Abstract
The introduction of TNFα blockers has revolutionized the treatment of ankylosing spondylitis (AS). The objectives of this review are to summarize the most up-to-date data on long-term efficacy and safety of infliximab in AS, with special emphasis on axial and extra-articular disease, predictors of response, and radiological response. The general consensus of this literature search was that infliximab is highly efficacious in the treatment of AS. Most studies have demonstrated good clinical outcomes after 3 years of treatment, as measured by Spondyloarthritis International Society response in 75%–85% of treated AS patients. Reports on the long-term effects of infliximab as documented by radiological findings, however, are controversial. While some studies reported a similar progression rate as that of the historical OASIS cohort, others have suggested that infliximab may halt new bone formation. The long-term safety of infliximab is well known, mainly from data stored in national registries. While it has been suggested that side effects of infliximab may be fewer in AS compared to rheumatoid arthritis, data on this issue are sparse, with most of the information on long-term safety pertaining to rheumatoid arthritis. It can however be concluded that the long-term efficacy of infliximab is apparently maintained in AS and with an acceptable safety profile.
Introduction
Ankylosing spondylitis (AS) is a chronic inflammatory disease primarily affecting the axial skeleton, including the sacroiliac joints and the spine. It is more common in young men, with a prevalence of 0.15%–0.8% in the general population.Citation1 The main symptoms are inflammatory low-back pain, which is characterized by rest pain, especially at night and early morning and accompanied by morning stiffness. Extra-axial involvement can also be present, and includes peripheral arthritis (most commonly asymmetrically oligoarthritis of large joints), enthesitis, and dactylitis.Citation2,Citation3 Extra-articular involvement is not uncommon, and includes anterior uveitis, inflammatory bowel disease, psoriasis, and others.Citation4–Citation6
Managing patients with AS consists of patient education, exercise, lifestyle modifications, and medications.Citation7 In addition to the aid provided by the clinician, patient education and support can be obtained from support groups. Smoking is correlated with poor functional outcome, and it is one example of a modifiable risk factor.Citation8
Nonsteroidal anti-inflammatory drugs are usually the first-line therapy and the only medication required by many patients. Approximately 70%–80% of AS patients report a substantial relief of the back pain and stiffness compared to 10%–15% of patients with mechanical back pain.Citation9 In contrast, synthetic disease-modifying antirheumatic drugs (DMARDs), such as methotrexate, sulfasalazine, and corticosteroids, have failed to demonstrate a significant role in the treatment of axial disease,Citation10–Citation12 and are usually not recommended.
The introduction of anti-TNFα agents in 2000 represented a major advance in the treatment of AS. Early trials with infliximab and later on with other anti-TNFα agents demonstrated significant improvement in the clinical measures.Citation13,Citation14 Anti-TNFα agents are also indicated in patients with active disease who are already being treated with nonsteroidal anti-inflammatory drugs. Currently, the five anti-TNFα agents available in the USA and Europe for AS are infliximab, etanercept, adalimumab, golimumab, and certolizumab pegol. The aim of this review was to assess the long-term safety and efficacy of infliximab for the treatment of AS.
