Abstract
Immunocompromised patients are traveling at increasing rates. Physicians caring for these complex patients must be knowledgeable in pretravel consultation and recognize when referral to an infectious disease specialist is warranted. This article outlines disease prevention associated with international travel for adults with human immunodeficiency virus, asplenia, solid organ and hematopoietic transplantation, and other immunosuppressed states. While rates of infection may not differ significantly between healthy and immunocompromised travelers, the latter are at greater risk for severe disease. A thorough assessment of these risks can ensure safe and healthy travel. The travel practitioners’ goal should be to provide comprehensive risk information and recommend appropriate vaccinations or prevention measures tailored to each patient’s condition. In some instances, live vaccines and prophylactic medications may be contraindicated.
Introduction
International travel continues to grow at an astonishing rate, with more than one billion tourist arrivals reported globally in 2013.Citation1 Travel to a wide range of destinations has become easier and more attainable for an increasingly diverse population of global travelers. Immunocompromised travelers include those with primary immunodeficiencies, malignancy, or human immunodeficiency virus (HIV) infection and others receiving iatrogenic immunosuppression for organ transplantation, rheumatologic disorders, and autoimmune diseases. Studies estimate that anywhere from 16% to 48% of solid organ transplant (SOT) recipients have traveled to tropical regions and other areas where the risk of acquiring infectious diseases may be elevated.Citation2 While rates of infection are not higher in immunosuppressed travelers, severity of illness can be markedly increased.Citation3–Citation5
Severely immunocompromised travelers should seek consultation with an infectious diseases specialist whenever possible. Primary care physicians, rheumatologists, and transplant specialists must likewise be equipped to provide advice to this unique population of travelers, both pre- and postdeparture. In a study of 267 transplant recipients, 36% traveled outside the US and Canada and 66% sought pretravel care by their transplant physicians. Despite pretravel consultation, only 5% of those traveling to a hepatitis A endemic region received the hepatitis A vaccine.Citation4 Improved pretravel consultation can prevent potentially devastating infections. This review provides guidance on infectious disease prevention for clinicians charged with counseling and caring for immunocompromised travelers.
General precautions
Consultation for immunocompromised patients should begin several months ahead of departure in order to assess and mitigate risks associated with travel. A structured approach that accounts for each patient’s unique immunocompromised state improves pretravel guidance and interventions ().
Table 1 Items to discuss during the travel visit with an immuno-compromised traveler
Depending on their destination, travelers can be at heightened risk of exposure to endemic infectious diseases, many of which may be vector borne. Detailed country-specific – and even city-specific – information regarding existing infectious disease threats can be found in routinely updated resources provided by the Centers for Disease Control and Prevention (CDC).Citation5 Specific interventions to counter these threats, including immunizations and chemoprophylaxis, should hinge upon the likelihood that a traveler may come in contact with these diseases and their vectors. For example, travel to rural or heavily forested areas in tropical countries may increase the risk that a traveler will come in contact with mosquito vectors known to transmit certain diseases (eg, yellow fever, Japanese encephalitis), particularly during peak seasons coinciding with heavy rainfall. In contrast, travel limited to urban areas in developed countries may present few, if any, risks of exposure to mosquitoes.
If pretravel vaccination is advisable, the physician’s clinical assessment, based on the mechanism and degree of the traveler’s immunosuppression (),Citation5 should guide restrictions on what types of vaccines can be administered, what level of response to expect, and whether revaccination will be necessary. Inactivated vaccines are considered safe in all immunocompromised patients but may not reliably elicit protective responses. Live vaccines (eg, yellow fever) carry a significant risk of vaccine-related infection in patients with severe immunosuppression and are therefore discouraged in this subset of travelers. Vaccinations should be administered early on to achieve sufficient immunity before travel and allow time to receive boosters when indicated as part of a series. Serological testing for immunity conferred from these vaccinations, as well as from vaccinations received in the past, is warranted to determine if additional boosters are needed. Antibody response to vaccination is often muted in immunocompromised patients, as evidenced by lower protective titers maintained over shorter durations. Borderline protective titers may not be fully indicative of the level of protection conferred by vaccination because cellular immunity also plays an important role in preventing disease.Citation6
Table 2 Categories of immunocompromise based on type of immune suppression
If the individual is not a candidate for immunization, vaccines are unlikely to be protective, or the risk of disease acquisition is high, the patient may be advised to alter his or her travel itinerary. This is particularly true with live vaccines and their potential for adverse events, most notably with vaccination against yellow fever preceding travel to endemic areas in South America or Africa. Travel to high-risk destinations should be delayed for at least 1 year in several situations. These include patients with a history of transplantation or those on high doses of immune-modulating medications. In addition, immune-related diseases should stabilize or be brought under good control prior to travel. Immunizations should be given prior to the initiation of any immunosuppressive therapy if possible. Alternatively, immunizations can be deferred. The timing of deferred vaccinations depends on the nature of the immunosuppression. Specifically, vaccines should be deferred until 1) 1 month after discontinuation of tumor necrosis factor inhibitors or corticosteroids, 2) several months to 1 year after discontinuation of monoclonal antibody therapy (eg, rituximab), or 3) once other immunosuppressive drugs can be administered at lower doses or discontinued altogether.Citation6 Immune responses and the duration of immunity will vary with different vaccines in the immunocompromised population.Citation7–Citation9 In regions with ongoing outbreaks or emerging infectious diseases (eg, viral hemorrhagic fever), travel is strongly discouraged.
