Abstract
Chronic hepatitis C virus (HCV) infection causes end-stage liver diseases and hepato cellular carcinoma. In the USA, Canada, and Japan, simeprevir – one of the second-generation HCV NS3/4A protease inhibitors – in combination with peginterferon α-2a or 2b plus ribavirin has recently been approved for HCV genotype 1-infected patients and is now used in daily clinical practice. This review summarizes the mechanism of action of simeprevir and the results of clinical trials of simeprevir and peginterferon plus ribavirin for HCV genotype 1 patients. In general, the simeprevir and peginterferon plus ribavirin treatment is highly effective and its adverse events are similar to those of peginterferon plus ribavirin only, the exception being milder, reversible jaundice. In the near future, the development of interferon-free regimens with simeprevir is expected. Careful attention should be paid to new results of clinical trials with simeprevir.
Introduction
Hepatitis C virus (HCV) is one of the major causes of chronic liver disease, hepatocellular carcinoma, and end-stage liver diseases in the USA, European countries, and Japan.Citation1–Citation3 In untreated patients with HCV, the steady progression of hepatic fibrosis is observed in many cases.Citation4 Several studies indicated that once patients develop cirrhosis, hepatocellular carcinoma develops at approximately 1%–7% per year, and the rate is increasing.Citation5,Citation6 This clearly highlights the importance of HCV treatment.
HCV is a single-stranded RNA virus approximately 9.6 kb in length, and is of the Hepacivirus genus belonging to the Flaviviridae family.Citation7 The HCV genome encodes at least ten proteins: four structural (core, E1, E2, and p7) and six non-structural proteins (NS2, NS3, NS4A, NS4B, NS5A, and NS5B). Among them, HCV NS2 and NS3 are cysteine protease and serine protease, respectively. HCV genomes are translated into an open reading frame of approximately 3,011 amino acids in length. Then, viral and cellular proteases chop this protein into structural and non-structural proteins, which are important for HCV assembly and replication.Citation7
Peginterferon plus ribavirin treatment leads to only 40%–50% sustained virologic response (SVR) in HCV genotype 1-infected patients but approximately 80% SVR in HCV genotype 2-infected patients.Citation7 In 2011, boceprevir or telaprevir – both first-generation HCV NS3/4A protease inhibitors – in combination with peginterferon plus ribavirin became available for HCV genotype 1-infected patients.Citation8 However, the treatment with HCV NS3/4A protease inhibitors is often associated with serious adverse events, despite achieving 70%–80% SVR in these patients.Citation8–Citation10
In the USA, Canada, and Japan, simeprevir – one of the second-generation HCV NS3/4A protease inhibitors – in combination with peginterferon plus ribavirin has recently been approved for HCV genotype 1-infected patients. This review focuses on and discusses simeprevir-including treatment for HCV genotype 1 infection.
Simeprevir (TMC435)
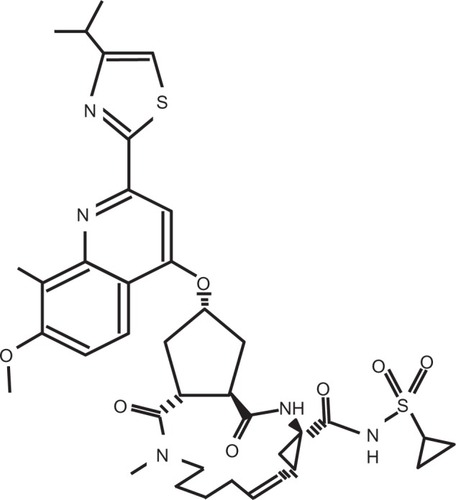
Simeprevir is an orally administered HCV NS3/4A protease inhibitor with a macrocyclic structure () and is one of the non-covalent inhibitors.Citation11 HCV NS3/4A protease inhibitors are divided into two classes: reversibly covalent and non-covalent. Boceprevir and telaprevir are linear peptidomimetic structures incorporating an α-ketoamide.Citation12 Simeprevir is a highly specific, potent HCV NS3/4A protease inhibitor, and biochemical assays have shown that HCV NS3/4A proteases of genotypes 1a and 1b, respectively, are inhibited with 0.5 nM and 0.4 nM of simeprevir.Citation13 In genotypes except HCV genotype 3, simeprevir has been shown to be effective in vivo.Citation14,Citation15 This compound has synergistic effects with interferon-α and HCV NS5B inhibitor, and it has additive effects with ribavirin in HCV replicon cells.Citation13 In rats, simeprevir