Abstract
Sporadic inclusion body myositis is the most common inflammatory muscle disorder preferentially affecting males over the age of 40 years. Progressive muscle weakness of the finger flexors and quadriceps muscles results in loss of independence with activities of daily living and eventual wheelchair dependence. Initial signs of disease are often overlooked and can lead to mis- or delayed diagnosis. The underlying cause of disease is unknown, and disease progression appears refractory to available treatment options. This review discusses the clinical presentation of inclusion body myositis and the current efforts in diagnosis, and focuses on the current state of research for both nonpharmacological and pharmacological treatment options for this patient group.
Introduction
Sporadic inclusion body myositis (IBM) is one of a group of inflammatory muscle disorders resulting in progressive muscle weakness. It is the most common inflammatory disorder preferentially affecting men (2:1 over females) over the age of 40–50 years.Citation1–Citation3 Incidence and prevalence of IBM have not been well characterized. A recent review estimates incidence of IBM between 0.9 and 3.2 per million per year; however, this varied dramatically based on geographic location.Citation4–Citation6 Reported prevalence also widely varies between 0.3 and 13.9 per 100,000, and again varied by geographical location.Citation5 The underlying cause of IBM is not yet fully understood and has been hypothesized to involve protein dysregulation, mitochondrial dysfunction, autoimmune factors, myonuclear degeneration, and impaired nucleic acid metabolism.Citation7–Citation14 Despite the continued investigation into the cause of IBM, histopathological characteristics of muscle have been traditionally used in confirming the diagnosis. The essential physical features for a diagnosis of IBM include initial muscle weakness presenting in a characteristic pattern of quadriceps and finger flexors in individuals over the age of 40 years. Griggs criteria was first developed and adopted to standardize diagnosis of this disease, followed by the Medical Research Council (MRC) criteria, and more recently the European Neuro Muscular Centre (ENMC) IBM research diagnostic criteria 2011.Citation15–Citation17 Griggs criteria defines IBM when rimmed vacuoles and inflammatory infiltrates are present; however, inconclusive biopsy results were often found in patients fitting the clinical presentation of IBM and refractory to any treatment. The MRC and ENMC criteria were developed to enhance ease of diagnosis when histopathological features were not found in muscle biopsies, hypothesized to potentially occur earlier in disease presentation.Citation16,Citation17 As treatments become available, the potential for earlier diagnosis has implications for earlier onset of treatment during a period when muscles may be more responsive.
There is currently no successful treatment to stop or delay the progression of disease in IBM. In the absence of a conclusive muscle biopsy, often IBM was differentiated from polymyositis or dermatomyositis by being refractory to immunosuppressive therapies.Citation18–Citation20 Current research is focused on identifying the fundamental causes of disease progression, as well as initial clinical trials aimed at reducing disease burden or treating symptoms of the disease.
Clinical description
IBM is characterized by progressive, often asymmetric, muscle weakness preferentially affecting skeletal muscle. The two muscle groups often initially affected are the quadriceps and finger flexors. The slowly progressive nature of the disease frequently leads to delayed diagnosis (discussed further), as both patients and clinicians attribute slight changes in function to the aging process.Citation21 Due to the initial quadriceps weakness, patients often report difficulty with stairs or falls as the first signs of the disease.Citation1 Later in the disease, ankle dorsiflexion and elbow extension weakness frequently develop, increasing susceptibility to falls and the difficulty of transfers, such as rising from a chair.