Chemoprophylaxis may be recommended primarily in the context of traveling to regions where malaria is endemic. Selection of an appropriate prophylactic regimen should take into account the potential for drug interactions with a traveler’s existing medications, including immunosuppressive agents and antivirals.
Travel vaccines
As stated earlier, the safety and efficacy of individual vaccinations depends on the timing and degree of immunosuppression. The following section describes precautions, indications, and advice for specific vaccinations as they relate to the immunocompromised traveler.
Yellow fever
Yellow fever (YF) is caused by a flavivirus that is transmitted by the Aedes aegypti mosquito and no treatment exists for this disease (). Infections occur throughout South America and sub-Saharan Africa. The case fatality of severe YF with hepatorenal disease ranges from 20% to 50% and the estimated number of deaths is 30,000 per year. Clinical presentations can include an asymptomatic illness or a febrile illness with headaches, myalgias, and jaundice. A hemorrhagic presentation can occur with multiorgan failure. Between 1997 and 2009, nine cases were reported in unvaccinated travelers; eight persons died. One case of infection has been documented in a vaccinated traveler.Citation10 A traveler’s risk for YF is based on the season of travel, level of immune competence, travel location, and anticipated travel activities (eg, jungle or safari tours increase risk). Despite the severity of the infection, the risk for travelers to most tourist destinations remains quite low. The calculated risk of acquiring YF during a 2-week trip to West Africa for an unvaccinated traveler is 50 cases per 100,000 population.Citation10
Figure 1 CDC maps of regions of Africa (A) and South America (B) at risk for yellow fever transmission.Citation49
Abbreviation: CDC, Centers for Disease Control and Prevention.
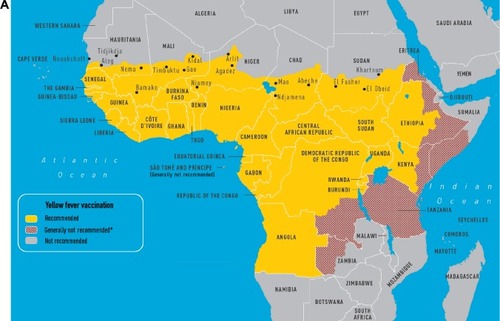
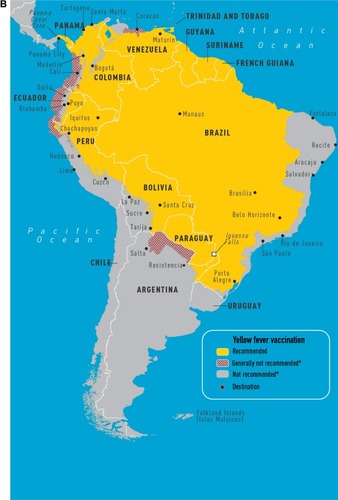
The 17D YF vaccine is a live vaccine. In immunocompetent individuals, neutralizing antibodies can be detected 10 days after vaccination. After 28 days, nearly 99% of vaccinated individuals demonstrate YF-neutralizing antibodies. Duration of protection is currently thought to be >10 years. Failure to create antibodies has been shown in those with malnutrition and HIV infection. The vaccine is contraindicated in travelers with severe immune deficits () due to the risk for life-threatening adverse reactions, including vaccine-associated viscerotropic disease (YEL-AVD) and vaccine-associated neurologic disease (YEL-AND). According to the Vaccine Adverse Event Reporting System, 43 adverse events occur per 100,000 doses distributed. The majority are nonserious events, including fever and pain at the injection site. From 2000 to 2006, 4.7 serious adverse events have occurred per 100,000 doses, which have been classified into YEL-AND, YEL-AVD, and anaphylaxis or hypersensitivity.Citation5,Citation10
YEL-AND is more common in children. In adults, YEL-AND manifests as Guillain-Barré syndrome, meningoencephalitis (neurotropic disease), acute disseminated encephalomyelitis, and a bulbar palsy. YEL-AND is rarely fatal. The reporting rate of YEL-AND is 0.4–0.8 cases per 100,000 doses distributed and, among persons ≥60 years, the rate is 1.6 cases per 100,000 doses.Citation10
A total of 57 cases of YEL-AVD from 14 countries has been reported since 2010. YEL-AVD is a disseminated process that presents with fever and multiorgan failure. Cases have primarily been reported with first-time vaccination and not with booster vaccinations. The reporting rate for YEL-AVD is 0.3–0.4 cases per 100,000 doses distributed, with higher rates reported among persons ≥60 years. Thymic diseases that cause immune deficiencies (ie, myasthenia gravis or thymoma) have been associated with YEL-AVD as four of the first 23 (17%) cases of YEL-AVD were associated with thymic disorders. Autoimmune diseases are thought to be a risk factor for YEL-AVD, and occurrences have been reported in patients with systemic lupus erythematosus, Addison’s disease, and Crohn’s disease. It is unclear if this is due to immunosuppressive treatment for the disease or the disease pathophysiology itself.Citation10,Citation11 These patients should be advised against travel to YF-endemic regions.
