periods of sedation, and limb braking, causing disuse muscle atrophyCitation13 and leading to CIM.Citation36
The patients in the ICU-AW group were found to have significantly higher BUN and Cr than those in the non-ICU-AW group. Patients with critical illnesses such as sepsis and severe pneumonia often suffer from acute renal impairment due to various causes, including systemic inflammatory responses, with significant increases in serum urea nitrogen and serum creatinine levels, reduced urine output, and retention of toxic metabolites and fluids in the body. The retention of toxic metabolites in the body can cause peripheral neuropathy, and patients with previous chronic kidney disease are also more likely to develop ICU-AW.Citation16,Citation37
Patients in the ICU-AW group had significantly lower serum albumin levels than those in the non-ICU-AW group, resulting in lower plasma colloid osmotic pressure. BNP indicates the body’s cardiac function and fluid load.Citation38 Patients in the ICU-AW group had a significantly higher BNP, possibly due to reduced cardiac function on the one hand and an increased fluid load on the other.Citation21 The superposition of these factors eventually led to muscle edema and reduced muscle strength in the ICU-AW group, resulting in ICU-AW.
Several studies have found that ICU-AW is not only a primary myopathy, a structural change in muscle, but may also be due to neuronal damage and axonal degeneration.Citation9,Citation39,Citation40 In this study, there were 47 patients with an MRC score of less than 48, and 29 of them completed EMG. A total of 18 patients in the ICU-AW group and all in the non-ICU-AW group failed to complete the EMG, either because they were too ill to go out for the examination or because their families refused. The patient’s EMG findings agreed with the degree of muscle strength loss and the degree of loss of limb circumference. All 29 patients showed neurogenic damage on EMG, with slow conduction velocity and reduced wave amplitude in peripheral nerves (including the phrenic nerve in some patients) and even undetectable motor and sensory potentials in some patients. These patients had no previous underlying neurological disease and no central nervous system damage, except for three patients who were associated with polytrauma. To reduce bias, EMG data from the injured limbs of these three trauma patients were excluded from the statistical analysis. Some studies have confirmed that diaphragmatic dysfunction is more common in patients with ICU-AW;Citation41,Citation42 this suggests that peripheral nerve injury, including the phrenic nerve, is also an important factor in ICU-AW. Because of the lack of EMG data in the non-ICU-AW group, EMG data were not included in the logistic regression analysis when building the predictive model for ICU-AW, which may lead to bias and limitations of this study.
In patients with sepsis, severe pneumonia, systemic inflammatory response, shock, microcirculatory disturbances, and mitochondrial dysfunction are usually present.Citation43 Microcirculatory changes include vasodilation and increased permeability, which allow leukocyte extravasation and tissue infiltration, local cytokine production, edema formation, and increased intercapillary distances.Citation35,Citation37 Nerve compression damage that may be caused by tissue edema needs to be clarified.Citation22 Impaired microcirculation leads to neuronal damage, axonal degeneration, and chronic membrane depolarization of terminal motor axons.Citation22 Mitochondrial dysfunction may affect energy production, leading to cellular energy deficiency. However, mitochondrial dysfunction in critical illness appears to be explained by impaired oxygen utilization due to direct mitochondrial damage further exacerbated by inflammation, hyperglycemia, and free radicals rather than impaired oxygen delivery.Citation22,Citation37 Mitochondrial dysfunction not only affects the energy supply but also amplifies the production of free radicals and reactive oxygen species, triggering a vicious cycle of macromolecular and organelle damage and causing peripheral nerve degeneration and muscle cell degeneration. One studyCitation44 confirmed E-selectin expression in the peripheral neurovascular endothelium of patients with severe polyneuropathy, suggesting the endothelial cell activation described in the sepsis model with microvascular leakage and an altered microvascular environment.Citation45 Hyperglycemia may exacerbate this problem by inducing neuronal mitochondrial dysfunction.Citation46,Citation47 In one trial, hyperglycemia in ICU patients was reported as an independent risk factor.Citation22 There is evidence that aggressive glycemic control can reduce the risk of severe disease polyneuropathy (and myopathy).Citation19,Citation48 In severe diseases such as sepsis, systemic inflammatory response, and shock, peripheral nerve damage and muscle cell degeneration may be induced by similar or unknown mechanisms, eventually leading to ICU-AW.