As the disease progresses, weakness expands throughout the upper and lower limbs, leading to eventual loss of independent ambulation and wheelchair use. Additionally, weakness of the distal, then proximal, muscles of the upper extremities quickly leads to loss of independence with activities of daily living, such as grooming, dressing, and hygiene activities.Citation22,Citation23 The rate of disease progression can vary widely, with some reports linking increased severity associated to a later age of onset.Citation3,Citation24,Citation25
Several natural history studies have reported declines in quadriceps strength ranging between 12.5% and 27.9% over 1 year.Citation3,Citation25–Citation28 Reports of composite quantitative muscle testing in patients with IBM appear to decline 9.2% in 1 year or 4% in 6 months.Citation27–Citation29 In a sample of 85 ambulatory individuals with IBM, quadriceps strength was computed as a percentage of age, sex, and height predictions. The deficits in this group ranged from 85% of predicted strength to less than 1%.Citation28 While these trends are useful in clinical trials, it is important for the clinician to remember that changes in strength and function are often not linear and that rates of decline or stability may change across the disease.Citation25,Citation27
The change in function and progressive disability has also been described. The mean or median loss of independent ambulation ranged between 7 and 14 years from the date of diagnosis in different cohorts, with an average change in distance walked on the 6-minute walk test (6MWT) decreasing 34% over a 4-year period of time.Citation3,Citation24,Citation27 The IBM functional rating scale was designed as a measure of disease severity and rates the difficulty to complete ten items related to swallowing, fine motor, and gross motor tasks.Citation30 Varying rates of decline using this outcome have been reported, ranging from 22.3% over 4 years to 13.8% over 1 year.Citation3,Citation27
In a subset of patients with IBM, insidious onset dysphagia creates swallowing difficulties and choking.Citation1 As with the initial muscle wasting, early signs of dysphagia can be overlooked as aging related choking or coughing associated with eating or drinking. In 40%–50% of patients, however, dysphagia becomes quite debilitating later in disease progression.Citation31,Citation32 Interestingly, although IBM is more common in men, there is some evidence suggesting that dysphagia is more commonly the initial presenting symptom in women.Citation33
Cardiac function appears to be spared in IBM, although case studies have been published reporting various cardiomyopathies coexisting in patients with IBM.Citation34–Citation37 While heart function may be preserved, there are reports of sleep disordered breathing being identified primarily later in disease progression, although not necessarily correlated to severity of peripheral muscle weakness.Citation38,Citation39 Respiratory decline has also been reported as the most common cause of death in a long-term follow-up of patients with IBM.Citation22
Diagnosis and markers of disease
Many patients with IBM report a difficulty finding the correct diagnosis. This is likely related to the rarity of this disease in combination with the slowly progressive changes often attributed to aging. The mean time from symptom onset to correct diagnosis ranges in published work between 3 and 5 years, with many patients receiving at least one incorrect diagnosis prior to the diagnosis of IBM.Citation21,Citation28,Citation40,Citation41 As described earlier there are multiple diagnostic criteria that have been developed to standardize diagnosis of IBM. Despite the wide use of these criteria among neuromuscular professionals, a recent study found that all criteria evaluated had high specificity (ranging 98%–100%), but they had wide-ranging sensitivities (11%–84%) with the potential of several false negatives or missing a diagnosis of IBM when present.Citation42
Traditionally, muscle biopsies have been used to confirm the diagnosis of IBM, requiring a combination of vacuolated muscle fibers, inflammatory myopathy with mononuclear cell invasion, amyloid accumulation, or 15–18 nm filaments.Citation15–Citation17 Often, a combination of clinical and pathological features is required to make the diagnosis in the presence of a nondefinitive biopsy. Many factors may contribute to the difficulty in observing hallmark pathological features in a biopsy, such as those related to biopsy site, distribution of pathology, or the effect of disease progression on distribution or occurrence of pathology.Citation16,Citation21,Citation43
In the continued pursuit to better understand the disease pathogenesis of IBM, several biomarkers have been evaluated for both their role in the disease process and as a potential for use in differentiating IBM from other inflammatory myopathies. Due to the presence of protein aggregation, inflammation, and cell death in muscle biopsy of patients with IBM, biomarkers related to inflammation, autophagy, and protein aggregation have been proposed, such as tau, β-amyloid, Clathrin, and Beclin I. While these have been specifically reported, others recommend targeting markers of general protein aggregation, such as LC3 and p62, as no one protein is consistently found in muscle cells of patients.Citation44–Citation49 Recently, autoantibodies have been investigated as a serological marker for IBM, with autoantibodies against cytoplasmic 5′ nucleotidase showing promise as they demonstrate moderate sensitivity (49%–76%) and high specificity (91%–96%) for IBM.Citation10,Citation50–Citation53 However, further research is needed as this marker was detected in other patients with other inflammatory myopathies or autoimmune disorders, so is not sensitive to IBM.Citation10
