Abstract
Among the host of distressing pathophysiological and psychosocial symptoms, fatigue is the most prevalent complaint in patients with systemic lupus erythematosus (SLE). This review is to update the current findings on non-pharmacological, pharmacological, and modality strategies to manage fatigue in patients with SLE and to provide some recommendations on optimal management of fatigue based on the best available evidence. We performed a systematic literature search of the PubMed and Scopus databases to identify publications on fatigue management in patients with SLE. Based on the studies reported in the literature, we identified nine intervention strategies that have the potential to alleviate fatigue in patients with SLE. Of the nine strategies, aerobic exercise and belimumab seem to have the strongest evidence of treatment efficacy. N-acetylcysteine and ultraviolet-A1 phototherapy demonstrated low-to-moderate levels of evidence. Psychosocial interventions, dietary manipulation (low calorie or glycemic index diet) aiming for weight loss, vitamin D supplementation, and acupuncture all had weak evidence. Dehydroepiandrosterone is not recommended due to a lack of evidence for its efficacy. In addition to taking treatment efficacy and side effects into consideration, clinicians should consider factors such as cost of treatment, commitments, and burden to the patient when selecting fatigue management strategies for patients with SLE. Any comorbidities, such as psychological distress, chronic pain, sleep disturbance, obesity, or hypovitaminosis D, associated with fatigue should be addressed.
Introduction
Systemic lupus erythematosus (SLE) is a chronic, multisystem, inflammatory, autoimmune connective tissue disorder. It is characterized by production of pathogenic autoantibodies and dysregulated immune responses by B-cells, T-cells, dendritic cells, and other immune cells, resulting in numerous clinical and serological manifestations.Citation1 A broad array of clinical presentations ranging from rash, oral ulcers, and arthralgias to life-threatening internal organ involvement such as lupus nephritis is common.Citation2 The course of SLE is extremely variable and is characterized by intermittent and unpredictable remissions and exacerbations (ie, flares) during which the immune system attacks various organs.Citation3 With recurrent flares (approximately 65% of patients per year),Citation3 SLE can lead to progressive disability. The cause of SLE is unknown, but is likely to be an interaction of genetic, environmental, and hormonal factors.Citation1 SLE affects mostly women, with an incidence about nine times higher than in men, and also has a higher prevalence in non-Caucasian populations.Citation4
Due to earlier diagnosis and better treatment options to control the disease and its complications, the 5-year survival of patients with SLE has exceeded 90% in most centers.Citation5 With a significant improvement in survival in this population, attention has additionally been directed toward improving health-related quality of life. Among the host of distressing pathophysiological and psychosocial symptoms, fatigue is the most prevalent complaint in approximately 50%–90% of patients with SLE,Citation6 with more than 50% of patients rating fatigue as the most debilitating symptom they experience.Citation7 Fatigue manifests as an overwhelming sense of extraordinary tiredness or exhaustion that is not completely relieved by rest or sleep.Citation8
In most cases the cause of fatigue is unknown. A number of factors associated with SLE-related fatigue have been reported, including pathochronobiological factors (sleep disturbance, physical inactivity), pathopsychosocial factors (anxiety, depression), chronic pain (fibromyalgia), obesity, and lack of perceived social support.Citation9–Citation14 Some studies have shown an association between disease activity (ie, cytokine and autoantibody levels) and fatigue,Citation9,Citation10,Citation15,Citation16 while others have reported that fatigue is not associated with any disease markers.Citation17,Citation18 Furthermore, fatigue was also reported in patients without clinical or laboratory evidence of active disease.Citation7
For many patients with SLE, fatigue disrupts normal daily functioning, decreases the ability to concentrate, and affects work, leisure, and social activities, leading to diminished quality of life,Citation6,Citation19 a high prevalence of work disability,Citation20,Citation21 and higher health care utilization.Citation22 At the same time, more than 80% of patients with SLE have reported that fatigue is not adequately addressed in their health care management plan.Citation23,Citation24
The aim of this review is to update the current findings on several non-pharmacological, pharmacological, and modality strategies to manage fatigue in patients with SLE and to provide some recommendations on optimal fatigue management based on the best available evidence.
Materials and methods
Literature search strategy
This systematic review was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.Citation25 The process began with the first author (HKY) performing a systematic electronic literature search of the PubMed and Scopus databases from their inception (1966 and 1996, respectively) to May 2014. Queries to identify potentially relevant publications on fatigue management in patients with SLE were based on Boolean combinations of the following search terms: ((lupus [tiab] OR SLE [tiab]) AND (fatigue [tiab] OR vitality [tiab] OR SF-36 [tiab] OR quality of life [tiab])). We also identified appropriate articles that were cited in review papers related to fatigue and lupus.
Study eligibility criteria
We limited this review to publications that were in the English language, with full text available, the majority of patients diagnosed with SLE, and adults (ie, 18+ years), although children could constitute a minority of the participants in a study. Only intervention and observational studies reporting a fatigue or vitality measure as one of the primary or secondary outcomes were included. Studies were excluded if they were case reports, dissertations, editorials, commentaries, or review articles, or if they employed primarily qualitative research methods.
Data extraction
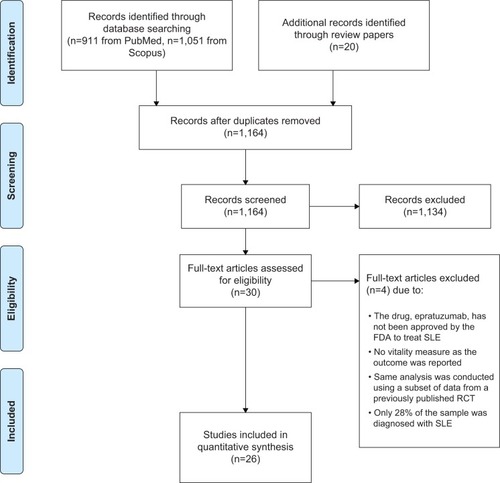
The first author (HKY) screened the titles and abstracts of all the articles that had been retrieved to determine if they met the eligibility criteria, and appraised the methodological quality and evidence of each selected study. The second author (MC) subsequently reviewed the accuracy and quality of the appraisal. Any disagreements were discussed until consensus was reached. The flow diagram in describes the process used to select articles for this study, and the results of this literature search.
