Abstract
Chordomas are rare, locally aggressive skull base neoplasms known for local recurrence and not-infrequent treatment failure. Current evidence supports the role of maximal safe surgical resection. In addition to open skull-base approaches, the endoscopic endonasal approach to clival chordomas has been reported with favorable albeit early results. Adjuvant radiation is prescribed following complete resection, alternatively for gross residual disease or at the time of recurrence. The modalities of adjuvant radiation therapy reported vary widely and include proton-beam, carbon-ion, fractionated photon radiotherapy, and photon and gamma-knife radiosurgery. As of now, no direct comparison is available, and high-level evidence demonstrating superiority of one modality over another is lacking. While systemic therapies have yet to form part of any first-line therapy for chordomas, a number of targeted agents have been evaluated to date that inhibit specific molecules and their respective pathways known to be implicated in chordomas. These include EGFR (erlotinib, gefitinib, lapatinib), PDGFR (imatinib), mTOR (rapamycin), and VEGF (bevacizumab). This article provides an update of the current multimodality treatment of cranial base chordomas, with an emphasis on how current understanding of molecular pathogenesis provides a framework for the development of novel targeted approaches.
Introduction
Chordomas are rare primary bone neoplasms that typically originate from the spine or skull base. Conventional practice generally involves maximal safe surgical removal, often followed by focused radiation therapy. Despite this, many patients ultimately succumb to local treatment failure and recurrence. To date, no chemotherapeutic agent has demonstrated sufficient efficacy to constitute part of the first-line therapy for these locally aggressive neoplasms. Progress in the understanding of chordomas’ molecular pathogenesis has given rise to efforts to treat chordomas with targeted therapies. This article provides an essential review on cranial base chordomas, outlines current surgery and radiation-treatment paradigms, and further examines how the evolving understanding of molecular pathogenesis has informed potential new targeted chemotherapeutic approaches.
Description and epidemiology
Chordomas are rare neoplasms, occurring with an annual age-adjusted incidence of 0.02 per 100,000 person-years, and account for 1%–4% of all primary malignant bone tumors.Citation1,Citation2 Arising from embryonic notochord remnants, chordomas occur at any point along the skeletal neuraxis,Citation2,Citation3 with the sacrum and cranial base being the most frequently affected areas.Citation4 Cranial base chordomas account for 35%–49% of all chordomas.Citation3 Consistent with their site of origin, chordomas predominantly present in the extradural space and produce symptoms secondary to local growth and bony destruction. Of note, chordomas can rarely present as a solitary intradural lesion in the retroclival space, and while they behave in similar fashion to their classic extradural counterparts,Citation5 intradural lesions must be distinguished from a more benign variant of intradural notochordal tumor termed ecchordosis physaliphora, which can have very subtle radiologic and pathologic differences from more malignant chordomas.Citation6,Citation7
The median age at presentation for cranial chordomas is in the sixth decade, slightly younger for sacral chordomas,Citation2 and with rare occurrences in the pediatric population.Citation2,Citation8 Given the predilection for growth in the clivus and cavernous sinus regions, the most frequent clinical presentations of skull-base chordomas are cranial nerve deficits (eg, abducens or oculomotor nerve palsy).Citation9
The median survival of cranial base chordomas is estimated at 6.29 years,Citation4,Citation10 with 5-year overall survival (OS) and progression-free survival (PFS) rates of 78.4% and 50.8%, respectively.Citation11 The lethality of skull-base chordoma is largely due to local progression, although systemic metastasis has been reported in 12.5% of skull base/craniocervical tumors.Citation12 A large retrospective study of cranial chordomas recently suggested a trend toward improvement in survival over time,Citation3 with 5-year OS for the 1975–1984, 1985–1994, and 1995–2004 epochs of 48.5%, 73.0%, and 80.7%, respectively. The reasons for such improvements are unclear, but may reflect earlier detection and treatment (lead-time bias); better treatment modalities, including the addition of endoscopic approaches both as an adjunct to open procedures and as a standalone procedure, especially for midline clival tumors; refinements in the safety of open skull-base approaches; and use of greater sophistication in radiation therapy-delivery techniques, allowing for safer prescription of the requisite higher dose for chordomas.
Current treatment paradigm for skull-base chordomas
There is no uniform consensus regarding the optimal standard treatment for skull-base chordomas, and evidence regarding various paradigms stem from retrospective series over often long and inconsistent treatment eras. While it is generally agreed that a maximal safe removal of the tumor should be done first,Citation13 a number of other practice differences exist, including the type of radiation therapy, the indications for radiation therapy (eg, after complete vs only incomplete resection; upfront or at the time of recurrence), and the management of metastases. To date, no chemotherapeutic agent have been approved for the first-line treatment of skull-base chordomas.Citation14 At the time of recurrence, the respective roles of repeat resection, radiation therapy, and chemotherapy remain unclear.
Surgery
The principal goals of surgery beyond histologic confirmation of the lesion are to achieve a maximal safe resection, provide symptomatic improvement, and to facilitate adjuvant treatment, such as radiotherapy, by minimizing the treatment volume and maximizing the distance between the target volume and critical surrounding neurovascular structures. In the skull base, surgical resection of chordomas remains a challenge, due to anatomic constraints in accessing the skull base, as well as their locally aggressive growth pattern and involvement of surrounding neurological structures, such as the brain stem, cranial nerves, and internal carotid and/or vertebral arteries. These factors often exceed the ability of any single surgical approach to achieve maximal resection, thus necessitating complex combined or multiple approaches. Understandably, the majority of surgical series describe a practice of piecemeal intralesional resection to normal dural and/or bony margins, as opposed to en bloc resection with margins as described for their sacral counterparts.Citation15
Despite these limitations, the literature supports the benefit of surgical resection on survival for chordomas. In a large review of 962 patients with spinal, sacral, or cranial base chordomas identified from the Surveillance, Epidemiology, and End Results (SEER) database, Jawad and Scully found surgical resection to be an independent predictor of OS.Citation16 In addition, in a meta-analysis of 23 retrospective studies, 807 patients with cranial base chordomas were analyzed together, with a mean follow-up of 53.6 months.Citation11 The rate of complete resection ranged widely across studies, from 0% to 73.7%. Those patients with a complete resection had a 5-year OS of 95% (vs 71% without) and 5-year PFS of 87% (vs 50% without). Despite a seemingly clear benefit of complete resection in this study, a few stipulations should be mentioned. The majority of included studies reported the routine use of adjuvant radiotherapy, including after complete resection. Most outcomes reported were for patients who had undergone open resection, with only a small minority of cases undergoing endoscopic endonasal resection. The careful assessment of a complete resection can be difficult, and may have differed across studies. For example, a complete resection may refer to the soft-tissue component of the tumor, versus a resection of the tumor and all affected surrounding bone and dura.