Additionally, we observed that patients in the ICU-AW group had significantly longer durations of mechanical ventilation, ICU stay, and overall hospital stay compared to the non-ICU-AW group. Mechanical ventilation represents a complex risk factor. The duration of mechanical ventilation before diagnosis is considered an independent risk factor for ICU-AW due to ventilator-induced diaphragmatic weakness and injury.Citation22,Citation49 Additionally, ICU-AW has been shown to prolong the duration of mechanical ventilation.Citation50,Citation51 Consequently, this can easily create a vicious cycle. Our study also found that the ICU-AW group experienced significantly higher morbidity and mortality rates. Prolonged ICU and total hospital stays further indicate increased hospitalization costs.
In the preliminary phase of this study, a review of existing research and literature on ICU-AW was conducted. Previous studiesCitation20–24 exploring the risk factors associated with ICU-AW have identified several key factors. These findings provide a foundation for developing predictive models and help identify potential variables. The results of these studies are crucial for creating effective predictive tools and intervention strategies. This study employed statistical methods such as logistic regression modeling to systematically collect clinical data, incorporating potential risk factors as variables in the analysis. The aim was to identify meaningful and predictive risk factors for ICU-AW and attempt to develop a predictive model for its occurrence.
This study identified low ALB level as a risk factor for ICU-AW. Although there are currently no targeted treatments for ICU-AW, early interventions such as neuromuscular electrical stimulation and other rehabilitation therapies may help prevent or mitigate ICU-AW during hospitalization.Citation52,Citation53 Additionally, factors such as the duration of mechanical ventilation, parenteral nutrition, hyperglycemia, steroid use, and medication can be partially managed and controlled.Citation30 The low ALB level identified in our study is a modifiable risk factor. Increased attention from clinical staff and timely albumin supplementation may help reduce the incidence and severity of ICU-AW.
In summary, this study aims to improve our understanding of the underlying mechanisms of ICU-AW. By assisting healthcare professionals in early identification of high-risk ICU-AW patients in clinical practice, we hope to enable timely interventions that enhance patient outcomes.
Limitations and Recommendations
There are still some limitations in this study: (1) the sample size is small; therefore, the results may be biased; (2) although the diseases of the enrolled patients were all common critical illnesses in the ICU, there may be significant differences in the diseases of the patients between the two groups, which may affect the study results; and (3) some trauma and surgical patients were included in this study. Although the EMG data of the trauma side of the limb were excluded from the statistical analysis, it still cannot be excluded that trauma and limb surgery impacted the study results. (4) Due to the critical condition of some patients and other reasons, not all participants completed EMG examinations, leading to the exclusion of EMG data from the binary logistic regression analysis. (5) In this study, due to the critical condition and complexity of the patients, it was not possible to analyze the impact of medications and underlying diseases on the development of acquired muscle weakness. (6) In this study, there was a difference in age between the two groups, which may have caused bias, and it is proposed to increase the sample size in subsequent studies to eliminate bias. In subsequent studies, we will further investigate the effects of factors such as drugs on the occurrence of ICU-AW, and we propose to reduce bias by increasing the sample size and extending the duration of the study.