was extensively distributed to the liver and intestinal tract, absolute bioavailability was 44% after a single oral administration, and compound concentrations were detected in both plasma and liver at 8 hours.Citation13 It was reported in both in vivo and in vitro studies that amino acids V36M, Q41R, F43S, T54S, Q80K/R/L, R155K, A156T/V, and D168N/A/V/E/H/T were resistance mutations to simeprevir.Citation16 R155K and D168E are the common resistance mutations in HCV genotypes 1a and 1b, respectively,Citation17–Citation19 although further studies will be needed. To prevent the resistant variants from emerging, simeprevir should be used in combination with peginterferon plus ribavirin, or other classes of direct-acting antivirals against HCV.Citation20
Simeprevir with peginterferon plus ribavirin for treatment-naïve HCV genotype 1 patients
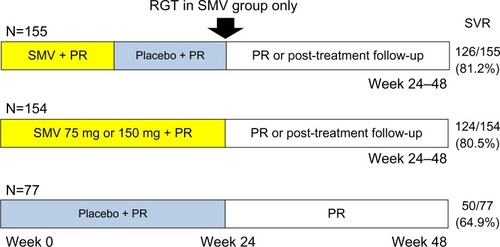
The Phase IIb, double-blind, placebo-controlled PILLAR trial (A Phase II Study of TMC435 in Combination with Peginterferon α-2a and Ribavirin in Patients Infected with Genotype 1 HCV Who Never Received Treatment; NCT00882908) examined the efficacy and safety of two different doses of simeprevir administered once daily (QD) for two different durations in combination with peginterferon plus ribavirin in treatment-naïve HCV genotype 1 patients.Citation21 A total of 386 patients were randomly assigned to one of five groups (): simeprevir (75 mg or 150 mg QD) for 12 or 24 weeks, or placebo, and peginterferon plus ribavirin. Patients in the simeprevir arms were treated for 24 or 48 weeks according to response-guided therapy (RGT) criteria. SVR rates, measured at 24 weeks after the end of treatment for each treatment group, are shown in . SVR rates were 74.7%–86.1% in the simeprevir groups versus 64.9% in the placebo control group (P<0.05).Citation21 For patients treated with simeprevir 75 mg, the SVR rates were lower for genotype 1a (66.2%) than for genotype 1b (88.9%), although the SVR rates in genotype 1a (82.4%) and 1b (83.8%) patients were similar with simeprevir 150 mg.Citation21 For the 332 patients with METAVIR scores of F0–F2, SVR rates were 81.7% with simeprevir 75 mg, 84.6% with simeprevir 150 mg, and 64.3% with placebo control. For the 53 patients with METAVIR scores of F3, SVR rates were 63.0% with simeprevir 75 mg, 75.0% with simeprevir 150 mg, and 71.4% with placebo control. Multiple regression analysis showed that treatment with simeprevir was associated with higher rates of SVR.Citation21
Figure 2 PILLAR study design and results. SVR rates were measured at 24 weeks after the end of treatment.
Abbreviations: PILLAR, Protease Inhibitor TMC435 Trial Assessing the Optimal Dose and Duration as Once-Daily Antiviral Regimen; PR, peginterferon plus ribavirin; RGT, response-guided therapy; SMV, simeprevir; SVR, sustained virologic response.
Among simeprevir-treated patients infected with HCV genotype 1a, higher SVR rates were observed in patients without the Q80K polymorphism at baseline.Citation21 It was reported that differences in the type of emerging mutations were observed between genotype 1a- (mainly emerging R155K alone or in combination with other mutations at NS3 positions 80 and/or 168) and 1b- (mainly D168V) infected patients.Citation21
SVR rates were 83.9% and 50.0%–78.1% with simeprevir 75 mg, 97.1% and 66.7%–80.0% with simeprevir 150 mg, and 100% and 50% in the control group (interleukin 28B [IL28B] major and minor genotype, respectively).Citation21 Other than mild reversible hyperbilirubinemia, without serum aminotransferase abnormalities, associated with higher doses of simeprevir, the adverse event profile was similar between simeprevir and control groups.Citation21 This studyCitation21 showed no differences in SVR between the 12- and 24-week durations of simeprevir, and it was also demonstrated that simeprevir allows the majority of treatment-naïve HCV genotype 1 patients to shorten their treatment duration to at least 24 weeks.