Results
Based on the studies reported in the literature, we identified nine intervention strategies that have the potential to alleviate fatigue in patients with SLE. These strategies were psychosocial intervention, exercise, diet manipulation, vitamin D supplementation, N-acetylcysteine (NAC), dehydroepiandrosterone (DHEA), belimumab, ultraviolet-A1 (UVA-1) phototherapy, and acupuncture. The results of our critical review on the clinical efficacy evidence of each strategy to improve fatigue or vitality are summarized as follows.
Psychosocial intervention
Approximately 40% of patients who have SLE remain distressed over time, and since there is a significant association between distress and fatigue, these patients may benefit from psychosocial interventions.Citation26 Psychosocial interventions for fatigue management in patients with SLE included cognitive behavioral therapy,Citation27,Citation28 psychoeducation,Citation29,Citation30 counseling,Citation30,Citation31 psychotherapy,Citation29,Citation32 and biofeedback,Citation28 with the aim of reducing interference by the disease with activities of daily living, improving interpersonal relationships, social support, self-efficacy, and coping skills for stress management.Citation33 These interventions focused primarily on reducing psychological distress (ie, stress, anxiety and depressive symptoms), fatigue, and pain, and consisted of skills training in relaxation, pain control, problem-solving, coping, social interaction (eg, interpersonal relationship, assertiveness), and cognitive restructuring.
Of the seven psychosocial intervention studies identified, two were non-randomized controlled trials (non-RCTs, one being a single group pretest-posttest design studyCitation29 and one being a non-equivalent control group pretest-posttest design study),Citation33 and five were RCTs.Citation27,Citation28,Citation30–Citation32 The two non-RCTs demonstrated a decrease in fatigue or an improvement in vitality.Citation29,Citation33 Of the five RCTs, only one demonstrated a reduction in fatigue.Citation30 The other four trials showed that psychosocial interventions resulted in either no significant decrease in fatigue when compared with standard usual careCitation27,Citation28,Citation32 or were equally effective in reducing fatigue when compared with placebo (ie, symptom monitoring only).Citation28,Citation31
The RCT that demonstrated a decrease in fatigue compared a psychoeducational intervention with an attention placebo control.Citation30 Patients and their partners assigned to the intervention group (n=64) received a 1-hour session with a nurse educator and subsequent monthly telephone counseling for 6 months, whereas participants (n=58) in the attention placebo group received a 45-minute video presentation about lupus and monthly telephone monitoring. The authors used a four-item visual analog scale (VAS) to assess levels of patient fatigue when performing various daily activities.Citation34 Rating on the scale ranged from 0 (no fatigue) to 10 (extreme fatigue). There was no significant difference in fatigue scores between the intervention and placebo groups at 6 months (ie, immediate post-telephone counseling); however, a significant difference in fatigue scores was observed between the intervention and placebo groups at 12 months (6 months without intervention for both groups), with the intervention group reporting less fatigue.Citation30 This phenomenon may suggest that the findings were not reliable and simply reflected sample attrition.
Overall, there was only a 0.7-point improvement in fatigue score from baseline to 12 months in the intervention group.Citation30 This amount of reduction did not reach the minimal clinically important difference (MCID) for the fatigue VAS, which was estimated to be 1.3 points.Citation35 Furthermore, as revealed in a previous meta-analysis study,Citation36 pooled analysis of the effects of psychosocial interventions on fatigue with another RCTCitation28 indicated that no significant difference in fatigue scores between the intervention and control groups was observed.
Based on the available evidence, psychosocial interventions seem effective in reducing psychological distress and pain,Citation36 and in improving problem-focused coping ability, self-efficacy, and perceived social support.Citation33 However, evidence to support psychosocial interventions in alleviating fatigue in patients with SLE was very weak. Furthermore, no immediate or sustained (12-month follow-up) effects of psychosocial intervention on physical function, mental health, and SLE disease activity were observed.Citation36
Exercise
Since physical inactivity was found to be significantly associated with fatigue in patients with SLE,Citation9,Citation12 exercise is a logical strategy to alleviate fatigue. Studies of exercise for patients with SLE used primarily aerobic exercise, in which the exercise was conducted either at a specific site (ie, supervised) or at home (ie, unsupervised) with weekly telephone monitoring. Typically, exercise was performed three times per week for 30–50 minutes, achieving an intensity of 60%–80% of the participant’s maximum heart rate, and the duration of the study ranged from 8 to 12 weeks. The primary aims of the exercise programs were to increase exercise tolerance, aerobic capacity, and perceived physical function, and to reduce fatigue.
Of the seven exercise studies identified, three were non-RCTs (one being a non-equivalent control group pretest-posttest design studyCitation37 and two being single group pretest-posttest design studies),Citation38,Citation39 and four were RCTs.Citation34,Citation40–Citation42 Three of the RCTsCitation34,Citation40,Citation42 and the non-equivalent control group studyCitation37 demonstrated that exercise significantly reduced fatigue when compared with the controls. The RCT that did not show a significant difference in fatigue was likely due to inadequate statistical power as the sample size of each group was only 5.Citation41 For this trial,Citation41 following the 2-month supervised exercise program, the magnitude of fatigue reduction, as measured by the nine-item Fatigue Severity Scale (FSS),Citation43 exceeded the threshold (ie, 0.6 points) of the MCID for the FSS.Citation35,Citation41 Both single group studies showed significant improvement in fatigue after exercise.Citation38,Citation39 In addition, exercise has been shown to be safe and does not aggravate SLE disease activity.Citation34,Citation38,Citation40–Citation42
The findings of these studies highlighted the role of exercise in reducing fatigue in patients with SLE; however, few studies evaluated long-term exercise adherence in patients with SLE or the sustained effect of exercise on reducing fatigue in this population. Therefore, large-scale RCTs with long-term follow-up are needed to support this conclusion.