Efficacy and clinical response
Axial disease
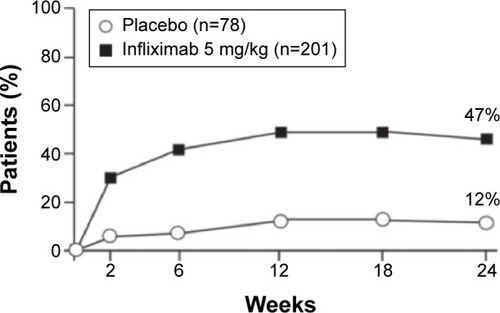
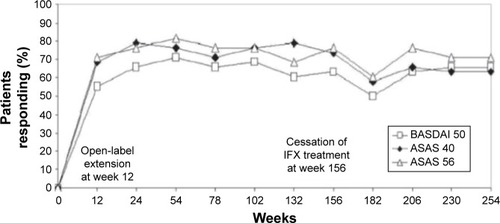
Infliximab, the first anti-TNFα agent introduced for the treatment of AS, usually induces rapid remission and decreases disease activity.Citation14,Citation15 Extra-articular manifestations of the disease also tend to respond to anti-TNFα therapy.Citation16 The first randomized controlled trial that demonstrated the efficacy of infliximab in AS was by Braun et al.Citation17 They randomized 69 patients with active AS to receive infliximab 5 mg/kg or placebo. After 12 weeks of treatment, 53% of the patients in the treatment group vs only 9% of the patients in the placebo group reported significantly decreased disease activity (as measured by a 50% reduction in the Bath Ankylosing Spondylitis Disease Activity Index [BASDAI]) (). Physical function and quality of life (as measured by the Bath Ankylosing Spondylitis Functional Index and Short-Form 36 Health Survey, respectively) significantly improved as well (P<0.0001). That first 12-week placebo-controlled trial was followed by a 3-year open-label extension that was completed by 43 of the 69 patients (62%). According to the study protocol, infliximab was discontinued after 3 years in order to evaluate time to flare. Most of the patients relapsed within 18–24 weeks, and 42 of them were restarted on infliximab, with a good response for most. Good clinical response (BASDAI <50%) was sustained for approximately 60% of the patients for 5 years ().Citation18 Other measures that were carried out in the extended trial and also showed promising results included Assessment of the Spondyloarthritis International Society (ASAS) 40 response, which was reached in 75% and 63% of patients at the end of 3 and 5 years, respectively. Similarly, an ASAS 5/6 response was achieved in 76% and 71% of patients at the end of 3 and 5 years, respectively. A BASDAI score <3 units (low disease activity) was attained in 57.9% of patients after 5 years. Those authors concluded that the high relapse rate and the short period to flare after discontinuation of the drug suggested that continuous therapy is necessary in patients with severe active AS.
Figure 1 Ankylosing spondylitis: disease activity.
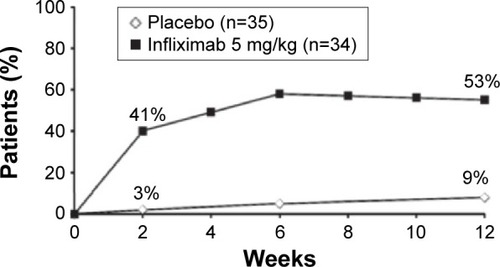
Figure 2 Ankylosing spondylitis: improvement in disease activity.
Abbreviations: ASAS, Assessment of Spondyloarthritis International Society; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; IFX, infliximab.
The ASSERT trial compared infliximab 5 mg/kg vs placebo in 279 AS patients.Citation19 In that randomized controlled trial, the clinical response was rapid and good activity scores were recorded, with values that were comparable to Braun et al ().Citation16 The European Ankylosing Spondylitis Infliximab Cohort (EASIC), a European multicenter study of long-term outcomes in patients with AS who were treated with infliximab, is an open-label continuous study based on the ASSERT trial patients.Citation20 Patients from the ASSERT trial continued into an open-label phase aimed at the evaluation of long-term safety and efficacy. Most of the patients (86%) continued to receive infliximab for 5 years, and they had good outcomes: only four of 103 patients dropped out of the study due to lack of efficacy. Venetsanopoulou et alCitation21 also investigated infliximab’s long-term efficacy, in addition to other parameters, such as tolerability, adverse events, and drug discontinuation. Thirty-five AS patients were enrolled and followed for 4 years. The BASDAI 50 response was 48.6% after both the third and fourth years, the ASAS 40 was 54.3% and 48.6% after the third and fourth years, respectively, and the ASAS 5/6 was 42.9% after both the third and fourth years. Drug survival was 94.3% after the first year, 91.4% after the second year, and 85.7% and 77.9% after the third and fourth years, respectively. Extra-articular manifestations were also found to be halted by infliximab.
Extra-articular manifestations
Approximately 20%–30% of AS patients develop uveitis.Citation5 One meta-analysis showed that infliximab significantly reduced the incidence of uveitis compared with placebo (P=0.005).Citation16 Up to 60% of AS patients have subclinical gut inflammation, which might later evolve into inflammatory bowel disease.Citation22 Another meta-analysis showed that AS patients treated with infliximab had reduced incidences of flares or new-onset inflammatory bowel disease compared with etanercept (P=0.001) and adalimumab (P=0.02).Citation23 Bone density was also shown to have improved under 24 weeks of infliximab treatment, as had been demonstrated in the ASSERT trial (P<0.001),Citation24 although the effect on vertebral fractures still remained to be evaluated. Arends et al’sCitation25 observational study evaluated the effects of anti-TNFα on bone turnover and bone density in AS patients by comparing bone markers and bone density of treated and untreated patients. After 3 years, 65 of the 111 patients (65%) were still under anti-TNFα therapy. Significant positive changes in bone metabolism compared to baseline values were noted in the treated patients, including increased alkaline phosphatase (a marker of bone formation), decreased serum collagen telopeptide (a marker of bone resorption), and increased lumbar spine and hip-bone mineral density.