Conclusions and Future Perspectives
In summary, the risk factors for ICU-AW may be related to low serum ALB levels and MRC scores. The ICU-AW group exhibited varied peripheral nerve damage and slow conduction velocities on EMG. However, it may also be associated with a severe systemic inflammatory response, renal function, BNP, prolonged mechanical ventilation, and peripheral nerve damage, which needs to be further confirmed by follow-up studies. ICU-AW significantly prolongs the duration of patient treatment with mechanical ventilation, the duration of supervised treatment in the ICU, and the hospitalization period, seriously affecting the patient’s prognosis. Subsequently, we need to conduct a prospective study with a larger sample size and duration of the study to establish a predictive model for ICU-AW and further validate the predictive model using large data. The ultimate goal is to provide early diagnosis and intervention for patients with ICU-AW, enhance treatment efficacy, and improve quality of life.
Ethics Approval and Informed Consent
This study was conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and its amendments, and the protocol was approved by the Clinical Research Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine (No. 2023-0323). Due to the retrospective nature of the study, the requirement for written informed consent was waived, and patient information was anonymized before analysis. All procedures were carried out in accordance with institutionally approved protocols.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
Acknowledgments
The study was supported by various colleagues from the hospital’s Department of Critical Care Medicine and Department of Orthopedics.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Abid H, Ryan ZC, Delmotte P, Sieck GC, Lanza IR. Extramyocellular interleukin‐6 influences skeletal muscle mitochondrial physiology through canonical JAK/STAT signaling pathways. FASEB J. 2020;34(11):14458–14472. doi:10.1096/fj.202000965RR
- Mignemi NA, McClatchey PM, Kilchrist KV, et al. Rapid changes in the microvascular circulation of skeletal muscle impair insulin delivery during sepsis. Am J Physiol Endocrinol Metab. 2019;316(6):E1012–E1023. doi:10.1152/ajpendo.00501.2018
- Azoulay E, Vincent J-L, Angus DC, et al. Recovery after critical illness: putting the puzzle together—a consensus of 29. Critical Care. 2017;21(1). doi:10.1186/s13054-017-1887-7
- Macdonald JB. Severe Myopathy after Status Asthmaticus. Lancet. 1977;(8040):310. doi:10.1016/s0140-6736(77)90533-5
- Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794. doi:10.1001/jama.2010.1553
- Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–1882. doi:10.1016/S0140-6736(09)60658-9
- Herridge MS, Tansey CM, Matte A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304. doi:10.1056/NEJMoa1011802
- Voiriot G, Oualha M, Pierre A, et al. Chronic critical illness and post-intensive care syndrome: from pathophysiology to clinical challenges. Ann Intens Care. 2022;12(1). doi:10.1186/s13613-022-01038-0
- Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol. 2011;10(10):931–941. doi:10.1016/S1474-4422(11)70178-8
- Appleton RTD, Kinsella J, Quasim T. The incidence of intensive care unit-acquired weakness syndromes: a systematic review. J Intensive Care. 2014;16(2):126–136. doi:10.1177/1751143714563016
- Moisey LL, Mourtzakis M, Cotton BA, et al. Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Critical Care. 2013;17(5). doi:10.1186/cc12901
- Stevens RD, Marshall SA, Cornblath DR, et al. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med. 2009;37(10 Suppl):S299–S308. doi:10.1097/CCM.0b013e3181b6ef67