In addition, there are issues related to difficulty measuring fatigue and interpreting findings in these studies. Most exercise studies included several measures for fatigue, but often only one fatigue measure was found to be significantly different between the exercise and control groups.Citation34,Citation37,Citation40,Citation42 In addition, among these studies, no one particular fatigue measure consistently showed a significant difference between groups. For example, there were two exercise studies of similar research design conducted by the same group of researchers;Citation34,Citation40 both used two fatigue measures, ie, the seven-item fatigue subscale of the Profile of Mood StatesCitation44 and a four-item VAS for fatigue.Citation34 One study found that the fatigue subscale of Profile of Mood States scores, but not the VAS fatigue scores, were significantly different between the groups, while the other study found the opposite.Citation34,Citation40
Willingness to participate in an exercise program or to continue exercise regularly after the study program ended was a significant problem. In one home-based exercise study, patients received a free Wii console and Wii Fit balance board, mitigating the need for travel to a fitness center for exercise, and the accrual rate was only 43%.Citation39 In another exercise study, the researchers recruited 93 patients to participate, but mentioned that 31 eligible patients declined to participate due to lack of time.Citation42 In addition, about one fifth (18%) of the participants assigned to the exercise group did not attend any supervised exercise sessions.Citation42 This appears to indicate a lack of enthusiasm on the part of patients with SLE to participate in exercise training. Finally, less than a quarter (24%) of the participants in that study continued to exercise regularly at the 3-month follow-up.Citation42
Manipulation of diet
Between 40% and 50% of adults with SLE are classified as obese.Citation14,Citation45–Citation47 Obese patients with SLE are more likely to experience higher levels of fatigue.Citation13,Citation14 Davies et al conducted a randomized comparative trial to explore the safety and benefits of special diets with regard to weight loss and fatigue reduction in patients with SLE.Citation48 They compared a low glycemic index (GI) diet (n=11) versus a low calorie diet (n=12) in 23 patients for 6 weeks. Fatigue was measured using the FSS.Citation43 The results indicated that both diets were equally effective in reducing fatigue and helping patients to lose weight. Fatigue reduction was reported by seven of the eleven participants in the low GI diet group, and four of the 12 in the low calorie diet group. The fatigue score was reduced by 0.5 points in the low GI diet group, and 0.3 points in the low calorie diet group. However, the amount of reduction did not reach the MCID of 0.6 points for the FSS.Citation35 Both diets were well tolerated, with mild adverse effects (headache, constipation, increased bowel frequency, bloating), and SLE disease activity remained unchanged.
Vitamin D supplementation
Sunlight avoidance, use of sunscreen, renal insufficiency, and drugs (such as chloroquine and prednisone) prescribed to treat the symptoms of SLE interfere with vitamin D metabolism and put patients with SLE at risk for vitamin D deficiency.Citation49 In fact, hypovitaminosis D is common in patients with SLE.Citation49
Evidence regarding the association between vitamin D deficiency and fatigue is inconsistent. In an open-label observational study, 60 patients with SLE took dietary vitamin D3 supplements (400–1,200 IU per day) for 2 years, by which time significant improvement in fatigue scores, as measured by a 10-point VAS for fatigue, was observed.Citation50 There was a 0.8-point improvement in fatigue score, but this did not reach the MCID of 1.3 points for the fatigue VAS.Citation35 Due to the lack of a control group and the unmasked nature of this open-label study, strong bias related to self-reported fatigue levels was unavoidable.
In that study, a significant association between serum 25(OH)D and fatigue levels at 2-year follow-up was also observed.Citation50 In contrast, the majority of cross-sectional studies did not find any significant relationship between vitamin D and fatigue levels.Citation51–Citation54 Participants in these cross-sectional studies did not know their serum 25(OH)D level or the purpose of the investigation.Citation51–Citation54 Although few in number, the findings of these studies do not support vitamin D supplementation as having a significant direct effect on improving fatigue in patients with SLE.