To achieve maximal resection, traditional open skull-base approaches (summarized elsewhere)Citation17,Citation18 are most commonly used, but the less invasive endoscopic approaches have been increasingly adopted. The endoscope has been employed as an adjunct to open resection or as a standalone endonasal approach to resect midline skull-base chordomas.Citation13,Citation19–Citation27 Advances in such techniques as pituitary gland mobilization, posterior clinoidectomy, and skeletonization of the petrous carotid arteries have permitted access to the entire rostrocaudal extent of the clivus via this approach.Citation28 However, comparisons of endoscopic versus microscopic resection of cranial base chordomas are invariably prone to selection bias, and understandably long-term comparisons of the rate of complete resection, recurrence, and OS have to date not been published. In one systematic review,Citation29 26 open-surgery studies published from 1987 to 2010 were compared with 12 studies of either endoscope-assisted or fully endoscopic resection of cranial base chordomas from 2002 to 2010. No survival data were available for the endoscopic cohort, and follow-up for this latter group was limited to 18.5 months. The reported rate of complete resection was 61.0%, although the rate of petrous involvement and dural invasion was lower in the endoscopic group. The main limitation of this technique remains the risk of cerebrospinal fluid leakage and meningitis, largely mitigated by meticulous layered closure and vascularized mucosal flaps. Nevertheless, the risk in all recently published larger (n>10) series with an intradural tumor component remains significant: 8%–33% developed cerebrospinal fluid leakage and 0%–14% meningitis.Citation19,Citation20,Citation23,Citation30
Radiotherapy
Although radiotherapy is an important therapeutic adjunct for cranial base chordomas, issues regarding both the timing of adjuvant radiotherapy, specifically after complete resection or only for residual/recurrent disease, and optimal type of radiotherapy are largely unresolved in the literature. Two recent meta-analyses based on the SEER database failed to demonstrate a benefit in survival in patients who received radiotherapy;Citation31,Citation32 however, this was possibly due to a number of sources of error, including but not limited to not controlling for the presence or absence of postoperative residual disease, histologic subtype, tumor size, patient age, type and dose of radiotherapy, era of treatment, and other factors. The difficulty of answering these questions is evident, and the following discussion attempts to interpret the available literature as much as possible.
There is significant heterogeneity in the literature regarding the use of adjuvant radiotherapy following surgical resections described as “complete” or “gross total”. In one meta-analysis, among patients reported to have gross total resection of a skull-base chordoma, only 37.9% of patients were referred for adjuvant radiation therapy.Citation11 Despite gross total resection, however, the recurrence rate following surgical removal of skull-base chordomas can be high. The results of large surgical series show a 5-year PFS of 22.5%–74.2% with complete upfront resection, and pooled numbers are too small to assess the benefit of radiotherapy in this subgroup.Citation11 The concept of gross total resection in cranial base chordoma is difficult to define or prove, given the challenge surgeons have in identifying and removing microscopic invasion adjacent to the tumor, and of assessing microscopic disease on postoperative imaging. Histopathologic studies of chordoma-growth patterns point to an infiltrative propensity to invade bone and submucous tissue and along the loose connective tissue among local vessels, nerves, and muscles in multilayers or multilobular fashion.Citation33 Chordomas lack a fibrous capsule,Citation34 and tumor cells clearly invade intralesional fibrous septa, particularly in advanced stages of the disease. The rationale for adjuvant radiation therapy in light of these features is thus to improve local control.Citation13
Despite this, the evidence supporting the use of radiation therapy following complete surgical removal is mixed.Citation35 In one large series of cranial base chordomas in which aggressive microsurgical resection was performed,Citation36 53 of 74 (71.6%) patients had complete resection either after single- or multistage resection. Only patients with gross residual disease received adjuvant radiation therapy. Five-year recurrence-free survival was 47%. In a larger updated series by the same authors,Citation37 a trend toward routine adjuvant radiation therapy was observed, despite a similar rate of complete resection in early (1988–1999) and late (2000–2011) treatment eras. Five-year recurrence-free survival in the early and late-treatment eras was not statistically different: 55% and 59%, respectively. Five-year OS, however, was significantly better in the latter treatment era (93% vs 64%). Although there was a modest reduction in perioperative complications in the latter era, it is also conceivable that the improvement in survival may also at least in part be attributable to more routine use of adjuvant radiotherapy. This remains to be proven, however, in a comparative prospective fashion.
In addition to the timing of adjuvant radiotherapy, the optimal type of contemporary radiotherapy is controversial and as-yet unproven in the literature. Chordomas exhibit an increased dose–response relationship relative to other tumors.Citation38 Therefore, the principal challenge of delivering safe and effective radiation therapy is to achieve clinically relevant doses while mitigating toxicity to adjacent neurovascular structures, such as the optic and other cranial nerves, pituitary gland, and brain stem. Proton-beam therapy has classically been considered to be well-suited for chordomas, due to its unique radiobiological properties,Citation39 although evidence regarding its clinical superiority over other modern delivery techniques (eg, radiosurgery and fractionated stereotactic radiotherapy), radiation sources, and dose schedules has been questioned.Citation40 To date, in addition to proton-beam radiotherapy, fractionated photon radiotherapy, CyberKnife and gamma-knife radiosurgery, and carbon-ion radiotherapy have all been reported for cranial base chordomas. Superiority of one radiotherapy modality over another has yet to be demonstrated, although large cohort comparisons are lacking. A discussion of each form of radiotherapy used currently for cranial base chordomas follows.
