Abstract
Background
Neutropenia is a common toxicity in patients receiving myelosuppressive chemotherapy. In this prospective pilot study, we compared the efficacy and safety profiles of pegfilgrastim administered subcutaneously once per cycle and lenograstim administered subcutaneously daily six times per cycle, for primary neutropenia prophylaxis in women with breast cancer receiving adjuvant anthracycline-based chemotherapy.
Materials and methods
Twenty women were enrolled. All patients received epirubicin 100 mg/m2 with 5-fluorouracil 500 mg/m2 and cyclophosphamide 500 mg/m2 on day 1 and every 21 days thereafter, according to the FEC 100 chemotherapy regimen. Eight patients received a single dose of pegfilgrastim on day 2, while 12 patients were treated with daily administration of lenograstim from days five to ten. Absolute neutrophil count and duration of grade 3–4 neutropenia were monitored using seriated blood samples. The incidence of bone pain was evaluated using the visual analog scale (VAS).
Results
The incidence of grade 3–4 neutropenia was 75% in patients who received pegfilgrastim, and 25% in patients who received lenograstim. One case of febrile neutropenia was shown in pegfilgrastim patients. The mean duration of grade 3–4 neutropenia was 2 days in pegfilgrastim group versus 1.4 days in the lenograstim group. Bone pain was present in 37.5% of pegfilgrastim patients versus 58.3% of lenograstim patients. The mean duration of bone pain in the pegfilgrastim group was 4 days versus 6 days in the lenograstim group.
Conclusion
In our experience, a single injection of pegfilgrastim was less effective for controlling neutropenia than six daily injections of lenograstim. The safety profiles of pegfilgrastim and lenograstim were similar with a lower incidence of bone pain in patients treated with pegfilgrastim.
Introduction
Neutropenia is a frequent and serious complication in patients treated with myelosuppressive chemotherapy. The grade and primarily the duration of neutropenia determine the risk of infection which results in the need to reduce the dose and to delay treatment, compromising antineoplastic treatment efficacy and the patient’s prognosis.Citation1–Citation3 Moreover, febrile neutropenia (FN), despite the progress of antibiotic therapy, is still considered a medical emergency associated with significant morbidity, mortality, and high related costs.Citation4 In patients receiving chemotherapy, the use of primary prophylaxis with granulocyte-colony stimulating factor (G-CSF) reduces the duration, severity, and incidence of neutropenia because these agents are able to regulate the production and release of neutrophils from the bone marrow.Citation5,Citation6 The indications for the use of primary prophylaxis with G-CSF depend on the risk of FN which is related to the aggressiveness and spread of the tumor, the type of chemotherapeutic regimen used, and the characteristics of the patients. Several randomized clinical trials have demonstrated a significant reduction in the risk of FN with prophylactic use of G-CSF in patients subjected to conventional chemotherapy.Citation2,Citation7–Citation12 There are three forms of recombinant G-CSF: filgrastim, lenograstim, and pegfilgrastim.Citation13,Citation14
Filgrastim is a recombinant methionyl non-glycosylated form of human G-CSF produced in Escherichia coli, whereas lenograstim is derived from Chinese hamster ovary (CHO) cells and glycosylated. Both require a daily administration to maintain their therapeutic effects because of their short circulating half-life (~3.5 hours). Therefore, a sustained duration form of filgrastim, pegfilgrastim, was developed.
The elimination of filgrastim and lenograstim occurs through renal excretion and by specific neutrophil regulatory mechanisms.Citation15,Citation16 Pegfilgrastim, due to PEGylation, has a reduced renal clearance and a long plasma half-life (~33 hours); therefore, this characteristic extends its pharmacological effect.