Predictors of response
Others studies have tried to evaluate the predictors of good response to anti-TNFα agents. Arends et alCitation26 evaluated 220 patients with AS who started therapy with infliximab (n=32), etanercept (n=137), or adalimumab (n=51). ASAS 20 scores were 68% and 63% at 3 and 6 months, respectively, and ASAS 40 scores were 49% and 46% at 6 and 3 months, respectively, while BASDAI 50 was achieved by 49% and 50% of the patients at 3 and 6 months, respectively. The percentage of the responders was comparable between the different anti-TNFα-agent groups. Baseline predictors of response were younger age, male sex, higher Ankylosing Spondylitis Disease Activity Score, higher erythrocyte sedimentation rate (ESR) level, higher C-reactive protein (CRP) level, presence of peripheral arthritis, higher patient global assessment of disease activity, and lower modified Schober test score. Baseline predictors of discontinuation of TNFα-blocking therapy were female sex, absence of peripheral arthritis, higher BASDAI, lower ESR level, and lower CRP level.Citation27 Vastesaeger et al also evaluated predictors of response based on the ASSERT and GO-RAISE trials; they evaluated 635 anti-TNFα patients. Algorithm-based prediction models were created, and concluded that age, Bath Ankylosing Spondylitis Functional Index score, enthesitis, CRP, and HLA-B27 genotype were identified as predictors, and they suggested using this model in selecting patients for biologic therapy.Citation28 An important question is whether efficacy is maintained despite dose reduction of infliximab and extended infusion interval. Mörck et alCitation29 investigated whether efficacy is maintained after reduction of the infliximab dose from 5 mg/kg to 3 mg/kg and if the interval between infusions is prolonged from 6 weeks to 8 weeks in 19 AS HLAB27-positive patients. Those authors found that efficacy was maintained, based on BASDAI and inflammatory parameters, despite the extended intervals and dose reduction. Several studies that tried to cease the anti-TNFα agent completely in AS patients and in nonradiographic axial spondylarthritis suggested that relapse is almost inevitable. In one study, 41 of 42 AS patients relapsed after discontinuing infliximab within 1 year, and most of the patients gained remission after reintroduction of the drug.Citation30 Similar results were demonstrated in 24 AS patients treated with etanercept.Citation31
What are the reasons for good initial response and for failure to respond or develop infusion reactions after a certain period? One of the possible explanations is immunogenicity. Plasencia et alCitation32 evaluated the relevance of antibodies to infliximab in 94 patients with spondylarthritis who were undergoing infliximab treatment. Antidrug antibody formation was found to be associated with poor clinical response, the appearance of infusion reactions, and discontinuation of treatment. Kneepkens et alCitation33 evaluated the same question using adalimumab in AS patients, and found that adalimumab levels were related to clinical response (as measured with Ankylosing Spondylitis Disease Activity Score) and influenced by antidrug antibodies.
Radiological response
Axial inflammatory activity of AS demonstrated in magnetic resonance imaging has been well documented to respond to anti-TNFα therapy.Citation21,Citation34 The answer to the more inter esting question whether structural damage can be halted due to suppressed inflammation is still pending. The difficulty in answering this question comes both from slow radiological progression and inability to conduct long-run placebo-controlled trials, and thus most of the data come from observational trials and comparisons to historical cohorts.
Louie et al showed that studies with a relative short time frame of up to 2 years of treatment mostly failed to show a positive effect of anti-TNFα therapy on radiological progression, while emerging longer observational studies are starting to demonstrate markers of halted progression.Citation35 A study by van der Heijde et alCitation36 that was done using the ASSERT trial data specifically examined the effect of infliximab on radiographic progression in AS patients compared to the OASIS data. AS patients who received infliximab from baseline through week 96 did not show a significant difference in the inhibition of structural damage progression at year 2, as assessed by the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS) system, when compared with radiographic data from the OASIS cohort historical control.