Charged heavy-particle therapy, such as with protons, takes advantage of a number of physical properties to deliver a high dose of radiation to tumors, while sparing falloff exposure to surrounding tissues. For cranial base chordomas, 5-year recurrence-free survival using proton-based therapy ranges from 59% to 73%.Citation39,Citation41,Citation42 Amichetti et al performed a systematic review of the literature comparing proton-based radiotherapy with other available forms of radiation therapy for skull-base chordomas.Citation43 There were no direct comparative studies, and the results of seven proton-beam therapy series including 416 patients were reviewed along with ten studies and 191 patients who underwent conventional forms of photon-based treatment. Mean dose delivery for proton-beam therapy was 66–83 cobalt gray equivalent. The average 5-year local control and OS were 69.2% and 79.8%, respectively. Only one study reported 10-year recurrence free survival and OS, which was 54%.Citation39 This was found to be superior to conventional photon radiotherapy, which had 33.5% and 53.5% average local control and OS at 5 years, respectively. The years of treatment for the photon group were from 1938 to 2005, and significantly earlier than included proton-beam studies.
Carbon-ion radiotherapy exerts similar physical characteristics as proton-based treatment, but with higher relative biological effectiveness, which is of interest in relatively radio-resistant tumors, such as chordomas.Citation44–Citation50 Early series from carbon-ion facilities in Germany and Japan have reported 3-year recurrence-free survival of 70%–80.6% for skull-base chordomas.Citation44,Citation47 A single-center randomized clinical trial of proton- versus carbon-radiation therapy in patients with cranial base chordomas is ongoing,Citation48 although other preliminary data for sacral chordomas suggest a lack of difference in clinical efficacy between ion type.Citation49 The primary disadvantages of proton and carbon ion-based radiotherapy are global availability and investment cost.Citation50
The results of more modern studies of fractionated photon-based radiotherapy for skull-base chordomas appears closer to proton-based therapy compared to earlier studies. Techniques are nevertheless variable from one study to another, including whether photon therapy is administered alone or in combination with particle therapy.Citation51,Citation52 In a recent small series of fractionated photon-based radiotherapy,Citation53 5-year OS was 76.4%, and 37.5% of patients were free of progression at 5 years. All patients either had gross residual disease after surgery or were being treated for symptomatic recurrences, however. Similar results were obtained by Debus et alCitation54 who treated 37 patients with chordomas of the skull base or upper cervical spine. Local control was 50% and OS 82% at 5 years. In places where proton-beam or carbon-ion radiotherapy centers are not available, photon-based radiotherapy appears to confer grossly similar local control rates, although true comparative studies would be of great interest.
Smaller volume tumors have also been amenable to stereotactic radiosurgery (SRS), and early results have suggested comparable outcomes to other radiation modalities for residual or recurrent cases.Citation55 SRS has been shown to be valuable in treating small residual postoperative and recurrent chordomas.Citation56 Krishnan et al and Kano et al reported that using an SRS boost (15.0 Gy median margin dose) with or without fractionated radiation therapy (50.4 Gy median margin dose), the 5-year tumor-control rate was 55% and 65%, respectively.Citation55,Citation57 Liu et al and Hasegawa et al had a 5-year treated tumor-control rate of 66% and 72% when using 12.7 Gy and 15 Gy median margin dose, respectively. In both series, OS was 80% and 84% at 5 years, respectively.Citation58,Citation59 In a multicenter study of 71 patients who underwent gamma-knife SRS for chordomas, an 80% 5-year actuarial OS and 66% treated tumor-control rate after SRS was reported for the combined cohort. In this study, the median patient age was 45 years, the median SRS target volume was 7.1 cm3, and the median tumor-margin dose was 15.0 Gy.Citation56
Irrespective of the type of radiotherapy administered, a number of factors have been identified that may influence the response rate of radiotherapy. These include age, sex, tumor heterogeneity, extent of resection, presence of necrosis in the pretreatment biopsy, elevated tumor volume, and radiotherapy dose delivered.Citation60,Citation61 Among cases with subtotal resection, a residual tumor volume under 25–30 cm3 appears associated with better local control using adjunctive radiation therapy.Citation29,Citation41,Citation42,Citation62 In one study of 42 patients with skull-base chordomas, a gross tumor volume ≤25 mL was associated with excellent local control using spot-scanning-based proton radiotherapy.Citation63 The typical at-risk structures during dosimetric planning include the optic apparatus, pituitary gland, and brain stem, and need to be carefully mapped pretreatment.
Investigation of novel therapeutic targets
Despite the challenges posed by the rarity of chordoma, advances in the molecular understanding of chordomas have led to the identification of promising targetable pathways and prognostic markers. These include brachyury, receptor tyrosine kinases (RTKs), and downstream pathways. lists some of the known pathways implicated in the pathogenesis of chordoma and the accompanying table () lists the matched targeted therapeutics. Loss of chromosome 1p36, 9p loss of heterozygosity, and an elevated Ki67 proliferative index all correlate with aggressive behavior and shorter OS in skull-base chordomas.Citation64,Citation65 Additionally, loss of chromosome 1q, gain of 2p, and aberrant brachyury copy-number changes and subsequent protein expression are associated with disease recurrence.
Figure 1 Signaling pathways thought to be implicated in chordoma pathogenesis.