Moreover, pegfilgrastim acts on hematopoietic cells by binding to specific cell surface receptors thereby stimulating proliferation, differentiation, commitment, and end cell functional activation thus allowing a single administration of the drug per chemotherapeutic cycle.Citation17,Citation18 Several clinical trials involving patients with breast cancer undergoing chemotherapy have compared the efficacy of pegfilgrastim with filgrastim.Citation11,Citation18–Citation20 These studies have shown that the incidence of neutropenia, both mild and severe (G3–G4), is not different between filgrastim and pegfilgrastim and consequently the efficacy of the two drugs is similar.
The most frequent side effect of G-CSF is bone pain, mainly due to the increase in mass of bone marrow and to the release of pro-inflammatory cytokines. Its incidence varies from 15% to 39% of treated patients.Citation2,Citation21 Some studies have compared the incidence of bone pain between PEGylated and non-PEGylated formulations of G-CSF. These clinical trials have shown, especially in breast cancer patients, that there is no statistically significant difference in the incidence of bone pain between filgrastim and pegfilgrastim.Citation11,Citation18,Citation22 However, some of these studies have demonstrated that the incidence of bone pain varies between the first and the last cycle of chemotherapy.
All the above cited studies enrolled patients with breast cancer undergoing combination chemotherapy with anthracyclines and taxanes. To our knowledge, no trial has ever compared PEGylated and not-PEGylated formulations of G-CSF in terms of efficacy and toxicity in primary prophylaxis in patients receiving epirubicin 100 mg/m2 with 5-fluorouracil 500 mg/m2 and cyclophosphamide 500 mg/m2 on day 1 and every 21 days thereafter (FEC 100).
Patients and methods
Twenty female patients (mean age 54 years) were enrolled following the inclusion and exclusion criteria listed in . All patients were evaluated in terms of treatment efficacy and toxicity. All patients gave signed consent to participate in the study and for the treatment of personal data. Twelve patients received primary prophylaxis with lenograstim and eight patients with pegfilgrastim. Patient characteristics are listed in . The measurement of clinical pain intensity was made using the visual analog scale (VAS).
Table 1 Inclusion and exclusion criteria
Table 2 Demographic and medical characteristics of patients
Study design
This study investigated whether a single dose per cycle of pegfilgrastim is as safe and effective as multiple doses per cycle of lenograstim, in patients receiving their first cycle of adjuvant chemotherapy for breast cancer according to the FEC 100 regimen (epirubicin 100 mg/m2 with 5-fluorouracil 500 mg/m2 and cyclophosphamide 500 mg/m2 on day 1 and every 21 days thereafter). Patients were assigned to one of two G-CSF administrations: a single subcutaneous injection containing a fixed dose of 6 mg pegfilgrastim on day 2 or six daily subcutaneous injections of lenograstim (263 μg) from day 5 to day 10, after administration of chemotherapy.
Seriated blood samples for determination of complete blood count with differential were performed on days 1, 8, 10, and 12, continuing daily until a rise of absolute neutrophil count (ANC) ≥1.0 × 109/L after the expected nadir. The patients’ oral temperature was recorded daily and all patients were monitored for occurrence of adverse events during the study.
Objectives
The primary endpoint was to compare the incidence and duration of G3–G4 neutropenia (defined as ANC <0.5 × 109/L) in the two groups of treatment during the first cycle of chemotherapy. Secondary endpoints were to assess the incidence of FN, hospitalization rate and the incidence, duration, and intensity of bone pain between the two treatment groups.
Results
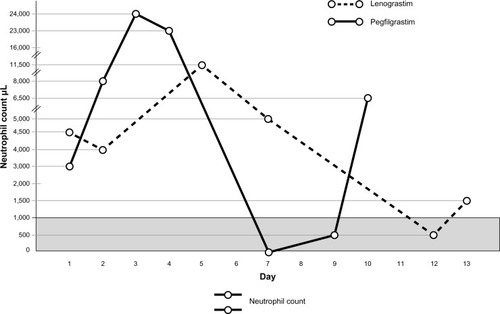
In those patients treated with lenograstim, the overall incidence of neutropenia was 41.6% and severe neutropenia (G3–G4) occurred in 25% (8.3% neutropenia G3 and 16.7% neutropenia G4) of patients. The mean duration of severe neutropenia (G3–G4) was 1.4 days. No patient experienced FN, defined as ANC <0.5 × 109/L and an oral temperature ≥38.2°C.