N-acetylcysteine
NAC, an amino acid precursor of glutathione, serves as an inhibitor of autoimmune inflammatory processes.Citation55 The data suggest that patients with SLE have low levels of glutathione.Citation56 Lai et al conducted a double-blind, placebo-controlled, randomized trial to explore the safety and benefits of orally formulated NAC in patients with SLE.Citation57 Thirty-six patients received either placebo (n=9) or one of three daily doses (1.2 g, 2.4 g, or 4.8 g) of NAC for 3 months (n=9 in each dose group). After 1–3 months, significant improvement was achieved with the two higher NAC doses, ie, 2.4 g/day and 4.8 g/day, in terms of anti-DNA, disease activity, and fatigue as measured by the Fatigue Assessment ScaleCitation58 when compared with placebo. In addition, for the 2.4 g/day group, the amount of fatigue reduction exceeded the MCID of 4 points for the Fatigue Assessment Scale.Citation59
Regardless of the mean baseline score, participants who received NAC 2.4 g/day or 4.8 g/day, or placebo all rated their fatigue level reduced to about 24 at 3 months. However, the mean ± standard deviation score on the Fatigue Assessment Scale in a random sample from an adult Dutch population was 19.2±6.5.Citation58 According to this pioneer work, a 2.4 g/day dose of NAC is effective for reducing fatigue but may not be potent enough to reduce fatigue in patients with SLE to a level that is comparable with that in the general population. The 2.4 g/day dose was tolerated by all participants, but the 4.8 g/day dose was less well tolerated, with one third of the participants reporting headache, heartburn, nausea, and vomiting.Citation57
Dehydroepiandrosterone
DHEA is a naturally occurring mild androgen with immunomodulatory properties.Citation60 The data suggest that patients with SLE have lower mean serum DHEA levels,Citation61 and lower levels of DHEA might relate to increased fatigue.Citation62 Beneficial health effects of DHEA supplementation in patients with SLE include reducing disease activity and improving global well-being and functioning.Citation63–Citation66 However, several double-blind, randomized, placebo-controlled trials have assessed the effect of orally formulated DHEA (200 mg per day) on fatigue specifically, and concluded that DHEA has either no effect on reducing fatigue or the same effect as placebo.Citation66–Citation69 Possible side effects in DHEA users include acne, hirsutism, weight gain, and menstrual changes, making this therapy less attractive to female lupus patients.Citation60
Belimumab
Belimumab is a fully humanized monoclonal antibody that binds to the soluble form of B-lymphocyte stimulator protein and inhibits its biological activity.Citation70 B-lymphocyte stimulator protein, also known as B-cell activating factor, is an immunomodulatory cytokine that promotes B-cell proliferation and survival.Citation71 B-lymphocyte stimulator protein levels are high in patients with SLE and correlate with changes in disease activity.Citation72
Two large multisite international collaboration trials assessed the efficacy of belimumab in patients with active SLE.Citation73,Citation74 They compared two doses of belimumab (1 and 10 mg/kg) with placebo over 1 year. The pooled analysis of data from these two randomized, double-blind, placebo-controlled clinical trials in 1,684 patients showed that both doses of belimumab administered intravenously once a month significantly reduced disease activity and corticosteroid use, and improved scores in the four-item vitality scale of the Medical Outcome Study 36-item Short-Form Health Survey (SF-36) and Functional Assessment of Chronic Illness Therapy Fatigue (FACIT-Fatigue) scale at week 52 when compared with placebo.Citation75 Mean score improvements in both FACIT-Fatigue and SF-36 vitality scales exceeded the MCIDs of their respective scales.Citation75 The SF-36 vitality scale score at week 52 was comparable with age-matched and sex-matched US norms.Citation75 In fact, significant differences in FACIT-Fatigue scale scores were observed between belimumab and placebo beginning at week 8.Citation76
In addition, extending the first phase of these two trials, 296 patients who completed the 52-week, double-blind treatment continued to receive monthly belimumab 10 mg/kg for 4 years in an open-label study.Citation77 The results indicated that the number of patients experiencing fatigue after 4 years of belimumab therapy decreased by four-fold (to 7.3%) compared with placebo or baseline.Citation77
Belimumab is effective for reducing fatigue in patients with active SLE and without severe nephritis or central nervous system involvement.Citation78 Adverse effects in belimumab users include headache, insomnia, arthralgia, gastrointestinal complaints (nausea, diarrhea), infection (upper respiratory tract, urinary tract infections), cellulitis, psychiatric disorders (anxiety, depression with increased suicidal ideation), and transient ischemic attack.Citation77,Citation79
Ultraviolet-A1 phototherapy
With the discovery of the immunosuppressive properties of long-wavelength ultraviolet radiation (ie, a UVA-1 range of 340–400 nm), this modality serves as a potential adjuvant therapy for SLE.Citation80 Polderman et al conducted a randomized, double-blind, placebo-controlled crossover trial to evaluate the safety and benefits of UVA-1 phototherapy.Citation81 In two consecutive 12-week periods, eleven patients with mild to moderate SLE received low-dose (6 J/cm2) whole-body UVA-1 phototherapy and a placebo light treatment five times per week for the first 3 weeks, or vice versa. Patients who received the UVA-1 phototherapy showed a significant improvement in the SF-36 vitality scale score compared with the same patients who received placebo. A 16-point increase in vitality score after UVA-1 phototherapy was observed.Citation81 Score improvement exceeded the threshold (ie, 3–5 points) of the MCID for the SF-36 vitality scale.Citation35,Citation82 However, none of the other seven subscales or total SF-36 scores showed a significant difference following UVA-1 phototherapy when compared with placebo. Based on the P-value (0.03) provided, the comparison between the vitality score following UVA-1 phototherapy and placebo would not be statistically significant, had corrections for chance been made.
UVA-1 phototherapy did not achieve a significant improvement in disease activity when compared with placebo;Citation81 however, a subsequent study conducted by the same group of researchers reported that UVA-1 (when doubling the intensity) was more effective than placebo in reducing SLE disease activity.Citation83 No signs of photosensitivity or other adverse events were reported at the lower dose;Citation81 however, as might be expected, two of 12 patients in the subsequent study had transient skin reactions (ie, erythema).Citation83 Serious adverse effects of UVA-1 may include photocarcinogenesis, phototoxicity, and induction of photodermatoses.Citation84 Contraindications to UVA-1 phototherapy are disorders of photosensitivity, long-term use of immunosuppressants and photosensitizing agents, history of skin cancer, or receiving radiation therapy.Citation84 It appears that UVA-1 phototherapy may be effective in reducing fatigue in patients with SLE, although larger-scale RCTs with long-term follow-up would be needed to confirm the previous findings and ensure its safety in lupus patients, which is uncertain.