As techniques for genetic analysis continue to advance, new findings relating genes and polymorphisms to disease susceptibility or severity of disease progression are being investigated. Changes in HLA-DRB1, HLA-DRB3, HLA-DRA, and BTNL2 have been associated with susceptibility of developing IBM, with HLA-DRB1, HLA-DRB3, HLA-DRA, and BTNL2 being reported with increased susceptibility to IBM and HLA-DRB1 increasing risk up to ten times in a Western Australian population.Citation54–Citation57 Alternatively, HLA-DRB loci DRB4 and DRB5 and a polymorphism in the TOMM40 gene appear to have a protective effect in reducing risk of IBM.Citation58 Additionally, allele changes in the TOMM40 gene were also associated with later onset of symptoms, up to 4 years; and when these changes were combined in carriers of the APOE genotype ε3/ε3, onset was delayed 5 years.Citation59
Additionally, use of MRI to assist or confirm diagnosis of IBM has been investigated.Citation60–Citation63 Fatty infiltration compared to inflammation was a hallmark of IBM compared to other inflammatory myopathies such as polymyositis.Citation61,Citation63 Muscles of the thigh, including vastus intermedius and medialis, were characteristically affected, while the rectus femoris and posterior compartment of the thigh were often spared or less involved.Citation60,Citation62,Citation63 The medial gastrocnemius was frequently involved with inconsistent involvement of the finger flexors, posterior tibialis, and soleus muscles.Citation60,Citation62,Citation63 A blinded assessment of MRI scans in 137 patients with myopathies (17 with definite IBM) reported the ability to detect definite IBM of the typical pattern with 59% sensitivity and 100% specificity.Citation60 Sensitivity improved when considering the diagnosis of IBM for both typical and consistent patterns (sensitivity of 94%). Authors reported similar accuracy for patients both early and late in disease progression.Citation60
Treatment options
Nonpharmacologic treatment options
Exercise
Historically, exercise has been recommended cautiously to avoid the effect of overwork leading to more rapid muscle breakdown; however, recent research is pushing the field to think of exercise differently. Although, there have been no large randomized-controlled trials evaluating the effect of exercise in IBM to date, several small studies have been published reporting safety of a low-to-moderate resistance program.Citation2,Citation31–Citation33,Citation64 These studies were limited by small sample sizes and relatively short follow-up periods. While the study completed by Arnardottir reported no improvement in strength or function after the home program, they reported a benefit to using exercise to prevent loss of muscle strength over time.Citation65 Separate cohorts of studies (ranging from case studies to seven patients enrolled) investigating possible exercise regimes in IBM reported improvements in strength, function, and aerobic capacity.Citation2,Citation32,Citation33,Citation64 While additional research is needed in a larger randomized cohort, initial results from small studies indicate that exercise appears safe and may produce the benefit of mild increases or maintenance of strength and function. In a disease that has appeared refractory to all treatment options, these findings suggest a possible tangible treatment for patients to temporarily reduce the progression of IBM over time. Implementation of any exercise regimen should be supervised by a trained physical therapist to ensure safety in an aging population with unique patterns of muscle weakness.
Orthoses
The presenting quadriceps weakness is often accompanied by anterior tibialis muscle weakness leading to progressive foot drop. Falls frequently occur in this population due to knee collapse or tripping due to foot drop or uneven surfaces. There have not been any large studies investigating use of orthoses in IBM, although bracing has the potential to reduce fall occurrence in this cohort. Bernhardt evaluated use of a specific stance control orthosis unilaterally in a small cohort of patients with IBM. The braces were well-tolerated; however, they resulted in slower walking velocity and cadence, which may not be preferable for all patients.Citation49 Patients with less weakness and those wearing the orthosis more frequently reported more stability and fewer falls. Further research is needed into the optimal orthotic intervention in this population, which is likely to be individualized to specific patient needs. A lightweight orthosis providing additional stability for the quadriceps muscles, such as one with anterior shell support, may also assist in reducing occurrence of foot drop and falls.