In those patients treated with pegfilgrastim we detected an overall incidence of severe neutropenia of 75% (12.5% neutropenia G3 and 62.5% neutropenia G4), while its mean duration was 2 days. One patient showed FN, which was treated with the administration of oral antibiotics. Hospitalization of patients or use of intravenous antibiotic therapy was not necessary in any cases ().
Figure 1 Neutrophil count in pegfilgrastim and lenograstim groups after first cycle of chemotherapy.
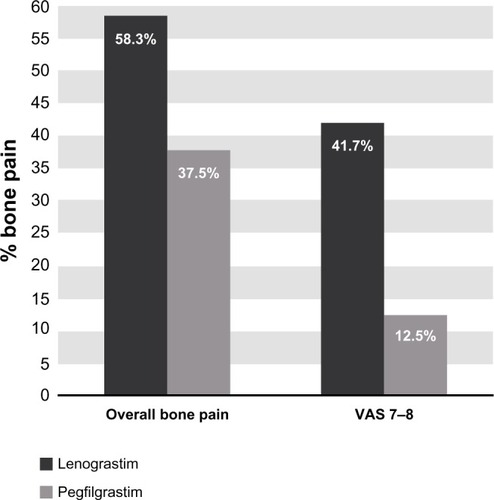
In regards to the emergence of bone pain as the most common side effect, in patients treated with lenograstim we found an incidence of 58.3%, specifically VAS 3–4 in 16.7% of patients and VAS 7–8 in 41.7% of patients. The average duration of bone pain was 6 days.
In patients treated with pegfilgrastim, bone pain was present in 37.5%. The average duration of bone pain was 4 days ().
Figure 2 Incidence of bone pain in lenograstim and pegfilgrastim groups.
A summary of our data regarding neutropenia and bone pain in the lenograstim group and pegfilgrastim group is listed in .
Table 3 Incidence of neutropenia and bone pain in lenograstim group and pegfilgrastim group
Discussion
In patients receiving chemotherapy, the use of G-CSF for primary prophylaxis reduces the duration and severity of neutropenia and its related complications.Citation5,Citation6
Currently, we have two types of granulocyte colony-stimulating factors: G-CSF in non-PEGylated formulations (lenograstim, filgrastim) and G-CSF in a PEGylated formulation (pegfilgrastim).
Our study compared the efficacy and side effects of lenograstim and pegfilgrastim, administered as primary prophylaxis in patients with breast cancer subjected to adjuvant chemotherapy according to the FEC 100 regimen.
The results showed that among patients treated with pegfilgrastim there was an incidence of severe neutropenia (G4) of 62.5% with a median duration of neutropenia of 2 days versus an incidence of 16.7% with an average duration of 1.4 days in the group treated with lenograstim. However, this difference did not result in an increase of the hospitalization rate of patients and in the pegfilgrastim group only one patient developed an episode of FN.
In the studies by Holmes et alCitation18,Citation20 and Green et al,Citation11 the incidence of severe neutropenia G4 in patients receiving primary prophylaxis with pegfilgrastim during the first cycle of chemotherapy was between 74% and 84%, with an average duration between 1.3 and 1.8 days. These results are essentially the same as reported in our study. The lower incidence of neutropenia G4 (62.5%) in our study can be explained by the different chemotherapy scheme used: generally, FEC 100 presents a lower incidence of neutropenia than a taxane/anthracycline combination such as doxorubicin and docetaxel, which was used by Holmes et alCitation18,Citation20 and Green et alCitation11 in the above mentioned studies.