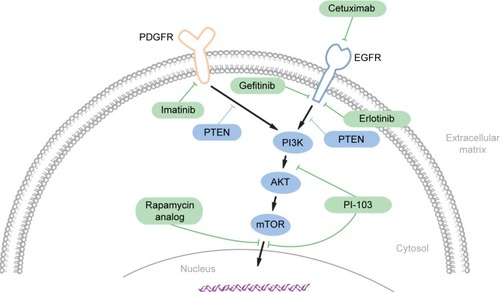
Table 1 Summary of targeted molecular agents trialed in skull-base chordomas
The brachyury or T gene located on chromosome 6q27 encodes a developmentally regulated transcription factor essential for notochordal development and formation of posterior mesodermal elements.Citation65,Citation66 Normally silenced in postdevelopmental tissue, brachyury is aberrantly reexpressed at high levels in chordoma cells,Citation67 providing a diagnostic adjunct in differentiating chordoma from other tumors with similar histology and geographical location.Citation68,Citation69 The impact of brachyury experimental gain and loss of function on increased and decreased cell proliferation, respectively, highlights its biological and functional importance for chordoma growth and progression.Citation70–Citation73
Several lines of evidence also suggest a causative role of brachyury overexpression in chordoma formation.Citation74 A study of four familial chordoma cohorts identified recurrent germ-line duplication within chromosome 6q27, which contains the brachyury gene.Citation75 However, the vast majority of chordoma patients lack a family history, and in these more common sporadic tumors, only 6.8% of patients (16 of 236) exhibited genomic amplification of brachyury.Citation70,Citation76,Citation77 Therefore, aberrant overexpression of brachyury in the latter group is potentially related to epigenetic changes rather than genomic amplification.Citation76 Also, a nonsynonymous single-nucleotide polymorphism or SNP (rs2305089) in brachyury is strongly associated with development of chordoma,Citation78 as well as worse OS in spinal chordoma patients.Citation79 Lastly, genomic copy-number status and subsequent protein expression of brachyury is associated with shorter PFS on a cohort of 37 skull-base chordomas.Citation65 However, a separate study published in the same year found no prognostic value of brachyury expression in a cohort of spinal chordomas.Citation80 Whether this represents inherent biological differences from tumors arising from the spine and skull base remains to be tested in additional studies.
Given the tight correlation between genomic aberrations of brachyury leading to its temporally aberrant reexpression in chordoma, brachyury may be considered to be a driver oncogene in chordoma.Citation65,Citation70,Citation71 As mentioned earlier, brachyury gain- and loss-of-function experiments have further established its putative role in the malignant growth of chordoma. Enforced silencing of brachyury in the JHC7 and UCH-1 chordoma cell lines result in growth cessation, senescence, and differentiation.Citation70,Citation71 Approximately 81.1% of 37 skull-base chordomas were found to be immunopositive for brachyury protein expression, indicating its potential central role in regulating growth in the majority of skull-base chordomas.Citation65 A potential mechanism by which brachyury may contribute to chordoma’s malignant phenotype may involve an activating role in epithelial–mesenchymal transition (EMT). EMT represents a mechanism for normal developmental and tissue-repair mechanisms, but can also be co-opted in cancer development.Citation81,Citation82 EMT can mediate infiltrative and invasive behavior in tumor cells via the selective activation of “mesenchymal” genetic programs, such as expression of the SNAIL gene. This is relevant in chordomas, as recent evidence has highlighted brachyury-mediated SNAIL activation in these tumors.Citation71,Citation74
In the absence of active therapeutic approaches that directly target brachyury, inactivation of downstream or interacting signaling pathways provides an alternative means to short-circuit its function. For instance, the extensive cross talk between brachyury and other progrowth signaling pathways is of therapeutic significance for targeting brachyury in chordoma. FGFR/MEK/ERK signal-transduction pathways appear to mediate downstream signaling of brachyury in chordomas.Citation83 Interestingly, these same pathways appear to effect positive feedback back on brachyury, which makes components of the FGFR/MEK/ERK pathways and brachyury potential therapeutic targets. A different study highlights the interaction between brachyury and the EGFR signaling pathway.Citation73
Reexpression of brachyury exclusively in chordoma, and its role in driving cancer behavior, makes brachyury an attractive target for therapeutic intervention. Since the protein is not expressed in nonchordoma normal tissues, targeting of the brachyury protein, its function, and associated signal-transduction pathways proves a very attractive clinical option. Recent advancements in the field of tumor immunology have rekindled the interest in its incorporation in cancer treatment.Citation84 The brachyury protein therefore represents a logical target for immunotherapy in chordoma, given its pivotal role in the initiation and progression of the disease.Citation69,Citation85
Protein tyrosine kinases (PTKs) mediate phosphorylation of selected tyrosine residues, which results in functional activation of many proteins and plays a crucial role in cancer development.Citation86 RTKs are specialized, transmembrane PTKs that mediate signaling via sampling of the external environment. RTKs are composed of extracellular domains that bind cognate environmental ligands and an intracellular domain that mediates the signaling event via dimerization and binding to other signaling molecules. Mutations and over-expression of RTKs, such as PDGFα and -β, EGFR, MET, and HER2/NEU are central to the development of many cancers; therefore, they are attractive targets of therapeutic intervention. Hyperactive RTKs not only result in growth proliferation but also other procancer “hallmarks”, such as enhanced cell survival and angiogenesis, which together contribute to tumorigenesis.Citation87
A large proportion of chordomas demonstrate EGFR overexpression, which is associated with aggressive clinical behavior. In a single study, 69% of chordoma samples expressed EGFR and 40% display EGFR amplification.Citation88 This is recapitulated in three other studies with a cumulative number of 79.6% (43 of 54) chordoma cases demonstrating EGFR expression.Citation89–Citation91 Given these observations, and the efficacy of EGFR inhibitors gefitinib and erlotinib in large-cell lung adenocarcinoma patients with activating EGFR mutations,Citation92 antibody-based blockade or pharmacologic inhibition of EGFR signaling in chordoma is a logical potential therapeutic option. Anecdotal reports of partial responses and clinical improvement in recurrent sacral and skull-base chordoma with adjuvant single-agent or combined EGFR antibody (cetuximab) and EGFR-specific (gefitinib or erlotinib) or broader-spectrum tyrosine-kinase inhibitors (lapatinib)Citation66,Citation93,Citation94 suggest that inhibition of EGFR protein and the downstream signaling pathway might offer a reasonable therapeutic option for some chordoma patients. However, larger, more controlled studies are required to define more precisely the indications for treatment and efficacy.