The Advisory Committee on Immunization Practices recommends vaccination for HIV-infected travelers with CD4 >200/mm3 as a “precaution” if travel is unavoidable. “Precaution” means that vaccine-related adverse events are higher in this population versus the general population and that the travel itinerary should be modified to avoid disease exposure. Duration of protection in these individuals is <10 years, unlike in healthy hosts, and the immunologic response is related to HIV viral load (versus CD4 count).Citation12,Citation13
Immunocompromised persons may not develop a response to the vaccine. Serologic testing should be obtained 1 month postvaccination. Serologic testing information can be obtained from either the state health department or the CDC. Conditions in which there is no contraindication or precaution for YF vaccination include presence of renal or liver diseases, asplenia, or diabetes mellitus.Citation5,Citation14 Those on corticosteroids can be given the vaccine under certain circumstances, including if they have been on corticosteroids for <2 weeks and are on a daily dose of prednisone (or equivalent) ≤2 mg/kg or ≤20 mg, on physiologic doses, on alternate-day dosing, or are using topical formulations ().Citation10 Immunization of SOT recipients is best pursued prior to transplant, as with all live vaccines.Citation2
In situations wherein YF vaccine is required for essential travel but is contraindicated, the traveler should be given a vaccine waiver as well as information on the symptoms of YF and mosquito avoidance. Travel should be deferred to low-risk seasons. The traveler should check with the respective country embassy or travel agent to determine if the waiver will be accepted.
Typhoid
Salmonella enterica serovar typhi is a gram-negative bacterium that causes fever, diarrhea, and abdominal pain. In severe cases, disease may result in sepsis, thrombocytopenia, or intestinal perforation. Transmission is via a fecal–oral route and most travelers to endemic regions should receive vaccination. It is the most common vaccine-preventable disease acquired during travel.Citation15
Currently, there are two available vaccines, an oral live-attenuated Ty21a vaccine and a parenteral Vi polysaccharide capsule vaccine. Both are directed against Salmonella typhi and effectiveness varies between 50% and 80%.Citation16 Additionally, Salmonella paratyphi A and B cause similar clinical symptoms and the live vaccine provides some cross-protection against these strains.Citation17
The live, oral vaccine is given as an enteric-coated capsule for four doses over 1 week and should be given at least 1 week prior to travel. It is licensed for adults and children aged 6 years or older. Protection lasts about 5–7 years and a booster is recommended every 5 years in persons traveling to endemic regions.Citation18 Adverse reactions are uncommon but include fever and headache. Less common reactions include nausea, vomiting, and urticarial rash.Citation19 However, as with all live vaccines, it is contraindicated in immunocompromised individuals.
In contrast, the Vi polysaccharide vaccine comprises a single intramuscular injection. It is indicated for adults and children aged 2 years or older, and seroconversion is achieved in >85% of immunocompetent adults. Vaccine efficacy lasts 2–3 years and booster vaccination is recommended every 2 years.Citation18,Citation20 Adverse reactions also include redness or pain at the injection site, muscle aches, and fevers. Coadministration of other intramuscular vaccines may produce increased redness at the Vi injection site. The only contraindication to its administration is a previous history of severe skin reaction associated with the Vi vaccine.Citation21
There are very limited data on the effectiveness of the Vi vaccine in the immunocompromised individual, but it should be administered to those traveling to endemic regions. HIV patients with CD4 counts <200/mm3 will have decreased antibody responses compared to those with CD4 counts >200/mm3.Citation22 In view of such limited data for the immunocompromised host, even after vaccination, it is of paramount importance that proper food and water hygiene measures be employed for disease prevention.