Pharmacologic intervention
There is no current treatment effective at halting or slowing disease progression in IBM. However, several treatments aimed at improving symptoms of IBM have been studied. Most research has focused on repurposing existing medications for use in patients with IBM, although development of novel compounds and therapies has begun in recent years ().
Figure 1 Progress of drug development and testing in IBM on a scale from not currently supported or recommended based on best available evidence to full approval.
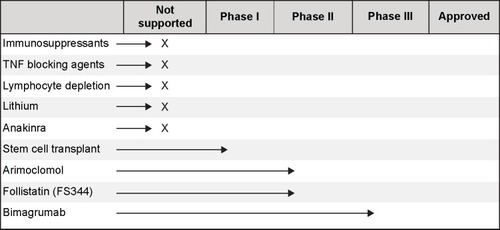
Immunosuppressants
Due to the presence of muscle inflammation in patients with IBM, a variety of immunosuppressants have been trialed in IBM cohorts. While one case study reported a sustained muscle strength improvement in a patient with IBM and anti-Jo-1 antibodies, larger studies have been inconclusive or have shown a decrement in muscle strength during prednisone treatment.Citation66,Citation67 Various combinations of prednisone with other compounds have also been trialed with mixed results, leading to negative consensus in the field for use of these compounds in IBM. Pilot studies of prednisone and mycophenylate, cyclosporin-A, or tacrolimus indicated potential for treatment; however, larger trials reported no long-term positive effect in IBM.Citation68,Citation69 Likewise, a trend toward improvement in dysphagia associated with IBM was reported in a trial of intravenous immunoglobulin (IVIg) and a small case series combining IVIg and prednisone;Citation70,Citation71 however, these changes have not been reported in the long-term in a larger cohort.Citation72,Citation73 Studies of IVIg in larger cohorts have reported inconclusive results, including minor strength gains in some muscles of the leg with the strength of other muscles declining over the same period.Citation71,Citation74 Additionally, methotrexate has been evaluated in IBM. However, a double-blind trial of methotrexate versus placebo reported no difference in disease progression measured by muscle strength over 48 weeks.Citation75 Additionally, there are reports of a connection between immunosuppressive therapies and potential disease exacerbation.Citation24,Citation76
Although small and unblinded trials have reported trends toward or improvement in few patients, these results have not been replicated in large-randomized controlled trials. While immunosuppressive therapy may have a potential for benefit in a small subset of patients, the characteristics of such a cohort have not been well defined. Current literature does not support the use of immunosuppressive therapy to maintain or improve muscle strength in IBM.
Tumor necrosis factor blocking agents
Tumor necrosis factor (TNF) blocking agents, etanercept and infliximab, have been evaluated in IBM, without dramatic improvements in disease progression. A small study comparing etanercept and two separate control groups (a natural history group and a placebo group from another trial) found no difference in strength at 6 months, but a slight improvement in the hand grip strength of the treated group at 12 months.Citation77 The clinical significance of this finding is unknown.
Infliximab was also tested in a cohort of patients with refractory myositis, including four patients with IBM. Treatment side effects were significant and resulted in drop out of 25% of the cohort. Additionally, no change in muscle strength was measured, and 23% of the cohort demonstrated increased muscle inflammation on MRI posttreatment. Thus, infliximab is not considered an alternative treatment option in patients with refractory myositis.Citation78 TNF blocking agents are likely not a viable treatment option as early trial results point to limited benefit which may not provide enough improvement to outweigh potential treatment risks and cost.