The PDGF receptor (PDGFR) is another PTK expressed in chordoma. Although PDGFR promotes chordoma cell proliferation through activation of the PI3K/AKT, RAS/ERK, and STAT pathways,Citation95 the PDGFRβ isoform is preferentially upregulated and localized in the stromal components of chordomas,Citation89,Citation96–Citation98 suggesting a role in providing a supportive tumor microenvironment. Therefore, responses to PDGFR inhibition may reflect the relative abundance of tumor-associated stromata. In practice, the PDGFR preferential PTK inhibitor imatinib has demonstrated modest activity in small uncontrolled case seriesCitation97,Citation99 of chordoma patients with advanced disease, prompting a Phase II trial of 56 patients that realized overall clinical benefit in 64% and stable disease in 70% of patients.Citation100 Despite some success with PDGFR inhibition in chordoma, a majority of treated patients do not show decline in tumor size, which may reflect the inability of targeting PDGFR expressing stromal elements to effect cytologic reduction of neoplastic chordoma cells. The presence of a prominent demineralized matrix in chordoma is another factor that may limit drug delivery and underlie cases that show suboptimal response to any systemic therapy.Citation96 These observations indicate the critical need to consider the role of the tumor microenvironment as a barrier to drug delivery when designing future treatment strategies.
Targeting PTKs in chordoma can be challenging, due to their multiplicity and functional redundancy. Strategies to address this challenge could include simultaneous inhibition of multiple activated PTKs or targeting common signaling pathways downstream of the PTKs. For instance, signaling from multiple RTKs, including EGFR and PDGFR, converges on the PI3K/AKT/mTOR pathway, which is in turn negatively regulated by the PTEN tumor suppressor. Components of the PI3K/AKT/mTOR pathway are activated,Citation95,Citation101,Citation102 and PTEN is suppressed in chordoma-tumor samples,Citation103 suggesting that inhibition of this pathway alone or in combination with PTK inhibitors could have therapeutic activity against chordoma. Preclinical experimental studies using inhibitors of mTORC1 (rapamycin), mTOR (MLN0128), and PI3K/AKT/mTOR (PI-103) support this approach,Citation101,Citation103,Citation104 while the addition of the mTOR inhibitor to imatinib showed additional clinical benefit in patients with advanced imatinib-resistant chordomas.Citation99 These preliminary results indicate the therapeutic potential of targeting downstream effectors of activated PTKs and their potential effectiveness in combination with other targeted therapies.
The rarity of skull-base chordomas limits not only the number of treatments that can be practically tested in clinical trials but also the statistical power required to identify meaningful differences in outcome. Therefore, the application of precision or personalized oncology based on the molecular profiling of aberrant pathways in individual patients holds great promise for advancing the treatment of chordoma patients.Citation105 For example, a chordoma patient treated with rapamycin on the basis of aberrant mTOR-pathway signaling identified in molecular profiling of patient-derived cells resulted in a sixfold reduction in tumor-growth rate.Citation106 Additional targets for chordoma therapy will emerge from ongoing efforts at molecular profiling. For example, in addition to the targets discussed earlier, proangiogenic pathways mediated by VEGFR-2 and iNOS with relevance to other cancers have been implicated in chordoma.Citation107 In a small clinical series, combined use of erlotinib and bevacizumab, a humanized anti-VEGF antibody, led disease stabilization in three chordoma patients.Citation108 Ultimately, the establishment of large and well-annotated databases will be crucial to realize the benefits of personalized chordoma therapy. As proposed for other cancers, ongoing iterative analysis of individual patient molecular profiles and treatment responses generated from these databases is expected to better inform therapeutic choices in a prospective fashion.
Conclusion
Management of chordomas affecting the base of the skull remains challenging, although epidemiologic data suggest an improvement in survival and local control in more recent eras. Complete safe removal after initial diagnosis appears to improve survival relative to incomplete resection. Endoscopic endonasal approaches may be useful in achieving safe resection of those chordomas affecting the midline clivus. Multiple modalities of radiation therapy have been used for chordoma, and results in well-selected individuals are comparable. To date, no chemotherapeutic agent has formed part of the standard treatment of chordomas. Based on an increasing knowledge of the molecular pathogenesis of skull-base chordomas, a number of targeted therapies have been attempted, with modest results for recurrent cases. Future systemic treatments based on affected pathways and application of the principals of personalized oncology will hopefully reduce the burden of this rare tumor.
Disclosure
The authors report no conflicts of interest in this work.