Hepatitis A
The hepatitis A virus is transmitted via the fecal–oral route and can lead to fulminant liver failure in the immunocompromised host. It is the second most common vaccine-preventable disease acquired during travel.Citation15 Estimated risk has been calculated among unvaccinated individuals to be 4–30 cases per 100,000 months of stay in a developing country.Citation23 The injectable vaccination requires two doses, administered 6–12 months apart, in SOT recipients who are a year or more post-transplant. The immunological response in SOT recipients is suboptimal and has a shorter duration compared to that in immunocompetent hosts. In 39 liver and 39 kidney transplants, seroconversion following two doses of the hepatitis A vaccine occurred in 72% and 97%, respectively, versus 100% in healthy controls. After 2 years, immunity persisted in 26%–59% of transplant recipients versus 100% of healthy controls.Citation24 Among rheumatoid arthritis patients on methotrexate and tumor necrosis factor inhibitors, vaccination with one dose of hepatitis A vaccine resulted in poor seroconversion rates.Citation2,Citation6 Thus, it is recommended that the immunocompromised traveler be vaccinated with two doses administered 6 months apart prior to departure. If time permits, then check for seroconversion 4 weeks prior to departure and, if the patient is seronegative, administer hepatitis A gamma globulin. If time does not permit adequate vaccination (eg, patient presents 2 weeks prior to travel), consider administering gamma globulin alone or in combination with hepatitis A vaccine.Citation2,Citation6 Immunocompromised travelers who receive routine intravenous immunoglobulin do not require vaccination against hepatitis A or measles.Citation5
Japanese encephalitis
Japanese encephalitis (JE) is caused by a flavivirus, resulting in meningitis, encephalitis, seizures, paralysis, and other neurologic disorders. It is transmitted by mosquitoes and is endemic in Southeast Asia, including several popular travel destinations in India and Thailand. In 2004, over 5.5 million travelers from the US visited JE-endemic countries. Case fatality is 20%–30% and neurologic sequelae exist in 30%–50% of survivors.Citation25 There is no treatment for JE apart from supportive care. Risk for disease acquisition depends on location of travel, activities during travel, and duration of travel. It is estimated that travel for prolonged periods in endemic countries puts one at similar risk for JE as that of the resident population (5–50 cases per 100,000 children per year). Outdoor activities in rural areas put travelers at greater risk, while travel limited to urban areas presents minimal risk for JE.Citation25 There is only one inactivated vaccine available in the US (Ixiaro) and it involves the administration of two intramuscular doses 28 days apart. Each dose costs nearly $300–$400 and, in terms of cost-effectiveness, understanding the detailed itinerary is important to determine JE risk and the need for vaccination. Vaccination is recommended for long-term travelers (>30 days) to endemic areas and short-term travelers during peak seasons, as well as for those traveling to rural areas, those who are likely to engage in outdoor activities, and those venturing into a JE-outbreak area. The vaccine is not recommended when short-term travel is restricted to urban areas or when travel occurs outside of JE transmission season.Citation25
Rabies
The rabies virus is transmitted by the bite of an infected mammalian host, resulting in fatal encephalitis in almost all cases. Canine rabies is enzootic in Asia, Africa, and South and Central America, as well as in other places. The risk of rabies among travelers in different studies has been estimated at 16–200 per 100,000 travelers.Citation5 The rabies vaccine consists of an injectable inactivated virus. It is recommended for those who plan on long-term (>30 days) travel, have frequent animal exposures, and travel to areas where medical care is not easily accessible. Immunologic response may be inadequate in some instances, and there are few data related to this vaccine in immunocompromised individuals. Even if adequate vaccination is provided, exposed travelers must present to health care providers for additional postexposure prophylaxis. Vaccination extends the window of opportunity in which one can delay postexposure prophylaxis but does not prevent infection. Patients exposed to the rabies virus should strongly consider medical evacuation.Citation26
Bacille Calmette-Guerin vaccine
Bacille Calmette-Guerin (BCG) is a live vaccine that can prevent active and severe tuberculosis but is contraindicated in the immunocompromised host. Vaccination can cause disseminated infection, termed BCGosis. The role for this vaccine even in healthy travelers remains controversial. Alternatively, pre- and posttravel detection of tuberculosis infection can be obtained through an interferon-gamma release assay, which may be superior to the purified protein derivative skin test, as the latter may be falsely negative in the immunocompromised patient.Citation5,Citation27
Routine vaccines
Routine vaccines should be recent for immunocompromised travelers. They are discussed separately in the following subsections.Citation5,Citation14,Citation28
Polio vaccine
Poliovirus causes myelitis with flaccid paralysis, and it has gained resurgence in selected Asian and African countries due to conflict; it is transmitted via a fecal–oral route. Two vaccine forms exist, the live oral and the injectable inactivated. The inactivated polio vaccine is recommended for immunocompromised individuals who are traveling to regions with polio outbreaks, in addition to their routine childhood series of vaccines. The live oral vaccine should not be given to household contacts of the immunosuppressed. The oral vaccine is no longer available in the US or Canada. Routine vaccination involves a childhood series and one booster; another booster should be given if vaccination was >10 years ago and travel involves going to a polio outbreak area or one with known circulating virus. Immunity is thought to last 5–10 years postvaccination in prior transplant recipients.Citation29
Meningococcal vaccine
The meningococcal vaccine is recommended for those traveling to high-risk settings such as the “meningitis belt” in sub-Saharan Africa during the winter (dry) months of December–June and for those traveling to Saudi Arabia for the Muslim pilgrimage of Hajj/Umrah. Vaccination is recommended with the quadrivalent polysaccharide (Menactra™). Immunocompromised travelers, especially those with primary complement deficiencies or asplenia, should be vaccinated every 5 years.Citation14,Citation30
Tetanus/diphtheria/pertussis vaccine
The acellular tetanus or Tdap vaccines can be given to those who have not received a booster within 10 years. Tetanus is common in most low-resource settings and, albeit rare among travelers, vaccination should be considered essential prior to travel. Diphtheria and pertussis should be considered while traveling to regions of the world where it is endemic and vaccination should be scheduled every 10 years. Seroprotection may wane more rapidly in SOT recipients, and diphtheria antibody titers should be assessed to determine the need for vaccination if they are below the protective titers.Citation2
Hepatitis B vaccine
Hepatitis B vaccination is essential when traveling to low-resource settings in case medical care is sought. Hepatitis B can be acquired through blood transfusions and other iatrogenic scenarios or through sexual contact. Factors affecting standard hepatitis B immune response include recent transplant and prior graft-versus-host disease.Citation28 A three-dose series can be given and serologic testing should be performed to detect a level ≥10 mIU (international units)/mL of anti-hepatitis B surface antibody. If levels are not adequate, then a second three-dose series can be given using the standard or high (40 μg) vaccine dose.Citation14
Influenza vaccine
Inactivated influenza vaccines should be administered annually unless there is profound immunosuppression, such as active treatment for transplant rejection or recent transplant prior to vaccination. In tropical regions, influenza occurs round the year, and so vaccination prior to departure for those unvaccinated within the year is prudent. Live attenuated influenza vaccines are contraindicated in these situations.