Simeprevir with peginterferon plus ribavirin for treatment-naïve HCV genotype 1b patients in Japan
The Phase II, multicenter, double-blind, DRAGON study (A Phase II Study of TMC435 in Patients with Chronic Hepatitis C; NCT00996476) examined the efficacy and safety of two different doses of simeprevir administered QD for two different durations in combination with peginterferon plus ribavirin in treatment-naïve HCV genotype 1b patients.Citation22 A total of 92 patients were randomly assigned to one of five groups (): simeprevir (50 mg or 100 mg QD) for 12 or 24 weeks, and peginterferon plus ribavirin. Patients in the simeprevir arms were treated for 24 or 48 weeks according to RGT criteria.
Figure 3 DRAGON study designs and results.
Abbreviations: DRAGON, A Phase II Study of TMC435 in Patients with Chronic Hepatitis C; PR, peginterferon plus ribavirin; RGT, response-guided therapy; SMV, simeprevir; SVR, sustained virologic response.
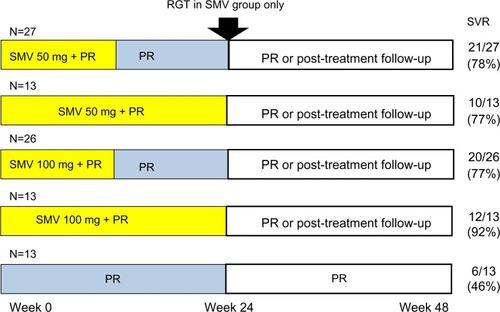
In the simeprevir groups, nine patients permanently discontinued all treatment due to adverse events (8/79, 10%) or virologic stopping criteria (1/79, 1.3%). In the peginterferon plus ribavirin for 48 weeks group, three patients permanently discontinued all treatment due to adverse events (2/13, 15%) or another reason (1/13, 7.7%).Citation22 Among the simeprevir groups, SVR rates ranged from 83%–90% ().Citation22 In the DRAGON study,Citation22 no emerging mutations were observed in the selected NS3 protease domain in the single patient with viral breakthrough. Among ten of eleven simeprevir-treated relapsers, mutations in the NS3 protease domain (Q80R, R155Q, and D168A/C/E/H/V, alone or in combination) emerged in six patients.Citation22 These results showed that simeprevir was highly effective for patients infected with HCV subgenotype 1b, to which almost all of HCV genotype 1 belongs.Citation23 The RGT approach might be beneficial for patients in Japan, although the majority of simeprevir-treated patients were eligible to stop all treatment at week 24.Citation22 The addition of simeprevir (100 mg QD) to peginterferon plus ribavirin demonstrated potent antiviral activity and improved the SVR rates with only 24-week treatment duration in treatment-naïve patients infected with HCV genotype 1 in Japan.
Simeprevir with peginterferon plus ribavirin for treatment-experienced HCV genotype 1 patients
The Phase IIb international, randomized, double-blind, placebo-controlled ASPIRE study (Antiviral Stat-C Protease Inhibitor Regimen in Experienced Patients; NCT00980330) evaluated the efficacy, tolerability, and safety of simeprevir QD in combination with peginterferon α-2a plus ribavirin in HCV genotype 1 patients who failed to respond to previous peginterferon/ribavirin treatment.Citation24 A total of 463 patients who did not respond (null response), had partial response, or relapsed after treatment with peginterferon plus ribavirin were randomly assigned to receive simeprevir (100 or 150 mg QD) for 12, 24, or 48 weeks plus peginterferon and ribavirin for 48 weeks (n=396), or placebo plus peginterferon and ribavirin for 48 weeks (n=66). SVR rates were significantly higher (P<0.01) in the simeprevir groups (61%–80%) than in those given placebo (23%) (simeprevir versus placebo: prior null response, 38%–59% versus 19%; prior partial response, 48%–86% versus 9%; and prior relapse, 77%–89% versus 37%).Citation24
SVR rates were higher in simeprevir-treated patients compared with control irrespective of HCV subgenotype, and were generally higher with simeprevir 150 mg than simeprevir 100 mg. In patients with simeprevir 150 mg, HCV genotype 1a and 1b patients had 63.1% and 80.4% SVR rates, respectively.Citation24 Regarding METAVIR scores, cirrhotic patients (METAVIR score F4) treated with simeprevir 150 mg achieved SVR rates of 73%, 82%, and 31% in prior relapse, prior partial response, and prior null response, respectively, compared with 0% for control patients.Citation24 There might be a benefit with simeprevir in previous null responders who have advanced stages of fibrosis and/or cirrhosis. Regarding IL28B genotype rs12979860, the SVR rates with 150 mg were 88% for CC, 74% for CT, and 61% for TT genotypes (versus 18%, 31%, and 14%, respectively, in the control group).Citation24 Regarding resistance mutations associated with simeprevir treatment, D168V was observed primarily in HCV genotype 1b and R155K in HCV genotype 1a.Citation24 Among patients infected with HCV genotype 1a, the presence of the Q80K polymorphism at baseline did not appear to influence the SVR rate in the simeprevir 150 mg group of the ASPIRE study.Citation24 Mild and transient increases in mean bilirubin were seen during the first weeks of simeprevir treatment. In vitro studies have demonstrated that simeprevir is an inhibitor of the bilirubin transporters OATP1B1 and MRP2.Citation22,Citation24 In treatment-experienced patients, 12, 24, or 48 weeks of simeprevir (100 mg QD or 150 mg QD) in combination with 48 weeks of peginterferon plus ribavirin significantly increased SVR rates.