Acupuncture
A systematic review on the effectiveness of acupuncture in reducing fatigue in patients with chronic fatigue syndrome indicated that about 92% of patients pooled from 13 studies who received acupuncture alone or augmented with moxibustion reported effectiveness in fatigue reduction.Citation85 Greco et al conducted a modified double-blind RCT to explore the safety and benefits of acupuncture in reducing fatigue and pain in patients with SLE.Citation86 They compared standardized acupuncture with minimal needling (sham) and usual care in 24 patients (eight in each group) during ten sessions over approximately 5 weeks.Citation86 Fatigue was measured using the FSSCitation43 and SF-36 vitality scale.Citation87 The results indicated that acupuncture and minimal needling were significantly superior to usual care in reducing fatigue. However, acupuncture and minimal needling were equally effective. The mean improvement scores on FSS and SF-36 vitality scale were 0.35 points and 1.6 points, respectively. However, the amount of reduction did not reach the MCID of 0.6 points for the FSS or 3–5 points for the SF-36 vitality scale.Citation35,Citation82 Only one of eight (13%) participants in the acupuncture group achieved clinical improvement in fatigue (vitality). Approximately 40% of participants in the acupuncture group reported an improvement in pain score, but no improvement in disease activity was observed.
Only transient adverse events, including local bruising, pain during needle insertion, dizziness, and lightheadedness were reported in the acupuncture and minimal needling groups.Citation86 Acupuncture is not recommended for SLE patients with advanced visceral organ involvement.Citation88
Discussion
Since fatigue management is part of the overall treatment of patients with SLE, selection of fatigue management strategies may often be dictated by the treatment that targets control of SLE disease activity. Strategies for fatigue management should be selected using those that are least invasive and with the fewest side effects. In addition to taking treatment efficacy and side effects into consideration, clinicians should consider factors such as cost of treatment, commitment, and burden to the patient when selecting fatigue management strategies with SLE (see ). These important factors need to be assessed when evaluating any strategies for fatigue management in patients with SLE using well-designed, large-scale RCTs with long-term follow-up in order to provide robust clinical evidence.
Table 1 Considerations of various intervention strategies on fatigue management in patients with SLE
Even though evidence of the efficacy of psychosocial interventions for reducing fatigue in patients with SLE is weak, such interventions are effective in reducing psychological distress and pain in this population,Citation36 and should be integrated into fatigue management. Based on the existing evidence, optimal management of fatigue for patients with SLE should start with lifestyle changes, which include aerobic exercise and dietary manipulation with the aims of increasing physical activity and weight control, respectively. Although clinicians should always encourage healthy choices by their patients, there are, undeniably, many barriers to modifying lifestyle and thus these changes are often difficult for patients with SLE to implement and sustain.
Since vitamin D supplementation for SLE patients with hypovitaminosis D is associated with significant improvement in disease activity,Citation89 clinicians frequently prescribe vitamin D supplementation as an adjuvant therapy for patients with SLE who are also diagnosed with low levels of serum 25(OH)D. Therefore, hypovitaminosis D-associated fatigue should be managed with vitamin D supplements.
Even though evidence for efficacy of the NAC supplement in reducing SLE disease activity and fatigue is still in the early phase of clinical trials, its use in fatigue management for patients with SLE is encouraging. Given that NAC supplements at 2.4 g/day have minimal side effects, and the cost of this supplement is affordable, it may be included in fatigue management.
Belimumab is a prescription medication that has been approved by the US Food and Drug Administration to treat SLE, and has been shown to reduce fatigue (ie, to increase vitality). However, side effects and the high cost of this medication are two factors that need to be considered when prescribing belimumab for fatigue management.
The use of UVA-1 phototherapy to manage fatigue for patients with SLE is somewhat encouraging, but the data are limited and safety concerns remain, as it may exacerbate SLE. The long-term efficacy and side effects of UVA-1 are unknown, so caution should be exercised in prescribing this modality to treat SLE. Furthermore, not all rheumatology clinics have the appropriate apparatus and personnel to provide this service.
For SLE patients with pain as a contributing factor associated with fatigue who do not want to rely on medications to control pain, acupuncture is an option to manage fatigue. Evidence to support its efficacy in fatigue reduction for patients with SLE is weak, and patients must be unafraid of acupuncture needling and be able to afford to pay out-of-pocket as it is not widely covered by insurance plans for treating SLE. Pain can also be simultaneously managed using meditation or biofeedback (part of psychosocial intervention).
Limitations
Given that this review was limited to papers published in the English language, with full text available, it is possible that we may have missed some important studies written in languages other than English, not indexed in these two databases (PubMed and Scopus), or as part of reporting bias. To reduce the probability of excluding appropriate studies relevant to this review, we used the tracking citations function of Scopus to locate additional appropriate articles that cited our selected studies. We did not include or evaluate a study of epratuzumab, an experimental drug administered at a dose of 360 mg/m2, which has been shown to improve the SF-36 vitality scale score at week 48, exceeding age-matched and sex-matched US normative values,Citation90 as epratuzumab has not yet been approved by the US Food and Drug Administration to treat SLE. We also did not include another RCT on the benefit of expressive writing on reduction of fatigue because only about one quarter of patients in that study had SLE (54/75 [72%] of the participants had rheumatoid arthritis) and no disease group stratum was formed before randomization,Citation91 so it is not possible to accurately interpret the beneficial findings of expressive writing and attribute that to SLE participants only.
Conclusion
Of the nine strategies for fatigue management in patients with SLE reviewed in this study, aerobic exercise and belimumab seem to have the strongest evidence for treatment efficacy. NAC and UVA-1 phototherapy demonstrated low-to-moderate levels of evidence. Psychosocial interventions, dietary manipulation (low calorie or glycemic index diet), vitamin D supplementation, and acupuncture all had weak evidence. Finally, DHEA is not recommended due to lack of evidence for its efficacy.