Pneumococcus vaccine
Immunization with both pneumococcal 13-valent conjugate (PCV13) and pneumococcal polysaccharide (PPVS23) vaccine is recommended for adults ≥65 years, followed by revaccination with PPV23 every 5 years. Vaccination with PCV13 is recommended in those <65 years who are immunocompromised or have asplenia, with revaccination at 5-year intervals with PCV13.Citation14,Citation31,Citation32
Measles, mumps, rubella vaccine
Measles is a viral disease spread via large respiratory droplets. While the US is disease free, outbreaks have occurred in Europe and in developing countries.Citation33 Measles, mumps, rubella (MMR) is a live virus vaccination and is contraindicated in persons with primary and secondary causes of humoral or cellular immune deficiencies, including those with HIV infection ≤200/mm3 (or T cell percentage <15). Measles inclusion body encephalitis, pneumonitis, and death have been reported with vaccination in immunocompromised individuals.Citation34 Serologic testing should be sought among SOT recipients prior to travel to endemic regions. For pre- and posttravel exposure, short-term immunoglobulin therapy can be given.Citation5,Citation6
Risk of vector-borne diseases and mosquito-related precautions
Vector-borne diseases include diseases transmitted by a range of insects, including mosquitoes, sand flies, ticks, and triatomes. Vaccinations or prophylactic medications are not available for many of these diseases, which are briefly discussed in the following subsections. Immunocompromised travelers are not necessarily at increased risk for infection from vector-borne diseases, but should they become ill, disease can be more severe.Citation2,Citation35 This remains especially true for malaria, leishmaniasis, and Chagas disease, while limited data exist for other infections such as dengue fever and chikungunya.Citation36–Citation39 Both leishmaniasis and Chagas disease can disseminate in immunocompromised patients, particularly HIV-affected and transplant patients.Citation36,Citation37 No vaccines or prophylaxis is available for either of these illnesses, and disseminated disease is refractory to treatment.
The dengue virus and the chikungunya virus (CHKV) are transmitted by mosquitoes. Dengue infections are quite common, accounting for roughly 10% of systemic febrile illnesses in travelers, suggesting that immunocompromised travelers are at risk.Citation40 Any increase in disease severity is poorly understood at this time. CHKV has emerged in recent years, first in the Reunion Islands, spreading to Italy and, most recently, to the Caribbean and the US.Citation41 As in dengue, limited information regarding the severity of CHKV in immunocompromised patients is available. Currently, no vaccines are available for either of the viruses, and supportive therapy is the only treatment option. For all of these illnesses, if the risk of infection is deemed high, travel should be delayed until disease incidence is decreased or immunosuppression is reversed; otherwise, travel should be avoided altogether.
Patients should strictly adhere to vector avoidance measures if travel to endemic areas is necessary.Citation5 This includes avoidance of mosquitos, especially during dusk and dawn when mosquitoes that transmit malaria are most likely to bite. Patients should wear long pants and shirtsleeves. A minimum of 20% diethyltoluamide should be applied. If indicated, patients should sleep under bed nets, especially in rural settings.
Malaria and chemoprophylaxis
Malaria is a major concern for travelers to endemic settings and the commonest cause of febrile illness in travelers.Citation40 Severe illness arises in immunocompetent but previously nonexposed adults and pregnant women. Conflicting evidence exists on whether or not HIV progresses in the setting of malaria, especially in HIV-positive travelers, but severe malaria is more common in HIV-positive patients.Citation38 Likewise, limited data are available for other forms of immunosuppression, but concern for severe disease persists.Citation5 All travelers to malaria-endemic regions should receive chemoprophylaxis based on CDC guidelines.Citation42 For immunosuppressed patients, care must be taken to evaluate for drug interactions.Citation2 Mefloquine can increase the concentration of calcineurin inhibitors, but it is safe in renal failure. Atovaquone/proguanil is generally considered safe in most circumstances, but its use may be limited by cost. Doxycycline may be useful but can reduce mycophenolate levels.