Phase III trials in Japanese patients infected with HCV genotype 1
Four Phase III Japanese studies of simeprevir QD demonstrated SVR 12 weeks after the end of treatment (SVR12) in HCV genotype 1 patients ().Citation25–Citation27
Figure 4 SVR12 in four Phase III Japanese hepatitis C virus genotype 1 patients treated with (A and B) simeprevir and ribavirin plus peginterferon α-2a or (C) peginterferon α-2b. (A) CONCERTO-1 trials for treatment-naïve patients; (B) CONCERTO-2 and CONCERTO-3 trials for prior non-responders and prior relapsers, respectively; and (C) CONCERTO-4 trials for treatment-naïve patients, prior non-responders, and prior relapsers. Data from Hayashi et al,Citation25 Izumi et al,Citation26 and Suzuki et al.Citation27
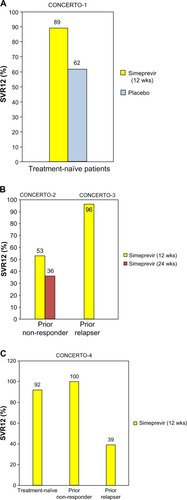
In CONCERTO-1 (A Study of the Efficacy of Combination Therapy Including Simeprevir [TMC435] in the Treatment of Genotype 1 Hepatitis C; ), 183 treatment-naïve patients were randomized to receive simeprevir or placebo for 12 weeks plus peginterferon α-2a and ribavirin for 24 or 48 weeks. In CONCERTO-2 (A Study of the Efficacy of Retreatment with Simeprevir [TMC435] in the Treatment of Genotype 1 Hepatitis C; ), 108 patients who failed to respond to previous treatment were randomized to two groups. In one group, patients received simeprevir plus peginterferon α-2a and ribavirin for 12 weeks, followed by RGT of peginterferon α-2a and ribavirin alone for 12 or 36 weeks. In the other group, patients received simeprevir plus peginterferon α-2a and ribavirin for 24 weeks, followed by RGT of peginterferon α-2a and ribavirin alone for up to 24 weeks. In CONCERTO-3 (A Study of the Efficacy of Retreatment with Simeprevir [TMC435] in the Treatment of Genotype 1 Hepatitis C; ), 49 patients with prior relapse received simeprevir and peginterferon α-2a and ribavirin for 12 weeks, followed by RGT of peginterferon α-2a and ribavirin alone for 12 or 36 weeks. In CONCERTO-4 (A Study of the Efficacy of Combination Therapy with Simeprevir [TMC435], Peginterferon α-2b, and Ribavirin in the Treatment of Genotype 1 Hepatitis C; ), instead of peginterferon α-2a, peginterferon α-2b was used for a total of 79 patients.Citation27 Simeprevir with peginterferon α-2a/2b plus ribavirin treatment was effective for not only treatment-naïve patients but also prior null responders and relapsers with HCV genotype 1b in Japan.