References
- Central Brain Tumor Registry of the United StatesCBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2007Hinsdale (IL)CBTRUS2011
- ChughRTawbiHLucasDRBiermannJSSchuetzeSMBakerLHChordoma: the nonsarcoma primary bone tumorOncologist200712111344135018055855
- ChambersKJLinDTMeierJRemenschneiderAHerrMGraySTIncidence and survival patterns of cranial chordoma in the United StatesLaryngoscope201412451097110224122844
- McMasterMLGoldsteinAMBromleyCMIshibeNParryDMChordoma: incidence and survival patterns in the United States, 1973–1995Cancer Causes Control200112111111227920
- AlOtaibiFGuiotMCMuanzaTDi MaioSGiant petroclival primary intradural chordoma: case report and systematic review of the literatureJ Neurol Surg Rep2014751e160e16925083378
- GoldenLDSmallJEBenign notochordal lesions of the posterior clivus: retrospective review of prevalence and imaging characteristicsJ Neuroimaging201424324524923464492
- GeorgeBBressonDBouazzaSChordomaNeuro-Chirurgie201460363140 French24856008
- MirraJMNelsonSDDella RoccaCMertensFChordomaFletcherDMUnniKMertensFPathology and Genetics of Tumours of Soft Tissue and BoneLyonIARC Press2002316317
- WalcottBPNahedBVMohyeldinACoumansJVKahleKTFerreiraMJChordoma: current concepts, management, and future directionsLancet Oncol2012132e69e7622300861
- McMasterMUpdate on the epidemiology of chordoma: SEER registry data 1973–2007Poster presented at: Third International Chordoma Research Workshop317–192011Bethesda, MD
- Di MaioSTemkinNRamanathanDSekharLNCurrent comprehensive management of cranial base chordomas: 10-year meta-analysis of observational studiesJ Neurosurg201111561094110521819197
- YasudaMBressonDChibbaroSChordomas of the skull base and cervical spine: clinical outcomes associated with a multimodal surgical resection combined with proton-beam radiation in 40 patientsNeurosurg Rev2012352171182 discussion 182–18321863225
- Fernandez-MirandaJCGardnerPASnydermanCHClival chordomas: a pathological, surgical, and radiotherapeutic reviewHead Neck201436689290623804541
- DiazRJCusimanoMDThe biological basis for modern treatment of chordomaJ Neurooncol2011104241142221384217
- BorianiSBandieraSBiaginiRChordoma of the mobile spine: fifty years of experienceSpine (Phila Pa 1976)200631449350316481964
- JawadMUScullySPSurgery significantly improves survival in patients with chordomaSpine (Phila Pa 1976)201035111712320042964
- Di MaioSSekharLNSkull base approachesEllenbogenRGAbdulraufSIPrinciples of Neurological Surgery: Expert Consult – Online and PrintPhiladelphiaElsevier2012
- RostomilyRCSekharLNElahiFChordomas and chondrosarcomasSekharLNFesslerRAtlas of Neurosurgical TechniquesBrainNew YorkThieme2006
- TanNCNaidooYOueSEndoscopic surgery of skull base chordomasJ Neurol Surg B Skull Base201273637938624294554
- ChibbaroSCorneliusJFFroelichSEndoscopic endonasal approach in the management of skull base chordomas – clinical experience on a large series, technique, outcome, and pitfallsNeurosurg Rev2014372217224 discussion 224–21524249430
- SaitoKTodaMTomitaTOgawaKYoshidaKSurgical results of an endoscopic endonasal approach for clival chordomasActa Neurochir (Wien)2012154587988622402876
- FraserJFNyquistGGMooreNAnandVKSchwartzTHEndoscopic endonasal transclival resection of chordomas: operative technique, clinical outcome, and review of the literatureJ Neurosurg201011251061106919698043
- DehdashtiARKarabatsouKGannaAWitterickIGentiliFExpanded endoscopic endonasal approach for treatment of clival chordomas: early results in 12 patientsNeurosurgery2008632299307 discussion 307–30918797360
- JiangWHZhaoSPXieZHZhangHZhangJXiaoJYEndoscopic resection of chordomas in different clival regionsActa Otolaryngol20091291718318607890
- ZhangQKongFYanBNiZLiuHEndoscopic endonasal surgery for clival chordoma and chondrosarcomaORL J Otorhinolaryngol Relat Spec200870212412918408411
- FrankGSciarrettaVCalbucciFFarnetiGMazzatentaDPasquiniEThe endoscopic transnasal transsphenoidal approach for the treatment of cranial base chordomas and chondrosarcomasNeurosurgery2006591 Suppl 1ONS50ONS5716888551
- FatemiNDusickJRGorgulhoAAEndonasal microscopic removal of clival chordomasSurg Neurol200869433133818234296
- Fernandez-MirandaJCGardnerPARastelliMMJrEndoscopic endonasal transcavernous posterior clinoidectomy with interdural pituitary transpositionJ Neurosurg20141211919924816325
- KomotarRJStarkeRMRaperDMAnandVKSchwartzTHThe endoscope-assisted ventral approach compared with open microscope-assisted surgery for clival chordomasWorld Neurosurg2011763–4318327 discussion 259–36221986431
- KoutourousiouMGardnerPATormentiMJEndoscopic endo-nasal approach for resection of cranial base chordomas: outcomes and learning curveNeurosurgery2012713614624 discussion 624–62522592328
- JonesPSAghiMKMuzikanskyAShihHABarkerFG2ndCurryWTJrOutcomes and patterns of care in adult skull base chordomas from the Surveillance, Epidemiology, and End Results (SEER) databaseJ Clin Neurosci20142191490149624852903
- BohmanLEKochMBaileyRLAlonso-BasantaMLeeJYSkull base chordoma and chondrosarcoma: influence of clinical and demographic factors on prognosis; a SEER analysisWorld Neurosurg201482580681425009165
- OikawaSKyoshimaKGotoTHistological study on local invasiveness of clival chordoma. Case report of autopsyActa Neurochir (Wien)2001143101065106911685615
- NakaTBoltzeCKuesterDIntralesional fibrous septum in chordoma: a clinicopathologic and immunohistochemical study of 122 lesionsAm J Clin Pathol2005124228829416040302
- JianBJBlochOGYangIHanSJArandaDParsaATA comprehensive analysis of intracranial chordoma and survival: a systematic reviewBr J Neurosurg201125444645321749184
- TzortzidisFElahiFWrightDNatarajanSKSekharLNPatient outcome at long-term follow-up after aggressive microsurgical resection of cranial base chordomasNeurosurgery200659223023716883163