Traveler’s diarrhea and food safety
Up to 50% of all travelers will experience a gastrointestinal illness.Citation5,Citation43 The most common cause is enterotoxigenic Escherichia coli (ETEC). While most food-borne illness is self-limited, immunocompromised travelers remain at risk for severe infection from bacteria and parasites.Citation5 For ETEC, the rapid onset of voluminous diarrhea can induce renal failure and subsequent medication toxicities.Citation2 Other bacterial infections of concern include Salmonella spp, Campylobacter spp, and Shigella spp. Prophylactic antibiotics such as ciprofloxacin or rifaximin may be useful, especially for short-term travel, but carry risks (eg, Clostridium difficile-associated diarrhea with ciprofloxacin). Breakthrough illness does occur and it is imperative that immunocompromised travelers take prophylactic antibiotics with them. The choice of agent must be balanced with possible drug interactions and the location of travel. Fluoroquinolones are frequently prescribed but run the risk of QT prolongation or spontaneous tendon rupture. Azithromycin is frequently prescribed due to high rates of fluoroquinolone resistance in Campylobacter in Southeast Asia.Citation43,Citation44 This medication can also prolong the QT interval and increase the serum concentrations of calcineurin inhibitors.Citation2
Parasitic infections occur in 1%–20% of travelers with diarrhea depending on the study and location.Citation2 Cryptosporidia, Giardia, and Cyclospora infections are all self-limited in immunocompetent patients but can cause prolonged severe diarrhea in immunocompromised patients. Treatments are of minimal efficacy, especially for cryptosporidia.Citation45
Patients should maintain good food safety practices to avoid infections with these agents as well as avoiding bacterial infections.Citation5 Any food that has not been thoroughly cooked should be avoided. Cooked food should be eaten immediately and not be allowed to sit, such as in buffet style dining. Undercooked shellfish and seafood should also be avoided to prevent infections with viruses (eg, hepatitis E, hepatitis A, and norovirus), parasites (eg, anisakiasis, paragonimiasis), and bacteria (eg, Vibrio species). Undercooked meat can lead to infections with gram-negative organisms, such as Escherichia coli 0157 and Campylobacter, and parasites, such as Toxoplasma, Taenia solium, Entameoba, and tapeworms. Fresh fruits that can be peeled are considered safe. Unpasteurized milk products, including soft cheeses, can place travelers at risk for gram-negative infections with Listeria and Brucella. Foods with raw eggs, including Hollandaise sauce and mayonnaise, put travelers at risk for Salmonella infection. Bottled or boiled drinking water is advised.
Avoidance of other infectious diseases
Fungal diseases
Fungi transmitted through respiratory inhalation, such as Cryptococcus, Histoplasma, Paracoccidioides, and Penicillium, may cause respiratory and/or disseminated disease in the immunocompromised host.Citation46 Caving and other outdoor activities that place travelers at risk should be avoided.
Vaccination of close contacts
Close healthy contacts of an immunosuppressed patient who have received a live vaccine can shed and transmit virus to the immunosuppressed host. In general, these close contacts should avoid receiving the live influenza and oral polio vaccines. Yellow fever, typhoid, and MMR have been shown to be safe in close contacts.
Insect-related precaution
Mosquitoes, sand flies, chiggers, and ticks can carry disease, and an insect repellent containing diethyltoluamide is advised. Application several times a day is necessary. Clothing to cover the arms, ankles, and legs is recommended despite temperature conditions. Ticks can carry rickettsial disease, tick-borne encephalitis, Lyme disease, and other diseases not previously mentioned.
Diseases from skin exposure
Travelers should avoid walking barefoot or swimming in freshwater, which can put them at increased risk for abrasions, infections, and parasitic disease. Strongyloides can penetrate intact skin and can cause a hyperinfection syndrome, a risk that is higher if the traveler is taking corticosteroids. Other parasitic infections include hookworm and schistosomasis. Leptospirosis, caused by a gram-negative bacterium, can result in fever as well as liver and renal failure, and is acquired through skin contact with infected rodent urine. Evidence supporting prophylaxis against leptospirosis is ambiguous; however, the CDC recommends doxycycline 200 mg weekly starting 1–2 days prior to and during high-risk exposures (ie, floods, heavy rainfall, and recreational water activities).Citation5,Citation47
Sexually transmitted diseases
Travelers need to use barrier precautions when engaging in sexual encounters. Recreational drugs and alcohol can create scenarios of increased HIV transmission risk in countries where prevalence is high.
Emerging epidemics
Emerging outbreaks such as the current Ebola epidemic will continue to occur. Specific information regarding prevention in such unique situations, especially in the setting of immunocompromised travelers, is beyond the scope of this article. Travelers in general are advised to avoid travel to affected regions. Physicians should be aware of ongoing epidemics, follow recommendations per the CDC, and advise immunocompromised travelers accordingly.
Noninfectious disease-related precautions
Several noninfectious disease-related precautions should be discussed during the travel consultation, including potential drug interactions, altitude sickness, dehydration, heat stroke, injury prevention, predeparture planning for medical evacuation, and thromboembolism precautions. Adjustments in immunosuppressive medication may be necessary during travel. Injury is the leading cause of preventable death in the traveler and is often related to motor vehicle accidents. Wearing seatbelts and arranging safe in-country transport predeparture is important.Citation5 Transplant recipients are at increased risk of skin cancer and photosensitivity while being on voriconazole.Citation2 Sunscreen lotions offering ultraviolet A protection and avoidance measures (eg, wearing a hat) are advised.
Immunocompromised travelers should identify health care facilities abroad where they may seek care in the event of an emergency. A list of facilities can be found on the CDC and US Department of State Web sites. Emergency evacuation insurance should be purchased and is usually different from routine health care coverage. Travelers should bring excess medications in case of travel delays accompanied by a letter of necessity. All medications and syringes should be carried in hand luggage and stored at the appropriate temperature. If medical care is required abroad, consultation with transplant, rheumatology, and/or infectious diseases specialists is crucial for medical decision making.