Phase III trials in treatment-naïve patients infected with HCV genotype 1
The QUEST (Phase III Trial of TMC435 in Treatment-naïve, Genotype 1 Hepatitis C-infected Patients) trials were Phase III, randomized, double-blind studies that evaluated simeprevir with peginterferon α-2a (QUEST-1) or peginterferon α-2a/2b (QUEST-2) plus ribavirin in treatment-naïve HCV genotype 1 adult patients. The pooled analysis consisted of 785 patients (IL28B TT, 14.6%; METAVIR F4, 10.4%; genotype 1a, 48.4%; Q80K+, 16.8%) (QUEST-1, n=394; QUEST-2, n=391).Citation28 The SVR12 rate was significantly superior with simeprevir/peginterferon plus ribavirin versus placebo/peginterferon plus ribavirin (80.4% versus 50.0%; P<0.001). In simeprevir-treated patients with rapid virologic response (defined as HCV RNA <25 IU/mL undetectable at week 4), 89.6% achieved SVR12. SVR12 rates of 94.7%, 92.3%, 85.4%, and 83.9% were achieved in patients treated with simeprevir who had IL28B genotype CC, baseline HCV RNA ≤800,000 IU/mL, genotype 1b, and METAVIR F0–F2, respectivelyCitation28 High rapid virologic response rates were observed in patients with IL28B minor genotype, METAVIR F4, and Q80K (68.8%, 66.7%, and 63.1%, respectively) with high SVR rates (77.4%, 75.0%, and 79.2%, respectively).Citation28 Results from these studies suggest that simeprevir also conferred clinical benefit among patients with unfavorable baseline characteristics, such as IL28B minor genotype, METAVIR F4, and HCV genotype 1a/Q80K+.
Phase III trials in HCV genotype 1 patients who relapsed after previous interferon-based therapy
The double-blind, multicenter, Phase III PROMISE (Phase III Trial of TMC435 in Genotype 1 Hepatitis C-infected Patients Who Relapsed After Previous Therapy) study evaluated simeprevir plus peginterferon α-2a/ribavirin in HCV genotype 1 patients with prior relapse.Citation29 The “intent-to-treat” population consisted of 393 patients. SVR12 was significantly higher with simeprevir (150 mg QD)/peginterferon α-2a plus ribavirin than placebo/peginterferon α-2a/ribavirin (79.2% versus 36.8%; P<0.001). With simeprevir (150 mg QD) treatment, SVR12 was 88.7%, 85.9%, and 82.0% in patients with IL28B CC genotype, HCV genotype 1b, and METAVIR F0–F2, respectively. The incidence and profile of adverse events were generally similar between the simeprevir and placebo treatment groups. Bilirubin increases with simeprevir/peginterferon α-2a plus ribavirin were mild, transient, and without concomitant increases in alanine aminotransferase/aspartate aminotransferase.Citation29 Simeprevir might be useful for the treatment of the different patient subpopulations with prior relapse on interferon, including patients with IL28B minor genotype and METAVIR score F4.Citation29
Other clinical trials with simeprevir
The ATTAIN (An Efficacy, Safety, and Tolerability Study for TMC435 Versus Telaprevir in Combination With Peginterferon α-2a and Ribavirin in Chronic Hepatitis C Patients Who Were Null or Partial Responders to Prior Peginterferon α-2a and Ribavirin Therapy) Phase III trial examined the efficacy, safety and tolerability of simeprevir versus telaprevir in combination with peginterferon α-2a and ribavirin in chronic hepatitis C patients who were null or partial responders to prior treatment.Citation30 The results have not yet been published.
The COSMOS (A Study of TMC435 in Combination With PSI-7977 [GS7977] in Chronic Hepatitis C Genotype 1-Infected Prior Null Responders To Peginterferon/Ribavirin Therapy or HCV Treatment-naïve Patients) Phase II, randomized, open-label study evaluated the efficacy and safety of simeprevir 150 mg QD and sofosbuvir 400 mg QD – an HCV NS5B nucleotide polymerase inhibitor – with or without ribavirin for 12 or 24 weeks in HCV genotype 1 patients with METAVIR score F0–F2 who were prior null responders to peginterferon plus ribavirin (cohort 1) or treatment-naïve patients and prior null responders with F3–F4 (cohort 2).Citation31 Totals of 80 (cohort 1) and 87 (cohort 2) patients began the treatment. In cohort 1, SVR12 was 96.3% (26/27) and 92.9% (13/14) in patients treated with simeprevir plus sofosbuvir with and without ribavirin for 12 weeks, and SVR12 was 79.2% (19/24) and 100% (14/14) in patients treated with simeprevir plus sofosbuvir with or without ribavirin for 24 weeks.Citation31 In cohort 2, SVR4 was 83.3% (20/24) and 93.3% (14/15) in patients treated with simeprevir plus sofosbuvir with and without ribavirin for 12 weeks.Citation31 Simeprevir plus sofosbuvir with or without ribavirin for 12 or 24 weeks might result in a high SVR in HCV genotype 1 patients including null responders and patients with cirrhosis.Citation31