- Di MaioSRostomilyRSekharLNCurrent surgical outcomes for cranial base chordomas: cohort study of 95 patientsNeurosurgery20127061355136022157545
- PearlmanAWFriedmanMRadical radiation therapy of chordomaAm J Roentgenol Radium Ther Nucl Med19701082332341
- MunzenriderJELiebschNJProton therapy for tumors of the skull baseStrahlenther Onkol1999175Suppl 2576310394399
- BradaMPijls-JohannesmaMDe RuysscherDCurrent clinical evidence for proton therapyCancer J200915431932419672149
- HugEBLoredoLNSlaterJDProton radiation therapy for chordomas and chondrosarcomas of the skull baseJ Neurosurg199991343243910470818
- CastroJRLinstadtDEBaharyJPExperience in charged particle irradiation of tumors of the skull base: 1977–1992Int J Radiat Oncol Biol Phys19942946476558040010
- AmichettiMCianchettiMAmelioDEnriciRMMinnitiGProton therapy in chordoma of the base of the skull: a systematic reviewNeurosurg Rev200932440341619319583
- Schulz-ErtnerDKargerCPFeuerhakeAEffectiveness of carbon ion radiotherapy in the treatment of skull-base chordomasInt J Radiat Oncol Biol Phys200768244945717363188
- OstroumovEHunterCJThe role of extracellular factors in human metastatic chordoma cell growth in vitroSpine (Phila Pa 1976)200732262957296418091487
- DuranteMLoefflerJSCharged particles in radiation oncologyNat Rev Clin Oncol201071374319949433
- TakahashiSKawaseTYoshidaKHasegawaAMizoeJESkull base chordomas: efficacy of surgery followed by carbon ion radiotherapyActa Neurochir (Wien)2009151775976919434365
- NikoghosyanAVKarapanagiotou-SchenkelIMünterMWJensenADCombsSEDebusJRandomised trial of proton vs carbon ion radiation therapy in patients with chordoma of the skull base, clinical phase III study HIT-1-StudyBMC Cancer20101060721054824
- MimaMDemizuYJinDParticle therapy using carbon ions or protons as a definitive therapy for patients with primary sacral chordomaBr J Radiol20148710332013051224288399
- PeetersAGruttersJPPijls-JohannesmaMHow costly is particle therapy? Cost analysis of external beam radiotherapy with carbonions, protons and photonsRadiother Oncol2010951455320106540
- TorresMAChangELMahajanAOptimal treatment planning for skull base chordoma: photons, protons, or a combination of both?Int J Radiat Oncol Biol Phys20097441033103919356861
- FeuvretLNoelGWeberDCA treatment planning comparison of combined photon-proton beams versus proton beams-only for the treatment of skull base tumorsInt J Radiat Oncol Biol Phys200769394495417889276
- BugociDMGirvigianMRChenJCMillerMMRahimianJPhoton-based fractionated stereotactic radiotherapy for postoperative treatment of skull base chordomasAm J Clin Oncol201336440441022772429
- DebusJSchulz-ErtnerDSchadLStereotactic fractionated radiotherapy for chordomas and chondrosarcomas of the skull baseInt J Radiat Oncol Biol Phys200047359159610837940
- KanoHIqbalFOSheehanJStereotactic radiosurgery for chordoma: a report from the North American Gamma Knife ConsortiumNeurosurgery201168237938921135744
- KanoHLunsfordLDStereotactic radiosurgery of intracranial chordomas, chondrosarcomas, and glomus tumorsNeurosurg Clin N Am201324455356024093573
- KrishnanSFooteRLBrownPDPollockBELinkMJGarcesYIRadiosurgery for cranial base chordomas and chondrosarcomasNeurosurgery200556477778415792516
- LiuALWangZCSunSBWangMHLuoBLiuPGamma knife radiosurgery for residual skull base chordomasNeurol Res200830655756118647493
- HasegawaTIshiiDKidaYYoshimotoMKoikeJIizukaHGamma knife surgery for skull base chordomas and chondrosarcomasJ Neurosurg2007107475275717937219
- TeraharaANiemierkoAGoiteinMAnalysis of the relationship between tumor dose inhomogeneity and local control in patients with skull base chordomaInt J Radiat Oncol Biol Phys199945235135810487555
- O’ConnellJXRenardLGLiebschNJEfirdJTMunzenriderJERosenbergAEBase of skull chordoma. A correlative study of histologic and clinical features of 62 casesCancer1994748226122677922977
- PotluriSJefferiesSJJenaRResidual postoperative tumour volume predicts outcome after high-dose radiotherapy for chordoma and chondrosarcoma of the skull base and spineClin Oncol (R Coll Radiol)201123319920820980136
- AresCHugEBLomaxAJEffectiveness and safety of spot scanning proton radiation therapy for chordomas and chondrosarcomas of the skull base: first long-term reportInt J Radiat Oncol Biol Phys20097541111111819386442
- HorbinskiCOakleyGJCieplyKThe prognostic value of Ki-67, p53, epidermal growth factor receptor, 1p36, 9p21, 10q23, and 17p13 in skull base chordomasArch Pathol Lab Med201013481170117620670138
- KitamuraYSasakiHKimuraTMolecular and clinical risk factors for recurrence of skull base chordomas: gain on chromosome 2p, expression of brachyury, and lack of irradiation negatively correlate with patient prognosisJ Neuropathol Exp Neurol201372981682323965741
- SinghalNKotasekDParnisFXResponse to erlotinib in a patient with treatment refractory chordomaAnticancer Drugs2009201095395519730087
- VujovicSHendersonSPresneauNBrachyury, a crucial regulator of notochordal development, is a novel biomarker for chordomasJ Pathol2006209215716516538613
- RomeoSHogendoornPCBrachyury and chordoma: the chondroid-chordoid dilemma resolved?J Pathol2006209214314616604512
- SchwabJHBolandPJAgaramNPChordoma and chondrosar-coma gene profile: implications for immunotherapyCancer Immunol Immunother200958333934918641983
- PresneauNShalabyAYeHRole of the transcription factor T (brachyury) in the pathogenesis of sporadic chordoma: a genetic and functional-based studyJ Pathol2011223332733521171078
- HsuWMohyeldinAShahSRGeneration of chordoma cell line JHC7 and the identification of brachyury as a novel molecular targetJ Neurosurg2011115476076921699479
- FernandoRILitzingerMTronoPHamiltonDHSchlomJPalenaCThe T-box transcription factor brachyury promotes epithelial- mesenchymal transition in human tumor cellsJ Clin Invest2010120253354420071775