On the other hand, Baraliakos et alCitation37 evaluated radiographic progression using conventional radiographs in AS patients treated with infliximab during a period of 4 years. The control group was the historical OASIS cohort. Although radiographic progression was demonstrated after 2 and 4 years of infliximab therapy in the AS patients’ (1.6±2.6) mSASSS, it was less than the progression documented for the OASIS cohort (4.4), suggesting that infliximab may decelerate the progression of structural changes. Another retrospective study by Baraliakos et alCitation38 evaluated the radiographic progression in AS patients treated with infliximab over 8 years compared to historical controls who had never been treated with TNFα blockers. Lateral cervical and lumbar radiographs were graded by blinded radiologists and scored by the mSASSS. The results demonstrated similar mSASSSs between the groups at 4 years, but showed a larger increase in the untreated group after 8 years (mean mSASSS was 0.9 per year in the treated group vs 1.5 in the untreated group). Less syndesmophyte formation was noted as well in the treated patients after 8 years, which argues against the TNF-brake hypothesis. Haroon et alCitation39 also investigated the effect of anti-TNFα agents on progressive spinal damage by conducting an observational study of 334 AS patients. Radiographic severity was assessed by the mSASSS. Patients with at least two sets of radiographs taken at a minimum of 1.5 years apart were included. TNFα-inhibitor treatment was associated with a 50% reduction in the odds of progression, with an odds ratio of 0.52 (95% confidence interval [CI] 0.30–0.88, P=0.02). Patients with a delay >10 years in starting therapy were more likely to experience progression compared to those who started earlier.
Safety
Safety considerations regarding therapy with anti-TNFα blockers are well characterized, due to over 15 years of experience and in great part due to national registries of thousands of patients receiving biological therapies for different indications. There are no major safety differences between the different anti-TNFα agents and indications, such as rheumatoid arthritis (RA), AS, and psoriatic arthritis. Therefore, this review covers safety issues regarding infliximab for all the aforementioned diseases. Patients receiving anti-TNFα agents often concomitantly use other agents, such as corticosteroids, azathioprine, mercaptopurine, and methotrexate, which may further contribute to side effects, such as infections. DMARDs and corticosteroid therapy are not part of the treatment guidelines of AS, unlike other inflammatory disease, such as RA and inflammatory bowel disease, and hence no assumption can be made about reduced adverse effects of AS patients treated with TNFα inhibitors compared to other diseases. There have been no publications of well-designed clinical studies to resolve this issue.
Serious adverse events with infliximab and other anti-TNFα agents include increased susceptibility for infectious diseases, such as tuberculosis (TB), invasive fungal infections, reactivation of hepatitis B virus, worsening of hepatitis C, and more. Allergic reactions ranging from mild infusion reaction to anaphylaxis have been documented, as have been malignancies, such as lymphoma and nonmelanoma skin cancers, as well as worsening of congestive heart failure, autoantibody formation, such as antinuclear antibody and anti-double-strand DNA antibody, neurological disorders, such as optic neuritis and seizures, and demyelinating disorders. In a recent report, Tong et al reported about adverse events of anti-TNFα therapy in 402 Han Chinese with AS.Citation40 Short- and long-term adverse events were evaluated regarding treatment with infliximab and recombinant human TNFα-receptor II:IgG Fc (rhTNFR-Fc). With regard to short-term adverse events, no significant differences between the two drugs were noted. Long-term adverse events occurred in 11% in the rhTNFR-Fc group vs 23.3% in the infliximab group (P=0.0013). Most long-term events were infectious disease, especially pneumonia and urinary tract infection, and the only significant difference regarding infectious disease between these groups was higher urinary tract infections in the infliximab arm. High baseline CRP and ESR was a predictor of long-term adverse events, and surprisingly elevated CRP and ESR were protective against short-term adverse events. summarizes the important adverse events of infliximab.