It was recognized that some “difficult-to-treat” patients would still exist,Citation32 and this would include HCV–human immunodeficiency virus (HIV) co-infected patients. For the shared modes of transmission, 20%–30% of HIV-infected patients in the USA are co-infected with HCV.Citation33 HIV infection facilitates HCV-related fibrosis progression, increasing the risk of cirrhosis and decompensated liver disease.Citation34 In HCV–HIV patients, the peginterferon plus ribavirin treatment led to only ~20% SVR in HCV genotype 1 patients.Citation32–Citation38 Telaprevir- or boceprevir-based triple therapy is a promising option for co-infected patients with well-controlled HIV infection, although these drugs may be associated with severe adverse events.Citation39–Citation42 Simeprevir with peginterferon plus ribavirin led to 57% SVR for prior null responders of HCV genotype 1/HIV co-infected patients.Citation43 Overall SVR12 was ~74%. Simeprevir is metabolized by cytochrome P450 3A4 enzyme and preclinical studies showed that it interacts with certain antiretrovirals. In that study, patients were not permitted to use HIV protease inhibitors or efavirenz, and most were on raltegravir and almost all took nucleoside/nucleotide reverse transcriptase inhibitors.Citation43 Simeprevir/peginterferon α-2a plus ribavirin could be useful for the treatment of some “difficult-to-treat” patients.Citation32 Previous studies suggested that amino acid substitutions in the HCV core region are a useful predictor of SVR in the peginterferon plus ribavirin combination treatment in patients infected with HCV genotype 1.Citation44–Citation46 Information from both IL28B single nucleotide polymorphism testing and HCV core protein substitutions may yield a more accurate baseline prediction of SVR in simeprevir with peginterferon plus ribavirin therapies.Citation47,Citation48 Although simeprevir shows cross-resistance with telaprevir at amino acid positions 155 and 156,Citation16,Citation17 most simeprevir-resistant mutations were reported to be at amino acid position 168.Citation49 Further studies will be needed to determine whether simeprevir is effective against the positively selected variants upon antiviral treatment.
Conclusion
This review summarized key preclinical and clinical data of simeprevir – an HCV NS3/4A protease inhibitor, its mechanism of action, resistance mutations, and its efficacy in clinical trials. In the USA, Canada, and Japan, simeprevir and peginterferon plus ribavirin have been approved for the treatment of HCV genotype 1 patients and are now used in daily clinical practice. In general, simeprevir and peginterferon plus ribavirin treatment is highly effective, with adverse events similar to those of peginterferon plus ribavirin, the exception being milder, reversible jaundice. In the near future, interferon-free regimens with simeprevir are sure to shed new light on HCV treatment. New results from clinical trials using simeprevir should be the focus of attention.
Acknowledgments
This work was supported by a Grant for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (24590955 to TK).
Disclosure
Tatsuo Kanda reports receiving lecture fees from Chugai Pharmaceutical, MSD K.K., Mitsubishi Tanabe Pharma, Ajinomoto, Bristol-Myers Squibb, Daiichi-Sankyo, and GlaxoSmithKline. Osamu Yokosuka reports receiving grant support from Chugai Pharmaceutical, Bayer, MSD K.K., Daiichi-Sankyo, Mitsubishi Tanabe Pharma, and Bristol-Myers Squibb. The other authors report no conflicts of interest in this work.