SLE-related fatigue is a complex phenomenon and a broad array of factors is commonly associated with fatigue. Optimal fatigue management should, therefore, start with a comprehensive evaluation of the patient as relates to these factors. Ideally, treatment strategies should be tailored to the individual patient’s physical and psychosocial health status, and their cultural background. Any comorbidities such as psychological distress, chronic pain, sleep disturbance, obesity, and hypovitaminosis D that are associated with fatigue should be addressed.
Disclosure
The authors report no conflicts of interest in this work.
References
- GualtierottiRBiggioggeroMPenattiAEMeroniPLUpdating on the pathogenesis of systemic lupus erythematosusAutoimmun Rev2010103720863908
- D’CruzDPKhamashtaMAHughesGRSystemic lupus erythematosusLancet200736958759617307106
- PetriMGenoveseMEngleEHochbergMDefinition, incidence, and clinical description of flare in systemic lupus erythematosus. A prospective cohort studyArthritis Rheum1991349379441859487
- DanchenkoNSatiaJAAnthonyMSEpidemiology of systemic lupus erythematosus: a comparison of worldwide disease burdenLupus20061530831816761508
- TragerJWardMMMortality and causes of death in systemic lupus erythematosusCurr Opin Rheumatol20011334535111604587
- SchmedingASchneiderMFatigue, health-related quality of life and other patient-reported outcomes in systemic lupus erythematosusBest Pract Res Clin Rheumatol20132736337524238693
- KruppLBLaRoccaNGMuirJSteinbergADA study of fatigue in systemic lupus erythematosusJ Rheumatol199017145014522273484
- AhnGERamsey-GoldmanRFatigue in systemic lupus erythematosusInt J Clin Rheumatol20127217227
- Da CostaDDritsaMBernatskySDimensions of fatigue in systemic lupus erythematosus: relationship to disease status and behavioral and psychosocial factorsJ Rheumatol2006331282128816758508
- JumpRLRobinsonMEArmstrongAEBarnesEVKilbournKMRichardsHBFatigue in systemic lupus erythematosus: contributions of disease activity, pain, depression, and perceived social supportJ Rheumatol2005321699170516142863
- OmdalRWaterlooKKoldingsnesWHusbyGMellgrenSIFatigue in patients with systemic lupus erythematosus: the psychosocial aspectsJ Rheumatol20033028328712563681
- MancusoCAPernaMSargentABSalmonJEPerceptions and measurements of physical activity in patients with systemic lupus erythematosusLupus20112023124221183562
- ChaiamnuaySBertoliAMFernandezMThe impact of increased body mass index on systemic lupus erythematosus: data from LUMINA, a multiethnic cohort (LUMINA XLVI) [corrected]J Clin Rheumatol20071312813317551377
- OeserAChungCPAsanumaYAvalosISteinCMObesity is an independent contributor to functional capacity and inflammation in systemic lupus erythematosusArthritis Rheum2005523651365916258902
- TayerWGNicassioPMWeismanMHSchumanCDalyJDisease status predicts fatigue in systemic lupus erythematosusJ Rheumatol2001281999200711550966
- WysenbeekAJLeiboviciLWeinbergerAGuedjDFatigue in systemic lupus erythematosus. Prevalence and relation to disease expressionBr J Rheumatol1993326336358339141
- OmdalRMellgrenSIKoldingsnesWJacobsenEAHusbyGFatigue in patients with systemic lupus erythematosus: lack of associations to serum cytokines, antiphospholipid antibodies, or other disease characteristicsJ Rheumatol20022948248611908560
- WangBGladmanDDUrowitzMBFatigue in lupus is not correlated with disease activityJ Rheumatol1998258928959598886
- PetterssonSLovgrenMErikssonLEAn exploration of patient-reported symptoms in systemic lupus erythematosus and the relationship to health-related quality of lifeScand J Rheumatol20124138339022646821
- BakerKPopeJEmployment and work disability in systemic lupus erythematosus: a systematic reviewRheumatology (Oxford)20094828128419153144
- ScofieldLReinlibLAlarcónGSCooperGSEmployment and disability issues in systemic lupus erythematosus: a reviewArthritis Rheum2008591475147918821664
- BexeliusCWachtmeisterKSkarePJönssonLVollenhovenRVDrivers of cost and health-related quality of life in patients with systemic lupus erythematosus (SLE): a Swedish nationwide study based on patient reportsLupus20132279380123761101
- Danoff-BurgSFriedbergFUnmet needs of patients with systemic lupus erythematosusBehav Med20093551319297299
- MosesNWiggersJNicholasCCockburnJPrevalence and correlates of perceived unmet needs of people with systemic lupus erythematosusPatient Educ Couns200557303815797150
- MoherDLiberatiATetzlaffJAltmanDGPRISMA GroupReprint – preferred reporting items for systematic reviews and meta-analyses: the PRISMA statementPhys Ther20098987388019723669
- DobkinPLDa CostaDFortinPRLiving with lupus: a prospective pan-Canadian studyJ Rheumatol2001282442244811708416
- Navarrete-NavarreteNPeralta-RamirezMISabio-SanchezJMEfficacy of cognitive behavioural therapy for the treatment of chronic stress in patients with lupus erythematosus: a randomized controlled trialPsychother Psychosom20107910711520090397
- GrecoCMRudyTEManziSEffects of a stress-reduction program on psychological function, pain, and physical function of systemic lupus erythematosus patients: a randomized controlled trialArthritis Rheum20045162563415334437
- HauptMMillenSJannerMFalaganDFischer-BetzRSchneiderMImprovement of coping abilities in patients with systemic lupus erythematosus: a prospective studyAnn Rheum Dis2005641618162315829575