- Piva S, Fagoni N, Latronico N. Intensive care unit-acquired weakness: unanswered questions and targets for future research. F1000Res. 2019;8:508. doi:10.12688/f1000research.17376.1
- Latronico N, Herridge M, Hopkins RO, et al. The ICM research agenda on intensive care unit-acquired weakness. Intensive Care Med. 2017;43(9):1270–1281. doi:10.1007/s00134-017-4757-5
- Zorowitz RD. ICU-acquired weakness: a rehabilitation perspective of diagnosis, treatment, and functional management. Chest. 2016;150(4):966–971. doi:10.1016/j.chest.2016.06.006
- Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. N Engl J Med. 2011;370(17):1626–1635. doi:10.1056/NEJMra1209390
- Mercuri E, Pichiecchio A, Allsop J, Messina S, Pane M, Muntoni F. Muscle MRI in inherited neuromuscular disorders: past, present, and future. J Magn Reson Imaging. 2007;25(2):433–440. doi:10.1002/jmri.20804
- Hernández-Socorro CR, Saavedra P, López-Fernández JC, Ruiz-Santana S. Assessment of muscle wasting in long-stay ICU patients using a new ultrasound protocol. Nutrients. 2018;10(12):1849. doi:10.3390/nu10121849
- Kelmenson DA, Quan D, Moss M. What is the diagnostic accuracy of single nerve conduction studies and muscle ultrasound to identify critical illness polyneuromyopathy: a prospective cohort study. Crit Care. 2018;22(1):342. doi:10.1186/s13054-018-2281-9
- Yang T, Li Z, Jiang L, Wang Y, Xi X. Risk factors for intensive care unit-acquired weakness: a systematic review and meta-analysis. Acta Neurol Scand. 2018;138(2):104–114. doi:10.1111/ane.12964
- Jolley SE, Bunnell AE, Hough CL. ICU-acquired weakness. Chest. 2016;150(5):1129–1140. doi:10.1016/j.chest.2016.03.045
- De Jonghe B, Sharshar T, Lefaucheur JP, et al. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288(22):2859–2867. doi:10.1001/jama.288.22.2859
- Chlan LL, Tracy MF, Guttormson J, Savik K. Peripheral muscle strength and correlates of muscle weakness in patients receiving mechanical ventilation. Am J Crit Care. 2015;24(6):e91–e98. doi:10.4037/ajcc2015277
- National Heart L, Blood Institute PCTN, Moss M, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380(21):1997–2008. doi:10.1056/NEJMoa1901686
- Camdessanche JP. End-plate disorders in intensive care unit. J Clin Neurophysiol. 2020;37(3):211–213. doi:10.1097/WNP.0000000000000659
- Ohbe H, Endo H, Kumasawa J. Characteristics of COVID-19 in multicenter ICUs in Japan. J Anesth. 2021;36(4):572–573. doi:10.1007/s00540-021-03028-1
- Teixeira JP, Mayer KP, Griffin BR, et al. Intensive Care unit–acquired weakness in patients with acute kidney injury: a contemporary review. Am J Kidney Dis. 2023;81(3):336–351. doi:10.1053/j.ajkd.2022.08.028
- Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi:10.1056/NEJMoa011300
- Polastri M, Oldani S, Pisani L, Nava S. Elastic band exercises for patients with intensive care unit-acquired weakness: a case report. Tanaffos. 2018;17(2):132–137.
- Tortuyaux R, Davion JB, Jourdain M. Intensive care unit-acquired weakness: questions the clinician should ask. Rev Neurol. 2022;178(1–2):84–92. doi:10.1016/j.neurol.2021.12.007
- Kleyweg RP, van der Meche FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barre syndrome. Muscle Nerve. 1991;14(11):1103–1109. doi:10.1002/mus.880141111
- Hai PD, Viet Hoa LT. The prognostic accuracy evaluation of mNUTRIC, APACHE II, SOFA, and SAPS 2 scores for mortality prediction in patients with sepsis. Crit Care Res Pract. 2022;2022:4666594. doi:10.1155/2022/4666594
- Van den Berghe G. On the neuroendocrinopathy of critical illness. Perspectives for feeding and novel treatments. Am J Respir Crit Care Med. 2016;194(11):1337–1348. doi:10.1164/rccm.201607-1516CI
- Callahan LA, Supinski GS. Sepsis-induced myopathy. Crit Care Med. 2009;37(10 Suppl):S354–S367. doi:10.1097/CCM.0b013e3181b6e439