Table 1 Main side effects of infliximab in patients with ankylosing spondylitis
Infections
Two RA registry-based studies assessed the safety profiles of biologic agents in comparison to synthetic DMARDs, and found no major differences in infectious adverse events between the two arms. The first assessmentCitation41 was based on the RADIUS registry, which evaluated 10,000 patients with RA. There were no unexpected safety signals and no trends of reasons for concern compared with the data that were recorded during earlier trials and in the early days of TNF-inhibiting therapies. Additionally, the incidences of serious adverse events and serious infections of anti-TNFα agents were comparable with those of methotrexate. Similar conclusions were drawn from the second assessment, which was an observational cohort of the Consortium of Rheumatology Researchers of North America registry, which included 18,305 RA patients.Citation42 There was no significant increase in adjusted risk for overall infections associated with anti-TNFα therapy compared with methotrexate, and infection-related safety profiles of the various biologic agents appeared to be similar.
Dixon et alCitation43 evaluated the rates of serious infection, including site-specific and bacterial intracellular infection, in RA patients receiving anti-TNFα therapy. That national prospective observational study used the British Society for Rheumatology Biologics Register, which included 7,664 anti-TNFα-treated and 1,354 DMARD-treated patients with severe RA. All serious infections that were stratified by site and organism were included in the analysis. The crude rates of serious infections were found to be similar among TNF inhibitors: at approximately 50 events per 1,000 person-years for etanercept, infliximab, and adalimumab. The incidence-rate ratio, adjusted for baseline risk, for the TNFα-inhibitor cohort compared with the DMARD cohort was 1.03 (95% CI 0.68–1.57), suggesting similar risk levels between the two arms. However, the types of serious infections were different between the groups, with 19 serious bacterial intracellular infections (ten Mycobacterium TB, two Legionella pneumophila, three Listeria monocytogenes, one Mycobacterium fortuitum, and three Salmonella) occurring exclusively in patients in the TNFα-inhibitor cohort.
After adjustment for baseline risk, anti-TNFα therapy was not associated with an increased risk of overall serious infections compared with DMARD treatment in patients with active RA. However, the frequency of serious skin and soft-tissue infections was higher in anti-TNFα-treated patients, with an adjusted relative risk of 4.28 (95% CI 1.06–17.17). Additional data on Mycobacterium TB infection came from the French RATIO registry, which showed an increased risk of infection in patients treated with infliximab and other anti-TNF therapies, although this risk might be lower with etanercept.Citation44 Of note, none of those cases received TB prophylaxis. A large randomized, placebo-controlled trial assessed the risk of serious infections after 22 weeks of treatment with infliximab 3 mg/kg or 10 mg/kg vs placebo in active RA patients receiving methotrexate.Citation45 There was a higher incidence of serious infections, with a relative risk of 3.1 (95% CI 1.2–7.9, P=0.013) in the unapproved higher-dosage arm compared to the placebo group, but not to the approved dosage group.
A prospective cohort study of the German RA registry RABBIT compared the rates of infections in patients treated with the biologic agents infliximab, etanercept, and anakinra with rates of infections in patients receiving conventional DMARDs. After adjustment for differences in the case-patient mix, the relative risks of serious adverse events were 2.2 (95% CI 0.9–5.4) for patients receiving etanercept and 2.1 (95% CI 0.8–5.5) for patients receiving infliximab compared with controls.Citation46 Other research also suggests an increased risk of herpes zoster infection in patients treated with TNF antagonists, except for etanercept.Citation47 Of note, most infections reported in clinical trials of TNFα inhibitors have been minor, and were treated with either outpatient antibiotic therapy and/or temporary withdrawal of the drug.Citation48
Malignancy
The use of anti-TNFα agents in malignancy has gained some attention, due to their mechanism of opposing tumor cytokines. However, although the first described action of TNF was to oppose tumor cells, it has also protumor action,Citation49 thus making this issue more complicated and leading to inconclusive results in different studies on this important aspect.
Moulis et alCitation50 conducted a meta-analysis of 33 double-blind randomized controlled trials in adult RA patients, and found no excessive risk of malignancy with any of five anti-TNFα agents during up to 2 years of treatment. However, there was a tendency toward an increased rate of nonmelanoma skin cancer in different reports.Citation51 Two other meta-analyses – one on etanercept aloneCitation52 and another on adalimumab, infliximab, and etanercept that included more than 26,000 patientsCitation53 – did not demonstrate a significant increase in the risk of malignancy. Other research has suggested an increased risk of lymphoma in anti-TNFα-treated patients, but those results were complicated by the association of RA and increased lymphoma risk.Citation54 Currently, no definite conclusion has been reached regarding lymphoma risk with TNFα inhibitors, and postmarketing pharmacovigilance continues to track the incidence of lymphoma among individuals who are treated with them.