Regarding the non-PEGylated G-CSF, Holmes et alCitation18,Citation20 and Green et alCitation11 found the incidence of severe neutropenia (G4) ranging between 76% and 83%, with an average duration between 1.6 and 1.8 days. However, in our study, the incidence of neutropenia G4 in patients treated with lenograstim was only 16.7% (mean duration 1.4 days), which is substantially lower compared to the data provided by Holmes et alCitation18,Citation20 and Green et al.Citation11 As discussed above, this discrepancy may be caused by the different chemotherapy schemed used (FEC 100 versus taxane/anthracycline). It is noteworthy that in the works by Holmes et alCitation18,Citation20 and Green et al,Citation11 an average number of eleven doses of filgrastim were administered, whereas in our study only six doses of lenograstim were administered. To date, there are no studies in literature comparing the efficacy of lenograstim versus pegfilgrastim with triplet chemotherapy FEC 100. In clinical trials using the adjuvant FEC 100 scheme in breast tumors, a steady supply of growth factors as primary prophylaxis was never provided, and therefore it is not possible to know exactly the efficacy of growth factors to prevent neutropenia in this setting. In some cases, G-CSF was administered as secondary prophylaxis or in other cases its use was banned during the course of the study.Citation23,Citation24
Regarding bone pain, in the lenograstim group there was a greater incidence of this side effect compared to pegfilgrastim group (58.3% versus 37.5%, respectively), with a longer duration (6 versus 4 days, respectively); this difference was more evident when considering severe pain with VAS 7–8 (lenograstim: 41.7% versus pegfilgrastim: 12.5%), which, however, was well controlled in all cases with non-steroidal anti-inflammatory drugs.
Some studies have shown that the incidence of bone pain was comparable between the G-CSF and PEGylated G-CSF, with an incidence around 30%–40% for both growth factors.Citation11,Citation18
More precisely, in the study by Green et al, the incidence of bone pain was 42% and 37% with filgrastim and pegfilgrastim, respectively, similar to our data, with a tendency toward a higher incidence in the filgrastim group.
In the study by Holmes et al,Citation18,Citation20 the incidence of bone pain was lower (25% in the pegfilgrastim group versus 26% in the filgrastim group) and comparable between the two growth factors.
However, in the two above mentioned works, the assessment of bone pain was not among the main endpoints of the study; thus, it is not possible to give a concrete explanation for the differences in the incidence of bone pain.
In contrast, a study by Kubista et alCitation22 investigated as a primary end point the assessment of bone pain in patients who were treated with the two growth factors with detailed data collection for each cycle of chemotherapy. Kubista et al,Citation22 specifically analyzed the incidence of bone pain for each chemotherapy cycle, highlighting how this effect tends to decline from the first cycle of treatment (filgrastim 34.2% versus 29.1% pegfilgrastim) to the last cycle (16.9% in both groups) for both growth factors and how in the filgrastim group bone pain was mainly present in the first few cycles but eventually overlapped with the pegfilgrastim group in the last few cycles. The study by Kubista et al,Citation22 compared filgrastim versus pegfilgrastim usage at the same standard doses as used in our study. The results showed an incidence of bone pain of 42.8% in the filgrastim group versus 36.7% in the pegfilgrastim group, with a non-statistically significant trend towards an increased incidence in the filgrastim group. In this respect, the results of this study coincide with ours.
In contrast to the study by Kubista et al,Citation22 in our study we evaluated bone pain only in the first cycle of chemotherapy. However, similarly to the aforementioned study, we found a higher incidence of bone pain in the non-PEGylated G-CSF lenograstim group (58.3%) compared to the PEGylated G-CSF pegfilgrastim group (37.5%). In our experience, the incidence of bone pain with non-PEGylated G-CSF (58.3%) is slightly greater than that shown in other studies, while the incidence of bone pain in the pegfilgrastim group is comparable.