- KarlsonEWLiangMHEatonHA randomized clinical trial of a psychoeducational intervention to improve outcomes in systemic lupus erythematosusArthritis Rheum2004501832184115188360
- AustinJSMaisiakRSMacrinaDMHeckLWHealth outcome improvements in patients with systemic lupus erythematosus using two telephone counseling interventionsArthritis Care Res199693913998997929
- DobkinPLDa CostaDJosephLCounterbalancing patient demands with evidence: results from a pan-Canadian randomized clinical trial of brief supportive-expressive group psychotherapy for women with systemic lupus erythematosusAnn Behav Med200224889912054324
- SohngKYEffects of a self-management course for patients with systemic lupus erythematosusJ Adv Nurs20034247948612752868
- Robb-NicholsonLCDaltroyLEatonHEffects of aerobic conditioning in lupus fatigue: a pilot studyBr J Rheumatol1989285005052590802
- GoligherECPouchotJBrantRMinimal clinically important difference for 7 measures of fatigue in patients with systemic lupus erythematosusJ Rheumatol20083563564218322987
- ZhangJWeiWWangCMEffects of psychological interventions for patients with systemic lupus erythematosus: a systematic review and meta-analysisLupus2012211077108722570339
- De CarvalhoMRSatoEITebexreniASHeidecherRTSchenk-manSNetoTLEffects of supervised cardiovascular training program on exercise tolerance, aerobic capacity, and quality of life in patients with systemic lupus erythematosusArthritis Rheum20055383884416342102
- Clarke-JenssenACFredriksenPMLillebyVMengshoelAMEffects of supervised aerobic exercise in patients with systemic lupus erythematosus: a pilot studyArthritis Rheum20055330831215818657
- YuenHKHolthausKKamenDLSwordDOBrelandHLUsing Wii Fit to reduce fatigue among African American women with systemic lupus erythematosus: a pilot studyLupus2011201293129921700656
- DaltroyLHRobb-NicholsonCIversenMDWrightEALiangMHEffectiveness of minimally supervised home aerobic training in patients with systemic rheumatic diseaseBr J Rheumatol199534106410698542209
- Ramsey-GoldmanRSchillingEMDunlopDA pilot study on the effects of exercise in patients with systemic lupus erythematosusArthritis Care Res20001326226914635294
- TenchCMMcCarthyJMcCurdieIWhitePDD’CruzDPFatigue in systemic lupus erythematosus: a randomized controlled trial of exerciseRheumatology (Oxford)2003421050105412730519
- KruppLBLaRoccaNGMuir-NashJSteinbergADThe fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosusArch Neurol198946112111232803071
- McNairDMLorrMDropplemanLFPOMS Manual: Profile of Mood StatesSan Diego, CA, USAEducational and Industrial Testing Service1992
- KatzPGregorichSYazdanyJObesity and its measurement in a community-based sample of women with systemic lupus erythematosusArthritis Care Res (Hoboken)20116326126820824801
- KatzPJulianLTonnerMCPhysical activity, obesity, and cognitive impairment among women with systemic lupus erythematosusArthritis Care Res (Hoboken)20126450251022337564
- KatzPYazdanyJJulianLImpact of obesity on functioning among women with systemic lupus erythematosusArthritis Care Res (Hoboken)2011631357136421702085
- DaviesRJLomerMCYeoSIAvlonitiKSangleSRD’CruzDPWeight loss and improvements in fatigue in systemic lupus erythematosus: a controlled trial of a low glycaemic index diet versus a calorie restricted diet in patients treated with corticosteroidsLupus20122164965522311939
- BreslinLCMageePJWallaceJMMcSorleyEMAn evaluation of vitamin D status in individuals with systemic lupus erythematosusProc Nutr Soc20117039940721861947
- Ruiz-IrastorzaGGordoSOlivaresNEgurbideMVAguirreCChanges in vitamin D levels in patients with systemic lupus erythematosus: effects on fatigue, disease activity, and damageArthritis Care Res (Hoboken)2010621160116520235208
- FragosoTSDantasATMarquesCD25-Hydroxyivitamin D3 levels in patients with systemic lupus erythematosus and its association with clinical parameters and laboratory testsRev Bras Reumatol201252606522286646
- StocktonKAKandiahDAParatzJDBennellKLFatigue, muscle strength and vitamin D status in women with systemic lupus erythematosus compared with healthy controlsLupus20122127127822004972
- ThudiAYinSWandstratAELiQZOlsenNJVitamin D levels and disease status in Texas patients with systemic lupus erythematosusAm J Med Sci20083359910418277116
- Ruiz-IrastorzaGEgurbideMVOlivaresNMartinez-BerriotxoaAAguirreCVitamin D deficiency in systemic lupus erythematosus: prevalence, predictors and clinical consequencesRheumatology20084792092318411213
- PerlAOxidative stress in the pathology and treatment of systemic lupus erythematosusNat Rev Rheumatol2013967468624100461
- ShahDSahSWanchuAWuMXBhatnagarAAltered redox state and apoptosis in the pathogenesis of systemic lupus erythematosusImmunobiology201321862062722940256
- LaiZWHanczkoRBonillaEN-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trialArthritis Rheum2012642937294622549432
- MichielsenHJDe VriesJVan HeckGLPsychometric qualities of a brief self-rated fatigue measure: The Fatigue Assessment ScaleJ Psychosom Res20035434535212670612
- de KleijnWPDe VriesJWijnenPADrentMMinimal (clinically) important differences for the Fatigue Assessment Scale in sarcoidosisRespir Med20111051388139521700440
- CrosbieDBlackCMcIntyreLRoylePLThomasSDehydroepiandrosterone for systemic lupus erythematosusCochrane Database Syst Rev20074CD00511417943841
- DerksenRHDehydroepiandrosterone (DHEA) and systemic lupus erythematosusSemin Arthritis Rheum1998273353479662752