Autoimmune disorders
Anti-TNFα agents are not effective for the treatment of MS, and many small studies and series described demyelination following the use of TNF inhibitors.Citation55 Lupus-like symptoms and autoantibodies have been reported with each of the TNF inhibitors, apparently with decreased prevalence with etanercept compared to monoclonal antibodies.Citation56 Alopecia areata was recently reported to be associated with treatment with infliximab, adalimumab, etanercept, and certolizumab.Citation57 Finally, an increased risk of psoriasis was reported in a retrospective cohort study of RA patients treated with TNF inhibitors.Citation58 Infusion reactions ranged from mild infusion reaction (nausea, headache, flushing) to anaphylaxis, which is more common in infliximab due to its chimeric structure. Overall, infusion reactions occur in approximately 4% of patients compared to 1.6% for placebo, and most of the reactions are mild to moderate.
Conclusion
The management of AS and other inflammatory diseases has changed dramatically since the introduction of infliximab and other anti-TNFα agents. After more than 15 years of experience, the efficacy and safety profile of infliximab for the treatment of AS is now well established. Infliximab is an efficient therapy for the treatment of the different aspects of active AS, including axial, articular, and extra-articular manifestations. The main concern with infliximab and other biological agents is the inhibition of the immune system and the subsequent increased susceptibility for bacterial, viral, and fungal infections. There is probably a mild increased risk for general infections and opportunistic agents as well, with TB being the main concern. TB screening should be done before commencing therapy that consists of any one of the anti-TNFα agents, and anti-TB therapy should be given in cases of latent TB. Screening for hepatitis B virus and hepatitis C virus should be done as well, since activation of latent hepatitis B virus infection and deterioration of hepatitis C virus have been documented as well. Other serious adverse effects, such as malignancy, autoimmunity, and congestive heart-failure exacerbation, have been described as well, but there are no specific guidelines for dealing with these important issues, and their role in decision making still needs to be better defined.
Disclosure
The authors report no conflicts of interest in this work.
References
- GranJHusbyGThe epidemiology of ankylosing spondylitisSemin Arthritis Rheum1993223193348511596
- BraunJSieperJThe sacroiliac joint in the spondyloarthropathiesCurr Opin Rheumatol199682752878864578
- LeeJJunJJungSHigher prevalence of peripheral arthritis among ankylosing spondylitis patientsJ Korean Med Sci20021766967312378021
- PetersMvan der Horst-BruinsmaIDijkmansBNurmohamedMCardiovascular risk profile of patients with spondylarthropathies, particularly ankylosing spondylitis and psoriatic arthritisSemin Arthritis Rheum20043458559215609262
- RosenbaumJAcute anterior uveitis and spondyloarthropathiesRheum Dis Clin North Am1992181431511561400
- BaetenDDe KeyserFMielantsHVeysEAnkylosing spondylitis and bowel diseaseBest Pract Res Clin Rheumatol20021653754912406426
- SmolenJBraunJDougadosMTreating spondyloarthritis, including ankylosing spondylitis and psoriatic arthritis, to target: recommendations of an international task forceAnn Rheum Dis20147361623749611
- DoranMBrophySMacKayKPredictors of long-term outcome in ankylosing spondylitisJ Rheumatol20033031632012563688
- SongIPoddubnyyDRudwaleitMSieperJBenefits and risks of ankylosing spondylitis treatment with nonsteroidal antiinflammatory drugsArthritis Rheum20085892993818383378
- ChenJLiuCLinJMethotrexate for ankylosing spondylitisCochrane Database Syst Rev2006CD00452417054209
- CleggDRedaDAbdellatifMComparison of sulfasalazine and placebo for the treatment of axial and peripheral articular manifestations of the seronegative spondylarthropathies: a Department of Veterans Affairs cooperative studyArthritis Rheum1999422325232910555027
- HaibelHFendlerCListingJCallhoffJBraunJSieperJEfficacy of oral prednisolone in active ankylosing spondylitis: results of a double-blind, randomized, placebo-controlled short-term trialAnn Rheum Dis20147324324623625982