In conclusion, in our experience, although this study was carried out on a small sample of patients, six doses of lenograstim were sufficient to reduce the risk of neutropenia in primary prophylaxis with chemotherapy FEC 100 and we found no necessity for a greater number of injections which is suggested by some guidelines.Citation25–Citation27 Furthermore, we found that a single injection of pegfilgrastim, which has a higher cost, was less effective in controlling neutropenia than six daily administrations of lenograstim.
However, in other studies it was demonstrated that in breast cancer patients treated with anthracycline-based (without taxanes) adjuvant chemotherapy even a smaller number of doses of non-PEGylated G-CSF is sufficient to reduce the risk of neutropenia.Citation28
Other studies investigating chemotherapy in other tumors besides breast cancer (including lung, ovarian, colorectal, and lymphoma) demonstrated that five doses of non-PEGylated G-CSF were effective in controlling the risk of neutropenia.Citation29–Citation31
With regard to the timing of administration of G-CSF, it should start 4–6 days before nadir, as was done in our study.Citation32
For bone pain, our study seems to confirm literature data; particularly that there is a trend towards an increased incidence with lenograstim, but essentially there is no difference in clinical relevance when comparison to pegfilgrastim.
Finally, we deem that further studies are required to determine the most efficient G-CSF and the most appropriate administration scheme, also in terms of cost/benefit, when used along with the FEC 100 regimen, considering that this chemotherapy protocol has been increasingly spreading in clinical practice and it is accompanied by a high risk of FN.
Disclosure
The authors report no conflicts of interest in this work.
References
- MorstynGCampbellLSouzaLMEffect of granulocyte colony stimulating factor on neutropenia induced by cytotoxic chemotherapyLancet1988185876676722895212
- CrawfordJOzerHStollerRReduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancerN Engl J Med199132531641701711156
- Trillet-LenoirVGreenJManegoldCRecombinant granulocyte colony stimulating factor reduces the infectious complications of cytotoxic chemotherapyEur J Cancer199329A33193247691119
- KudererNMDaleDCCrawfordJLymanGHImpact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic reviewJ Clin Oncol200725213158316717634496
- HeuserMGanserABokemeyerCAmerican Society of Clinical OncologyNational Comprehensive Cancer NetworkEuropean Organization for Research and Treatment of CancerUse of colony-stimulating factors for chemotherapy-associated neutropenia: review of current guidelinesSemin Hematol200744314815617631179
- LocatelliFPedrazzoliPRecombinant human G-CSF: how wide is the field of clinical applicability?Haematologica19958031992057545634
- Trillet-LenoirVGreenJManegoldCRecombinant granulocyte colony stimulating factor reduces the infectious complications of cytotoxic chemotherapyEur J Cancer199329A33193247691119
- GabriloveJLJakubowskiAScherHEffect of granulocyte colony-stimulating factor on neutropenia and associated morbidity due to chemotherapy for transitional-cell carcinoma of the urotheliumN Engl J Med198831822141414222452983
- VogelCLWojtukiewiczMZCarrollRRFirst and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III studyJ Clin Oncol20052361178118415718314
- Timmer-BonteJNAdangEMSmitHJCost-effectiveness of adding granulocyte colony-stimulating factor to primary prophylaxis with antibiotics in small-cell lung cancerJ Clin Oncol200624192991299716682725
- GreenMDKoelblHBaselgaJInternational Pegfilgrastim 749 Study GroupA randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapyAnn Oncol2003141293512488289
- VoseJMCrumpMLazarusHRandomized, multicenter, open-label study of pegfilgrastim compared with daily filgrastim after chemotherapy for lymphomaJ Clin Oncol200321351451912560443