- Derde S, Hermans G, Derese I, et al. Muscle atrophy and preferential loss of myosin in prolonged critically ill patients. Crit Care Med. 2012;40(1):79–89. doi:10.1097/CCM.0b013e31822d7c18
- Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591–1600. doi:10.1001/jama.2013.278481
- Friedrich O, Reid MB, Van den Berghe G, et al. The sick and the weak: neuropathies/myopathies in the critically ill. Physiol Rev. 2015;95(3):1025–1109. doi:10.1152/physrev.00028.2014
- Hall C. Essential biochemistry and physiology of (NT-pro)BNP. Eur J Heart Fail. 2004;6(3):257–260. doi:10.1016/j.ejheart.2003.12.015
- Latronico N, Friedrich O. Electrophysiological investigations of peripheral nerves and muscles: a method for looking at cell dysfunction in the critically ill patients. Crit Care. 2019;23(1):33. doi:10.1186/s13054-019-2331-y
- Batt J, Herridge MS, Dos Santos CC. From skeletal muscle weakness to functional outcomes following critical illness: a translational biology perspective. Thorax. 2019;74(11):1091–1098. doi:10.1136/thoraxjnl-2016-208312
- Dres M, Goligher EC, Heunks LMA, Brochard LJ. Critical illness-associated diaphragm weakness. Intensive Care Med. 2017;43(10):1441–1452. doi:10.1007/s00134-017-4928-4
- Supinski GS, Westgate P, Callahan LA. Correlation of maximal inspiratory pressure to transdiaphragmatic twitch pressure in intensive care unit patients. Crit Care. 2016;20(1):77. doi:10.1186/s13054-016-1247-z
- Lelubre C, Vincent JL. Mechanisms and treatment of organ failure in sepsis. Nat Rev Nephrol. 2018;14(7):417–427. doi:10.1038/s41581-018-0005-7
- Bolton CF. Neuromuscular manifestations of critical illness. Muscle Nerve. 2005;32(2):140–163. doi:10.1002/mus.20304
- Hanna JS. Sarcopenia and critical illness: a deadly combination in the elderly. JPEN J Parenter Enteral Nutr. 2015;39(3):273–281. doi:10.1177/0148607114567710
- Fenzi F, Latronico N, Refatti N, Rizzuto N. Enhanced expression of E-selectin on the vascular endothelium of peripheral nerve in critically ill patients with neuromuscular disorders. Acta Neuropathol. 2003;106(1):75–82. doi:10.1007/s00401-003-0704-3
- Rumora AE, Savelieff MG, Sakowski SA, Feldman EL. Disorders of mitochondrial dynamics in peripheral neuropathy: clues from hereditary neuropathy and diabetes. Int Rev Neurobiol. 2019;145:127–176. doi:10.1016/bs.irn.2019.05.002
- Van den Berghe G, Schoonheydt K, Becx P, Bruyninckx F, Wouters PJ. Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology. 2005;64(8):1348–1353. doi:10.1212/01.WNL.0000158442.08857.FC
- Jaber S, Petrof BJ, Jung B, et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med. 2011;183(3):364–371. doi:10.1164/rccm.201004-0670OC
- Garnacho-Montero J, Amaya-Villar R, García-Garmendía JL, Madrazo-Osuna J, Ortiz-Leyba C. Effect of critical illness polyneuropathy on the withdrawal from mechanical ventilation and the length of stay in septic patients*. Crit Care Med. 2005;33(2):349–354. doi:10.1097/01.Ccm.0000153521.41848.7e
- Hermans G, Van Mechelen H, Clerckx B, et al. Acute outcomes and 1-year mortality of intensive care unit-acquired weakness. A cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2014;190(4):410–420. doi:10.1164/rccm.201312-2257OC
- Burke D, Gorman E, Stokes D, Lennon O. An evaluation of neuromuscular electrical stimulation in critical care using the ICF framework: a systematic review and meta-analysis. Clin Respir J. 2016;10(4):407–420. doi:10.1111/crj.12234
- Zayed Y, Kheiri B, Barbarawi M, et al. Effects of neuromuscular electrical stimulation in critically ill patients: a systematic review and meta-analysis of randomised controlled trials. Aust Crit Care. 2020;33(2):203–210. doi:10.1016/j.aucc.2019.04.003