Conclusion
More immunocompromised patients are traveling worldwide and a dedicated predeparture travel visit to discuss infectious disease prevention and other noninfectious disease precautions is strongly recommended. While the list of precautions described herein may sound excessive, many simple measures can prevent life-threatening complications. This review is not meant to discourage travel, but instead to encourage safe travel. Clinical assessment and advice for vaccination begins with defining the mechanisms and degree of immunosuppression. Live vaccine formulations, which include YF, oral polio, MMR, BCG, and oral typhoid vaccines, are generally contraindicated in those with severe immunosuppression. Vaccines to be administered are based on the patient’s immunosuppression level, region of travel, activities during travel, risk of disease exposure, and duration of travel. Immunologic response and duration of vaccine-induced immunity is dependent on the type of vaccine and the host’s immune system. Routine vaccines should be updated prior to travel. Chemoprophylaxis against malaria, mosquito-oriented precautions, and food safety to prevent traveler’s diarrhea should be discussed. Consultation with an infectious diseases specialist is strongly recommended to optimize the pretravel care of all immunocompromised travelers. This article addresses the key issues regarding the immunocompromised traveler within the limitations in providing an exhaustive review of the subject matter.
Disclosure
The authors report no conflicts of interest in this work.
References
- United Nations World Tourism Organization (UNWTO)UNWTO Tourism Highlights 201420142 Available from: http://mkt.unwto.org/publication/unwto-tourism-highlights-2014-editionAccessed October 1, 2014
- RosenJTravel medicine and the solid-organ transplant recipientInfect Dis Clin North Am201327242945723714348
- BaatenGGGeskusRRKintJARoukenAHSonderGJvan den HoekASymptoms of infectious diseases in immunocompromised travelers: a prospective study with matched controlsJ Travel Med201118531832621896095
- BoggildAKSanoMHumarASalitIGilmanMKainKCTravel patterns and risk behavior in solid organ transplant recipientsJ Travel Med2004111374314769286
- Centers for Disease Control and Prevention (CDC)CDC Health Information for International Travel 2014New YorkOxford University Press2014
- AsklingHDalmVThe medically immunocompromised adult traveler and pre-travel counseling: status quo 2014Travel Med Infect Dis201412321922824821082
- van der VeldenAMClaessenAMvan Velzen-BladHVaccination responses and lymphocyte subsets after autologous stem cell transplantationVaccine200725518512851717996989
- ElkayamOCaspiDReitblattTCharboneauDRubinsJBThe effect of tumor necrosis factor blockade on the response to pneumococcal vaccination in patients with rheumatoid arthritis and ankylosing spondylitisSemin Arthritis Rheum200433428328814978666
- RosenauJHoomanNRifaiKHepatitis B virus immunization with an adjuvant containing vaccine after liver transplantation for hepatitis B-related disease: failure of humoral and cellular immune responseTranspl Int2006191082883316961775
- Centers for Disease Control and Prevention (CDC)Yellow fever vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP)MMWR Morb Mortal Wkly Rep201059712120075837
- DomingoCNiedrigMSafety of 17D derived yellow fever vaccinesExpert Opin Drug Saf20098221122119309249
- VeitONiedrigMChapuis-TaillardCSwiss HIV Cohort StudyImmunogenicity and safety of yellow fever vaccination for 102 HIV-infected patientsClin Infect Dis200948565966619191654
- PacanowskiJLacombeKCampaPPlasma HIV-RNA is the key determinant of long-term antibody persistence after yellow fever immunization in a cohort of 364 HIV-infected patientsJ Acquir Immune Defic Syndr201259436036722267015
- RubinLGLevinMJLjungmanPInfectious Diseases Society of America2013 IDSA clinical practice guideline for vaccination of the immunocompromised hostClin Infect Dis2014583e44e10024311479
- BoggildAKCastelliFGautretPGeoSentinel Surveillance NetworkVaccine preventable diseases in returned international travelers: results from the GeoSentinel Surveillance NetworkVaccine201028467389739520851081
- MahonBENewtonAEMintzEDEffectiveness of typhoid vaccination in US travelersVaccine201432293577357924837780
- MacLennanCAMartinLBMicoliFVaccines against invasive disease: current status and future directionsHum Vaccin Immunother20141061478149324804797
- Centers for Disease Control and Prevention (CDC)Typhoid immunization – recommendations of the Advisory Committee on Immunization Practices (ACIP)MMWR Morb Mortal Wkly Rep199443RR1417
- BegierEMBurwenDRHaberPBallRPostmarketing safety surveillance for typhoid fever vaccines from the vaccine adverse event reporting system, July 1990 through June 2002Clin Infect Dis200438677177914999618
- MartinLBVaccines for typhoid fever and other salmonellosesCurr Opin Infect Dis201225548949922825288