- BrandtJHaibelHCornelyDSuccessful treatment of active ankylosing spondylitis with the anti-tumor necrosis factor α monoclonal antibody infliximabArthritis Rheum2000431346135210857793
- BraunJBrandtJListingJTreatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trialLancet20023591187119311955536
- GraingerRHarrisonAAInfliximab in the treatment of ankylosing spondylitisBiologics2007116317119707326
- BraunJBaraliakosXListingJSieperJDecreased incidence of anterior uveitis in patients with ankylosing spondylitis treated with the anti-tumor necrosis factor agents infliximab and etanerceptArthritis Rheum2005522447245116052578
- BraunJBrandtJListingATwo year maintenance of efficacy and safety of infliximab in the treatment of ankylosing spondylitisAnn Rheum Dis20056422923415388511
- BraunJBaraliakosXListingJPersistent clinical efficacy and safety of anti-tumour necrosis factor-α therapy with infliximab in patients with ankylosing spondylitis over 5 years: evidence for different types of responseAnn Rheum Dis20086734034517967831
- van der HeijdeDDijkmansBGeusensPEfficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT)Arthritis Rheum20055258259115692973
- HeldmannFBrandtJvan der Horst-BruinsmaIEThe European Ankylosing Spondylitis Infliximab Cohort (EASIC): a European multicentre study of long term outcomes in patients with ankylosing spondylitis treated with infliximabClin Exp Rheumatol20112967268021906431
- VenetsanopoulouAIVoulgariPVAlamanosYPapadopoulosCGMarkatseliTEDrososAAPersistent clinical response of infliximab treatment, over a 4-year period in ankylosing spondylitisRheumatol Int20072793593917357804
- MielantsHVeysECuvelierCde VosMCourse of gut inflammation in spondylarthropathies and therapeutic consequencesBaillieres Clin Rheumatol1996101471648674145
- BraunJBaraliakosXListingJDifferences in the incidence of flares or new onset of inflammatory bowel diseases in patients with ankylosing spondylitis exposed to therapy with anti-tumor necrosis factor α agentsArthritis Rheum20075763964717471540
- VisvanathanSvan der HeijdeDDeodharAEffects of infliximab on markers of inflammation and bone turnover and associations with bone mineral density in patients with ankylosing spondylitisAnn Rheum Dis20096817518218495735
- ArendsSSpoorenbergAHoutmanPThe effect of three years of TNFα blocking therapy on markers of bone turnover and their predictive value for treatment discontinuation in patients with ankylosing spondylitis: a prospective longitudinal observational cohort studyArthritis Res Ther201214R9822546520
- ArendsSvan der VeerEKallenbergCGBrouwerESpoorenbergABaseline predictors of response to TNF-α blocking therapy in ankylosing spondylitisCurr Opin Rheumatol20122429029822418743
- ArendsSBrouwerEvan der VeerEBaseline predictors of response and discontinuation of tumor necrosis factor-alpha blocking therapy in ankylosing spondylitis: a prospective longitudinal observational cohort studyArthritis Res Ther201113R9421689401
- VastesaegerNvan der HeijdeDInmanRDPredicting the outcome of ankylosing spondylitis therapyAnn Rheum Dis20117097398121402563
- MörckBPulleritsRGeijerMBremellTForsblad-d’EliaHInfliximab dose reduction sustains the clinical treatment effect in active HLAB27 positive ankylosing spondylitis: a two-year pilot studyMediators Inflamm2013201328984524089587
- BaraliakosXListingJBrandtJClinical response to discontinuation of anti-TNF therapy in patients with ankylosing spondylitis after 3 years of continuous treatment with infliximabArthritis Res Ther20057R439R44415899030
- BrandtJListingJHaibelHLong-term efficacy and safety of etanercept after readministration in patients with active ankylosing spondylitisRheumatology (Oxford)20054434234815561737
- PlasenciaCPascual-SalcedoDNuñoLInfluence of immunogenicity on the efficacy of long-term treatment of spondyloarthritis with infliximabAnn Rheum Dis2012711955196022563028
- KneepkensEWeiJNurmohamedMYeoKExtended report: immunogenicity, adalimumab levels and clinical response in ankylosing spondylitis patients during 24 weeks of follow-upAnn Rheum Dis20157439640124326011
- BarkhamNKeenHCoatesLClinical and imaging efficacy of infliximab in HLA-B27-positive patients with magnetic resonance imaging-determined early sacroiliitisArthritis Rheum20096094695419333933