References
- DitahIDitahFDevakiPThe changing epidemiology of hepatitis C virus infection in the United States: national health and nutrition examination survey 2001 through 2010J Hepatol201460469169824291324
- FenoglioLSerrainoCCastagnaEEpidemiology, clinical-treatment patterns and outcome in 256 hepatocellular carcinoma casesWorld J Gastroenterol201319213207321623745022
- KimMNKimBKHanKHHepatocellular carcinoma in patients with chronic hepatitis C virus infection in the Asia–Pacific regionJ Gastroenterol201348668168823463401
- OmataMKandaTYuMLAPASL consensus statements and management algorithms for hepatitis C virus infectionHepatol Int201262409435
- SeeffLBNatural history of chronic hepatitis CHepatology2002365 Suppl 1S35S4612407575
- TakanoSYokosukaOImazekiFTagawaMOmataMIncidence of hepatocellular carcinoma in chronic hepatitis B and C: a prospective study of 251 patientsHepatology19952136506557875662
- KandaTImazekiFYokosukaONew antiviral therapies for chronic hepatitis CHepatol Int20104354856121063477
- KandaTNakamotoSNakamuraMDirect-acting antiviral agents for the treatment of chronic hepatitis C virus infectionJ Clin Transl Hepatol20142116
- D’AmbrosioRColomboMSafety of direct antiviral agents in real lifeDig Liver Dis201345Suppl 5S363S36624091117
- KumadaHToyotaJOkanoueTChayamaKTsubouchiHHayashiNTelaprevir with peginterferon and ribavirin for treatment-naïve patients chronically infected with HCV of genotype 1 in JapanJ Hepatol2012561788421827730
- TsantrizosYSTMC-435, an NS3/4A protease inhibitor for the treatment of HCV infectionCurr Opin Investig Drugs2009108871881
- RosenquistASamuelssonBJohanssonPODiscovery and development of simeprevir (TMC435), a HCV NS3/4A protease inhibitorJ Med Chem20145751673169324446688
- LinTILenzOFanningGIn vitro activity and preclinical profile of TMC435350, a potent hepatitis C virus protease inhibitorAntimicrob Agents Chemother20095341377138519171797
- MorenoCBergTTanwandeeTAntiviral activity of TMC435 monotherapy in patients infected with HCV genotypes 2–6: TMC435-C202, a Phase IIa, open-label studyJ Hepatol20125661247125322326470
- LenzOVijgenLBerkeJMVirologic response and characterisation of HCV genotype 2–6 in patients receiving TMC435 monotherapy (study TMC435-C202)J Hepatol201358344545123142061
- WuSKandaTNakamotoSImazekiFYokosukaOHepatitis C virus protease inhibitor-resistance mutations: our experience and reviewWorld J Gastroenterol201319478940894824379619
- LenzOVerbinnenTLinTIIn vitro resistance profile of the hepatitis C virus NS3/4A protease inhibitor TMC435Antimicrob Agents Chemother20105451878188720176898
- HalfonPLocarniniSHepatitis C virus resistance to protease inhibitorsJ Hepatol201155119220621284949
- XueWPanDYangYLiuHYaoXMolecular modeling study on the resistance mechanism of HCV NS3/4A serine protease mutants R155K, A156V and D168A to TMC435Antiviral Res201293112613722127068
- LenzOde BruijneJVijgenLEfficacy of re-treatment with TMC435 as combination therapy in hepatitis C virus-infected patients following TMC435 monotherapyGastroenterology201214351176117822885330
- FriedMWButiMDoreGJOnce-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naïve genotype 1 hepatitis C: the randomized PILLAR studyHepatology20135861918192923907700
- HayashiNSetoCKatoMKomadaYGotoSOnce-daily simeprevir (TMC435) with peginterferon/ribavirin for treatment-naïve hepatitis C genotype 1-infected patients in Japan: the DRAGON studyJ Gastroenterol201449113814724005956
- WuSKandaTNakamotoSPrevalence of hepatitis C virus subgenotypes 1a and 1b in Japanese patients: ultra-deep sequencing analysis of HCV NS5B genotype-specific regionPLoS One201389e7361524069214
- ZeuzemSBergTGaneESimeprevir increases rate of sustained virologic response among treatment-experienced patients with HCV genotype-1 infection: a Phase IIb trialGastroenterology2014146243044124184810
- HayashiNIzumiNKumadaHSimeprevir with peginterferon/ribavirin for treatment-naïve hepatitis C genotype 1 patients in Japan: CONCERTO-1, a phase III trialJ Hepatol Epub201449
- IzumiNHayashiNKumadaHOnce-daily simeprevir with peginterferon and ribavirin for treatment-experienced HCV genotype 1-infected patients in Japan: the CONCERTO-2 and CONCERTO-3 studiesJ Gastroenterol201449594195324626851
- SuzukiFHayashiNGotoSKumadaHSimeprevir with Peginterferon alpha-2b/Ribavirin for hepatitis C genotype 1 patients: the CONCERTO-4 studyKanzo201454suppl1A157 Japanese
- JacobsonIMDoreGJFosterGRSimeprevir (TMC435) with peginterferon/ribavirin for treatment of chronic HCV genotype 1 infection in treatment-naïve patients: efficacy in difficult-to-treat patient sub-populations in the QUEST-1 and 2 Phase III trials [abstract]Hepatology201358Suppl 1756A