- OvermanCLHartkampABossemaERFatigue in patients with systemic lupus erythematosus: the role of dehydroepiandrosterone sulphateLupus2012211515152122936125
- van VollenhovenRFEnglemanEGMcGuireJLAn open study of dehydroepiandrosterone in systemic lupus erythematosusArthritis Rheum199437130513107945493
- van VollenhovenRFParkJLGenoveseMCWestJPMcGuireJLA double-blind, placebo-controlled, clinical trial of dehydroepiandroster-one in severe systemic lupus erythematosusLupus1999818118710342710
- ChangDMLanJLLinHYLuoSFDehydroepiandrosterone treatment of women with mild-to-moderate systemic lupus erythematosus: a multicenter randomized, double-blind, placebo-controlled trialArthritis Rheum2002462924292712428233
- NordmarkGBengtssonCLarssonAKarlssonFASturfeltGRönnblomLEffects of dehydroepiandrosterone supplement on health-related quality of life in glucocorticoid treated female patients with systemic lupus erythematosusAutoimmunity20053853154016373258
- HartkampAGeenenRGodaertGLBijlMBijlsmaJWDerksenRHEffects of dehydroepiandrosterone on fatigue and well-being in women with quiescent systemic lupus erythematosus: a randomised controlled trialAnn Rheum Dis2010691144114719854713
- PetriMALahitaRGVan VollenhovenRFEffects of prasterone on corticosteroid requirements of women with systemic lupus erythematosus: a double-blind, randomized, placebo-controlled trialArthritis Rheum2002461820182912124866
- PetriMAMeasePJMerrillJTEffects of prasterone on disease activity and symptoms in women with active systemic lupus erythematosusArthritis Rheum2004502858286815452837
- DingHJGordonCNew biologic therapy for systemic lupus erythematosusCurr Opin Pharmacol20131340541223664092
- TownsendMJMonroeJGChanACB-cell targeted therapies in human autoimmune diseases: an updated perspectiveImmunol Rev201023726428320727041
- PetriMStohlWChathamWAssociation of plasma B lymphocyte stimulator levels and disease activity in systemic lupus erythematosusArthritis Rheum2008582453245918668552
- FurieRPetriMZamaniOBLISS-76 Study GroupA phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosusArthritis Rheum2011633918393022127708
- NavarraSVGuzmanRMGallacherAEBLISS-52 Study GroupEfficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trialLancet201137772173121296403
- StrandVLevyRACerveraRBLISS-52 and -76 Study GroupsImprovements in health-related quality of life with belimumab, a B-lymphocyte stimulator-specific inhibitor, in patients with autoantibody-positive systemic lupus erythematosus from the randomised controlled BLISS trialsAnn Rheum Dis20147383884423524886
- van VollenhovenRFPetriMACerveraRBelimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of responseAnn Rheum Dis2012711343134922337213
- MerrillJTGinzlerEMWallaceDJLBSL02/99 Study GroupLong-term safety profile of belimumab plus standard therapy in patients with systemic lupus erythematosusArthritis Rheum2012643364337322674457
- ManziSSanchez-GuerreroJMerrillJTBLISS-52 and BLISS-76 Study GroupsEffects of belimumab, a B lymphocyte stimulator-specific inhibitor, on disease activity across multiple organ domains in patients with systemic lupus erythematosus: combined results from two phase III trialsAnn Rheum Dis2012711833183822550315
- WallaceDJNavarraSPetriMABLISS-52 and -76, and LBSL02 Study GroupsSafety profile of belimumab: pooled data from placebo-controlled phase 2 and 3 studies in patients with systemic lupus erythematosusLupus20132214415423213069
- McGrathHJrUltraviolet-A1 irradiation decreases clinical disease activity and autoantibodies in patients with systemic lupus erythematosusClin Exp Rheumatol1994121291358039279
- PoldermanMCHuizingaTWLe CessieSUVA-1 cold light treatment of SLE: a double blind, placebo controlled crossover trialAnn Rheum Dis20016011211511156542
- SamsaGEdelmanDRothmanMLWilliamsGRLipscombJMatcharDDetermining clinically important differences in health status measures: a general approach with illustration to the Health Utilities Index Mark IIPharmacoeconomics19991514115510351188
- PoldermanMCle CessieSHuizingaTWPavelSEfficacy of UVA-1 cold light as an adjuvant therapy for systemic lupus erythematosusRheumatology (Oxford)2004431402140415304672
- GambichlerTTerrasSKreuterATreatment regimens, protocols, dosage, and indications for UVA1 phototherapy: facts and controversiesClin Dermatol20133143845423806161
- WangTZhangQXueXYeungAA systematic review of acupuncture and moxibustion treatment for chronic fatigue syndrome in ChinaAm J Chin Med20083612418306446
- GrecoCMKaoAHMaksimowicz-McKinnonKAcupuncture for systemic lupus erythematosus: a pilot RCT feasibility and safety studyLupus2008171108111619029279
- WareJEJrSherbourneCDThe MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selectionMed Care1992304734831593914
- ChouCTAlternative therapies: what role do they have in the management of lupus?Lupus2010191425142920947552
- Abou-RayaAAbou-RayaSHelmiiMThe effect of vitamin D supplementation on inflammatory and hemostatic markers and disease activity in patients with systemic lupus erythematosus: a randomized placebo-controlled trialJ Rheumatol20134026527223204220
- StrandVPetriMKalunianKEpratuzumab for patients with moderate to severe flaring SLE: health-related quality of life outcomes and corticosteroid use in the randomized controlled ALLEVIATE trials and extension study SL0006Rheumatology (Oxford)20145350251124273022
- Danoff-BurgSAgeeJDRomanoffNRBenefit finding and expressive writing in adults with lupus or rheumatoid arthritisPsychol Health200621651665