- LouieGHWardMMMeasurement and treatment of radiographic progression in ankylosing spondylitis: lessons learned from observational studies and clinical trialsCurr Opin Rheumatol20142614515024389865
- van der HeijdeDLandewéRBaraliakosXRadiographic findings following two years of infliximab therapy in patients with ankylosing spondylitisArthritis Rheum2008583063307018821688
- BaraliakosXListingJBrandtJRadiographic progression in patients with ankylosing spondylitis after 4 yrs of treatment with the anti-TNF-α antibody infliximabRheumatology (Oxford)2007461450145317623745
- BaraliakosXHaibelHListingJSieperJBraunJContinuous long-term anti-TNF therapy does not lead to an increase in the rate of new bone formation over 8 years in patients with ankylosing spondylitisAnn Rheum Dis20147371071523505240
- HaroonNInmanRLearchTThe impact of tumor necrosis factor α inhibitors on radiographic progression in ankylosing spondylitisArthritis Rheum2013652645245423818109
- TongQCaiQde MooijTAdverse events of anti-tumor necrosis factor α therapy in ankylosing spondylitisPLoS One201510e011989725764452
- GibofskyAPalmerWKeystoneESafety profiles of disease-modifying anti-rheumatic drugs and biologics in patients with rheumatoid arthritis: observations from the RADIUS registryArthritis Rheum200960Suppl 101593
- CurtisJChenLCushJThe risk for hospitalized and outpatient infections related to anti-TNF therapy and newer biologicsArthritis Rheum200960Suppl 102059
- DixonWGWatsonKLuntMHyrichKLSilmanAJSymmonsDPRates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: results from the British Society for Rheumatology Biologics RegisterArthritis Rheum2006542368237616868999
- TubachFSalmonDRavaudPRisk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: the three-year prospective French Research Axed on Tolerance of Biotherapies registryArthritis Rheum2009601884189419565495
- WesthovensRYocumDHanJThe safety of infliximab, compared with background treatments, among patients with rheumatoid arthritis and various comorbidities: a large, randomized, placebo-controlled trialArthritis Rheum2006541075108616572442
- ListingJStrangfeldAKarySInfections in patients with rheumatoid arthritis treated with biologic agentsArthritis Rheum2005523403341216255017
- StrangfeldAListingJHerzerPRisk of herpes zoster in patients with rheumatoid arthritis treated with anti-TNF-α agentsJAMA200930173774419224750
- KeystoneEAdvances in targeted therapy: safety of biological agentsAnn Rheum Dis200362Suppl 2ii34ii3614532146
- BalkwillFMantovaniACancer and inflammation: implications for pharmacology and therapeuticsClin Pharmacol Ther20108740140620200512
- MoulisGSommetABénéJCancer risk of anti-TNF-α at recommended doses in adult rheumatoid arthritis: a meta-analysis with intention to treat and per protocol analysesPLOS One20127e4899123155441
- MarietteXMatucci-CerinicMPavelkaKMalignancies associated with tumor necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysisAnn Rheum Dis2011701895190421885875
- BaecklundEEkbomASparénPFelteliusNKlareskogLDisease activity and risk of lymphoma in patients with rheumatoid arthritis: nested case-control studyBMJ19983171801819665898
- AsklingJFahrbachKNordstromBRossSSchmidCHSymmonsDCancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level dataPharmacoepidemiol Drug Saf20112011913021254282
- BaecklundEIliadouAAsklingJAssociation of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritisArthritis Rheum20065469270116508929
- MagnanoMRobinsonWGenoveseMDemyelination and inhibition of tumor necrosis factor (TNF)Clin Exp Rheumatol200422S134S14015552527
- MoulisGSommetALapeyre-MestreMMontastrucJIs the risk of tumour necrosis factor inhibitor-induced lupus or lupus-like syndrome the same with monoclonal antibodies and soluble receptor? A case/non-case study in a nationwide pharmacovigilance databaseRheumatology (Oxford)2014531864187124899660
- BénéJMoulisGAuffretMFessierCLefevreGGautierSTumor necrosis factor antagonists and alopecia: a case/non case in a nationwide pharmacovigilance databaseArthritis Rheum201264S788
- HarrisonMDixonWWatsonKRates of new-onset psoriasis in patients with rheumatoid arthritis receiving anti-tumour necrosis factor αtherapy: results from the British Society for Rheumatology Biologics RegisterAnn Rheum Dis20096820921518385277