- NelsonACPillayNHendersonSAn integrated functional genomics approach identifies the regulatory network directed by brachyury (T) in chordomaJ Pathol2012228327428522847733
- NibuYJosé-EdwardsDSDi GregorioAFrom notochord formation to hereditary chordoma: the many roles of brachyuryBiomed Res Int2013201382643523662285
- YangCSchwabJHSchoenfeldAJA novel target for treatment of chordoma: signal transducers and activators of transcription 3Mol Cancer Ther2009892597260519723879
- LeLPNielsenGPRosenbergAERecurrent chromosomal copy number alterations in sporadic chordomasPloS One201165e1884621602918
- ShalabyAAPresneauNIdowuBDAnalysis of the fibroblastic growth factor receptor-RAS/RAF/MEK/ERK-ETS2/brachyury signalling pathway in chordomasMod Pathol2009228996100519407855
- PillayNPlagnolVTarpeyPSA common single-nucleotide variant in T is strongly associated with chordomaNat Genet201244111185118723064415
- VargaPFisherCRhinesLPrognostic significance of T gene SNP s2305089 in individuals with spinal column chordomaPoster presented at: AACR Annual Meeting 2014April 5–9, 2014San Diego, CA
- ZhangLGuoSSchwabJHTissue microarray immunohistochemical detection of brachyury is not a prognostic indicator in chordomaPloS One201389e7585124086644
- KalluriRWeinbergRAThe basics of epithelial-mesenchymal transitionJ Clin Invest200911961420142819487818
- TamWLWeinbergRAThe epigenetics of epithelial-mesenchymal plasticity in cancerNat Med201319111438144924202396
- HuYMintzAShahSRQuinones-HinojosaAHsuWThe FGFR/MEK/ERK/brachyury pathway is critical for chordoma cell growth and survivalCarcinogenesis20143571491149924445144
- SharmaPWagnerKWolchokJDAllisonJPNovel cancer immunotherapy agents with survival benefit: recent successes and next stepsNat Rev Cancer2011111180581222020206
- PalenaCPolevDETsangKYThe human T-box mesodermal transcription factor brachyury is a candidate target for T-cell-mediated cancer immunotherapyClin Cancer Res20071382471247817438107
- GschwindAFischerOMUllrichAThe discovery of receptor tyrosine kinases: targets for cancer therapyNat Rev Cancer20044536137015122207
- HanahanDWeinbergRAHallmarks of cancer: the next generationCell2011144564667421376230
- ShalabyAPresneauNYeHThe role of epidermal growth factor receptor in chordoma pathogenesis: a potential therapeutic targetJ Pathol2011223333634621171079
- FasigJHDupontWDLaFleurBJOlsonSJCatesJMImmunohistochemical analysis of receptor tyrosine kinase signal transduction activity in chordomaNeuropathol Appl Neurobiol20083419510417973908
- WeinbergerPMYuZKowalskiDDifferential expression of epidermal growth factor receptor, c-Met, and HER2/neu in chordoma compared with 17 other malignanciesArch Otolaryngol Head Neck Surg2005131870771116103303
- PtaszyńskiKSzumera-CiećkiewiczAOwczarekJEpidermal growth factor receptor (EGFR) status in chordomaPol J Pathol2009602818719886182
- ChenZFillmoreCMHammermanPSKimCFWongKKNon-small-cell lung cancers: a heterogeneous set of diseasesNat Rev Cancer201414853554625056707
- LindénOStenbergLKjellénERegression of cervical spinal cord compression in a patient with chordoma following treatment with cetuximab and gefitinibActa Oncol200948115815918752082
- LaunaySGChetailleBMedinaFEfficacy of epidermal growth factor receptor targeting in advanced chordoma: case report and literature reviewBMC Cancer20111142321970335
- TamboriniEVirdisENegriTAnalysis of receptor tyrosine kinases (RTKs) and downstream pathways in chordomasNeuro Oncol201012877678920164240
- TamboriniEMiselliFNegriTMolecular and biochemical analyses of platelet-derived growth factor receptor (PDGFR) B, PDGFRA, and KIT receptors in chordomasClin Cancer Res200612236920692817145809
- CasaliPGMessinaAStacchiottiSImatinib mesylate in chordomaCancer200410192086209715372471
- OrzanFTerreniMRLongoniMExpression study of the target receptor tyrosine kinase of Imatinib mesylate in skull base chordomasOncol Rep200718124925217549375
- StacchiottiSMarrariATamboriniEResponse to imatinib plus sirolimus in advanced chordomaAnn Oncol200920111886189419570961
- StacchiottiSLonghiAFerraresiVPhase II study of imatinib in advanced chordomaJ Clin Oncol201230991492022331945
- SchwabJAntonescuCBolandPCombination of PI3K/mTOR inhibition demonstrates efficacy in human chordomaAnticancer Res20092961867187119528441
- PresneauNShalabyAIdowuBPotential therapeutic targets for chordoma: PI3K/AKT/TSC1/TSC2/mTOR pathwayBr J Cancer200910091406141419401700
- HanSPolizzanoCNielsenGPHornicekFJRosenbergAERameshVAberrant hyperactivation of akt and mammalian target of rapamycin complex 1 signaling in sporadic chordomasClin Cancer Res20091561940194619276265
- DaviesJMRobinsonAECowdreyCGeneration of a patient-derived chordoma xenograft and characterization of the phosphoproteome in a recurrent chordomaJ Neurosurg2014120233133624286145
- ShragerJTenenbaumJMRapid learning for precision oncologyNat Rev Clin Oncol201411210911824445514
- Ricci-VitianiLRunciDD’AlessandrisQGChemotherapy of skull base chordoma tailored on responsiveness of patient-derived tumor cells to rapamycinNeoplasia201315777378223814489
- Akhavan-SigariRGaabMRRohdeVExpression of vascular endothelial growth factor receptor 2 (VEGFR-2), inducible nitric oxide synthase (iNOS), and Ki-M1P in skull base chordoma: a series of 145 tumorsNeurosurg Rev2014371798823999886
- AsklundTSandströmMShahidiSRiklundKHenrikssonRDurable stabilization of three chordoma cases by bevacizumab and erlotinibActa Oncol201453798098424456503
- Di MaioSKongEYipSRostomilyRConverging paths to progress for skull base chordoma: review of current therapy and future molecular targetsSurg Neurol Int201347223776758
- HofHWelzelTDebusJEffectiveness of cetuximab/gefitinib in the therapy of a sacral chordomaOnkologie2006291257257417202828
- StacchiottiSTamboriniELo VulloSPhase II study on lapatinib in advanced EGFR-positive chordomaAnn Oncol20132471931193623559153