- MarcusLCFroeschleJEHillDRSafety of Typhim Vi vaccine in a postmarketing observational studyJ Travel Med200714638639117995534
- KroonFPvan DisselJTRavensbergenENibberingPHvan FurthRImpaired antibody response after immunization of HIV-infected individuals with the polysaccharide vaccine against Salmonella typhi (Typhim-Vi)Vaccine19991723–242941294510462227
- FioreAEWasleyABellBPAdvisory Committeee on Immunization Practices (ACIP)Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP)MMWR Morb Mortal Wkly Rep200655RR–712316410759
- GuntherMStarkKNeuhausRReinkePSchroderKBienzleURapid decline of antibodies after hepatitis A immunization in liver and renal transplant recipientsTransplantation200171347747911233913
- Centers for Disease Control and Prevention (CDC)Use of Japanese encephalitis vaccine in children: recommendations of the Advisory Committee on Immunization Practices 2013MMWR Morb Mortal Wkly Rep2013624589890024226626
- GibbonsRVRupprechtCEPostexposure rabies prophylaxis in immunosuppressed patientsJAMA2001285121574157511268256
- National Center for Immunization and Respiratory DiseasesGeneral recommendations on immunization – recommendations of the Advisory Committee on Immunization Practices (ACIP)MMWR Recomm Rep2011602164
- Danziger-IsakovLKumarDAST infectious diseases community of practice. Vaccination in solid organ transplantationAm J Transplant201313suppl 431131723465023
- LjungmanPAschanJGustafssonBLewensohn-FuchsIWiniarskiJRingdenOLong-term immunity to poliovirus after vaccination of allogeneic stem cell transplant recipientsBone Marrow Transplant200434121067106915489876
- Centers for Disease Control and Prevention (CDC)Updated recommendation from the Advisory Committee on Immunization Practices (ACIP) for revaccination of persons at prolonged increased risk for meningococcal diseaseMMWR Morb Mortal Wkly Rep200958371042104319779400
- TomczykSBennettNMStoeckerCCenters for Disease Control and Prevention (CDC)Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP)MMWR Morb Mortal Wkly Rep2014633782282525233284
- Centers for Disease Control and Prevention (CDC)Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP)MMWR Morb Mortal Wkly Rep2012614081681923051612
- Centers for Disease Control and Prevention (CDC)Increased transmission and outbreaks of measles – European region, 2011MMWR Morb Mortal Wkly Rep201160471605161022129994
- Merck & CoMeasles, Mumps, and Rubella Virus Vaccine Live2014 Available from: http://www.merck.com/product/usa/pi_circulars/m/mmr_ii/mmr_ii_pi.pdfAccessed October 1, 2014
- KottonCNTravel and transplantation: travel-related diseases in transplant recipientsCurr Opin Organ Transplant201217659460023147910
- JarvisJNLockwoodDNClinical aspects of visceral leishmaniasis in HIV infectionCurr Opin Infect Dis20132611923221770
- LattesRLasalaMBChagas disease in the immunosuppressed patientClin Microbiol Infect201420430030924602129
- GonzalezRAtaideRNanicheDMenendezCMayorAHIV and malaria interactions: where do we stand?Expert Rev Anti Infect Ther201210215316522339190
- WaggonerJJSodaEADeresinskiSRare and emerging viral infections in transplant recipientsClin Infect Dis20135781182118823839998
- FreedmanDOWeldLHKozarskyPEGeoSentinel Surveillance NetworkSpectrum of disease and relation to place of exposure among ill returned travelersN Engl J Med2006354211913016407507
- Centers for Disease Control and Prevention (CDC)Chikungunya virus in the United States2014 Available from: http://www.cdc.gov/chikungunya/geo/united-states.htmlAccessed July 22, 2014
- Centers for Disease Control and Prevention (CDC)Guidelines for Treatment of Malaria in the United States2014 Available from: http://www.cdc.gov/malaria/resources/pdf/treatmenttable.pdfAccessed July 22, 2014
- HillDRBeechingNJTravelers’ diarrheaCurr Opin Infect Dis201023548148720683261
- TribbleDRSandersJWPangLWTraveler’s diarrhea in Thailand: randomized, double-blind trial comparing single-dose and 3-day azithromycin-based regimens with a 3-day levofloxacin regimenClin Infect Dis200744333834617205438
- AbubakarIAliyuSHArumugamCHunterPRUsmanNKPrevention and treatment of cryptosporidiosis in immunocompromised patientsCochrane Database Syst Rev20071CD00493217253532
- PanackalAAHjjehRACetronMSWarnockDWFungal infections among returning travelersClin Infect Dis20023591088109512384843
- Brett-MajorDMLipnickRJAntibiotic prophylaxis for leptospirosisCochrane Database Syst Rev20093CD00734219588424
- KottonCNFreedmanDOAdvising Travelers With Specific Needs: Immunocompromised TravelerChapter 8: The Yellow Book2014 Available from: http://wwwnc.cdc.gov/travel/yellowbook/2014/chapter-8-advising-travelers-with-specific-needs/immunocompromised-travelersAccessed October 2, 2014
- GershmanMDStaplesJEInfectious Diseases Related to Travel: Yellow FeverChapter 3: Yellow Book2014 Available from: http://wwwnc.cdc.gov/travel/yellowbook/2014/chapter-3-infectious-diseases-related-to-travel/yellow-feverAccessed October 1, 2014