- HaoYChenJWangXZhuHRongZEffects of site-specific polyethylene glycol modification of recombinant human granulocyte colony-stimulating factor on its biologic activitiesBioDrugs200620635736217176123
- HonjoETamadaTMaedaYCrystallization of a 2:2 complex of granulocyte-colony stimulating factor (GCSF) with the ligand-binding region of the GCSF receptorActa Crystallogr Sect F Struct Biol Cryst Commun200561Pt 8788790
- MolineuxGKinstlerOBriddellBA new form of Filgrastim with sustained duration in vivo and enhanced ability to mobilize PBPC in both mice and humansExp Hematol199927121724173410641590
- LaytonJEHockmanHSheridanWPMorstynGEvidence for a novel in vivo control mechanism of granulopoiesis: mature cell-related control of a regulatory growth factorBlood1989744130313072475185
- JohnstonECrawfordJBlackwellSRandomized, dose-escalation study of SD/01 compared with daily filgrastim in patients receiving chemotherapyJ Clin Oncol200018132522252810893282
- HolmesFAO’ShaughnessyJAVukeljaSBlinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancerJ Clin Oncol200220372773111821454
- von MinckwitzGKümmelSdu BoisAGerman Breast GroupPegfilgrastim ± ciprofloxacin for primary prophylaxis with TAC (docetaxel/doxorubicin/cyclophosphamide) chemotherapy for breast cancer. Results from the GEPARTRIO studyAnn Oncol200819229229817846019
- HolmesFAJonesSEO’ShaughnessyJComparable efficacy and safety profiles of once-per-cycle pegfilgrastim and daily injection filgrastim in chemotherapy-induced neutropenia: a multicenter dose-finding study in women with breast cancerAnn Oncol200213690390912123336
- PettengellRGurneyHRadfordJAGranulocyte colony-stimulating factor to prevent dose-limiting neutropenia in non-Hodgkin’s lymphoma: a randomized controlled trialBlood1992806143014361381626
- KubistaEGlaspyJHolmesFAGreenMDHackettJNeumannTPegfilgrastim Study GroupBone pain associated with once-per-cycle pegfilgrastim is similar to daily filgrastim in patients with breast cancerClin Breast Cancer20033639139812636878
- MartínMRodríguez-LescureARuizAGEICAM 9906 Study InvestigatorsRandomized phase 3 trial of fluorouracil, epirubicin, and cyclophosphamide alone or followed by Paclitaxel for early breast cancerJ Natl Cancer Inst20081001180581418505968
- RochéHFumoleauPSpielmannMSequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNCLCC PACS 01 TrialJ Clin Oncol200624365664567117116941
- NCCN guidelines practice guidelines in oncology – v 2012
- SmithTJKhatcheressianJLymanGH2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guidelineJ Clin Oncol200624193187320516682719
- AaproMSCameronDAPettengellREuropean Organisation for Research and Treatment of Cancer (EORTC) Granulocyte Colony-Stimulating Factor (G-CSF) Guidelines Working PartyEORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumoursEur J Cancer200642152433245316750358
- PapaldoPLopezMMarollaPImpact of five prophylactic filgrastim schedules on hematologic toxicity in early breast cancer patients treated with epirubicin and cyclophosphamideJ Clin Oncol200523286908691816129844
- FalandryCCamponeMCartronGGuerinDFreyerGTrends in G-CSF use in 990 patients after EORTC and ASCO guidelinesEur J Cancer201046132389239820732287
- BadalamentiGIncorvaiaLProvenzanoSBronteGLetoGFulfaroFMalteseGLenograstim in preventing hemotherapy-induced febrile neutropenia in patients with soft tissue sarcomaAnticancer Res2013;33267968423393367
- SwansonGBergstromKStumpEMiyaharaTHerfindalETGrowth factor usage patterns and outcomes in the community setting: collection through a practice-based computerized clinical information systemJ Clin Oncol20001881764177010764438
- RostiGAppropriate administration of Granulocyte-Colony Stimulating Factors in prophylaxis and treatment of chemotherapy-induced febrile neutropeniaRev Heal Car201232127142