- FornsXLawitzEZeuzemSSimeprevir (TMC435) with peginterferon α-2a/ribavirin for treatment of chronic HCV genotype 1 infection inpatients who relapsed after previous interferon-based therapy: efficacy and safety in patient sub-populations in the PROMISE Phase III trial [abstract]Hepatology201358Suppl 1737A
- Janssen R&D IrelandTMC435HPC3001 – an efficacy, safety and tolerability study for TMC435 vs telaprevir in combination with pegINFα-2a and ribavirin in chronic hepatitis C patients who were null or partial responders to prior pegINFα-2a and ribavirin therapy (ATTAIN) Available from: http://clinicaltrials.gov/ct2/show/NCT01485991. NLM identifier: NCT01485991Accessed February 10, 2014
- JacobsonIMGhalibRHRodriguez-TorresMSVR results of a once-daily regimen of simeprevir (TMC435) plus sofosbuvir (GS-7977) with or without ribavirin in cirrhotic and non-cirrhotic HCV genotype 1 treatment-naïve and prior null responder patients: the COSMOS study [abstract]Hepatology201358Suppl 61379A
- KandaTYokosukaOOmataMAntiviral therapy for “difficult-to-treat” hepatitis C virus-infected patientsChin Med J (Engl)2013126234568457424286427
- SulkowskiMSHCV therapy in HIV-infected patientsLiver Int201333Suppl 1636723286848
- Perez-LatorreLSanchez-CondeMRinconDPrediction of liver complications in patients with hepatitis C virus-related cirrhosis with and without HIV coinfection: comparison of hepatic venous pressure gradient and transient elastographyClin Infect Dis201458571371824265358
- TorrianiFJRodriguez-TorresMRockstrohJKPeginterferon α-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patientsN Engl J Med2004351543845015282351
- ChungRTAndersenJVolberdingPPeginterferon α-2a plus ribavirin versus interferon α-2a plus ribavirin for chronic hepatitis C in HIV-coinfected personsN Engl J Med2004351545145915282352
- CarratFBani-SadrFPolSPegylated interferon α-2b vs standard interferon α-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trialJAMA2004292232839284815598915
- LagunoMMurillasJBlancoJLPeginterferon α-2b plus ribavirin compared with interferon α-2b plus ribavirin for treatment of HIV/HCV co-infected patientsAIDS20041813F27F3615316335
- SulkowskiMSShermanKEDieterichDTCombination therapy with telaprevir for chronic hepatitis C virus genotype 1 infection in patients with HIV: a randomized trialAnn Intern Med20131592869623685940
- SulkowskiMPolSMallolasJBoceprevir versus placebo with pegylated interferon α-2b and ribavirin for treatment of hepatitis C virus genotype 1 in patients with HIV: a randomised, double-blind, controlled Phase 2 trialLancet Infect Dis201313759760523768747
- Martel-LaferriereVBrinkleySBichoupanKVirological response rates for telaprevir-based hepatitis C triple therapy in patients with and without HIV coinfectionHIV Med201415210811524025147
- FiererDSDieterichDTMullenMPTelaprevir in the treatment of acute hepatitis C virus infection in HIV-infected menClin Infect Dis201458687387924336914
- HighleymanLEACS 2013: simeprevir with interferon effective for genotype 1 HIV/HCV coinfected people [webpage on the Internet]San Francisco, CAhivandhepatitis.com2013 [cited October 20, 2013]. Available from: http://www.hivandhepatitis.com/hcv-treatment/experimental-hcv-drugs/4367-eacs-2013-simeprevir-with-interferon-effective-for-hivhcv-coinfected-people-with-genotype-1-or-4Accessed February 10, 2014
- DonlinMJCannonNAYaoEPretreatment sequence diversity differences in the full-length hepatitis C virus open reading frame correlate with early response to therapyJ Virol200781158211822417522222
- AkutaNSuzukiFHirakawaMA matched case-controlled study of 48 and 72 weeks of peginterferon plus ribavirin combination therapy in patients infected with HCV genotype 1b in Japan: amino acid substitutions in HCV core region as predictor of sustained virological responseJ Med Virol200981345245819152407
- AlestigEArnholmBEilardACore mutations, IL28B polymorphisms and response to peginterferon/ribavirin treatment in Swedish patients with hepatitis C virus genotype 1 infectionBMC Infect Dis20111112421569441
- SorianoVPovedaEVispoELabargaPRallonNBarreiroPPharmacogenetics of hepatitis CJ Antimicrob Chemother201267352352922194301
- NakamotoSImazekiFFukaiKAssociation between mutations in the core region of hepatitis C virus genotype 1 and hepatocellular carcinoma developmentJ Hepatol2010521727819910070
- MaekawaSEnomotoNOnce-daily simeprevir in combination with pegylated-interferon and ribavirin: a new horizon in the era of direct-acting antiviral agent therapy for chronic hepatitis CJ Gastroenterol201449116316424356811