Lymphocyte depletion therapy
Alemtuzumab is a compound targeted against CD52, a glycoprotein expressed on mature T-cells. It has been proposed that use of this compound in IBM has the potential to reduce muscle weakness by decreasing muscle injury related to T-cell response. Results from a pilot trial of 13 patients receiving one course of alemtuzumab treatment lasting 4 days were promising.Citation79 Authors reported differential gain in strength at 6 months compared to the previous 12-month observation, and 4 of 13 showed an improvement in strength and 6 reported improved activities of daily living.Citation79 Concerns have been raised with this study design and the reported conclusions, recommending further analysis of this compound and evaluation in a blinded trial.Citation80 A case study of rituximab in a patient with comorbid rheumatoid arthritis resulted in muscle wasting and weakness of quadriceps muscles following prednisone treatment. Rituximab improved symptoms related to the rheumatoid arthritis leading to remission, but had no effect on muscle strength.Citation76
Other treatments
Lithium was proposed as a potential treatment in early preclinical studies. Muscles of patients with IBM are characterized by protein aggregates containing amyloid-β as well as phosphorylated tau. Early in vitro and mice studies reported reduced amyloid-β precursor protein levels and improved proteasome function indicating a potential benefit for patients with IBM.Citation81,Citation82 However, a 12-month study enrolling 15 participants produced no significant benefit in muscle strength.Citation83
Anakinra, an interleukin-1 receptor antagonist, was evaluated in patients with IBM with the goal of reducing amyloid deposits and improving muscle strength and function by blocking IL-1β. An open-label pilot study in four patients resulted in no significant improvement or stabilization in hand grip strength or muscle strength measured by manual muscle testing.Citation84 In a separate study of 15 patients with refractory myositis (five with IBM) receiving daily self-administered injection of anakinra for 12 months, results were mixed.Citation85 Seven patients demonstrated improvement across the 12-month study period on the International Myositis Assessment and Clinical Studies group global assessment, with one patient with IBM showing improvement after 3 months. Three patients with IBM did not demonstrate improvement, and one withdrew from the study due to worsening of symptoms.Citation85 Further research is needed to determine efficacy in a larger cohort or to identify the specific characteristics of a potential subgroup of responders to this treatment.
Early work in stem cell transplantation is being evaluated for patients with IBM. Mesoangioblasts from muscle of patients with IBM display defective terminal differentiation with absence of MyoD mRNA expression in myotubes. This block can be corrected in vitro by transient MyoD transfection.Citation86,Citation87 A Phase I clinical trial is underway (expected enrollment of ten patients) and is designed to evaluate the effect of high dose cyclophosphamide and antithymocyte globulin with hematopoetic stem cells on progression of myositis.Citation88 Results are anticipated in 2017.
Arimoclomol induces increased production of heat shock protein, which is typically increased in vivo in response to cellular stress. A 4-month study in 24 patients with IBM was conducted to evaluate the safety and potential to increase muscle strength in this disease.Citation89 Initial reports indicate that arimoclomol was safe and could have a potential for treatment in IBM.Citation90 Further research is needed in a larger cohort to confirm these preliminary results.
Follistatin, a myostatin inhibitor, has the potential to increase muscle mass. A Phase I/IIa study is currently underway in patients with IBM.Citation91 The alternatively spliced isoform of follistatin (FS344) is injected into the quadriceps muscles of nine patients with IBM to evaluate safety and initial signs of efficacy. Results from a Phase I/IIa study in Becker muscular dystrophy show promising results, but have not yet been reported in the IBM cohort.Citation92
Bimagrumab acts to inhibit activin type II receptors in the myostatin pathway to potentially increase muscle size in patients with IBM.Citation93 Initial results of a safety study of this compound in eleven subjects with IBM receiving one dose of bimagrumab compared to three receiving placebo indicated that the compound was well-tolerated with significant improvements in muscle volume and lean body mass.Citation94 A 16-week observation phase in 12 subjects described a significant improvement in distance walked on the 6MWT peaking at 16 weeks.Citation94 A pivotal trial in 240 subjects with IBM is currently ongoing to evaluate changes in the distance walked on the 6MWT after 52 weeks.Citation95
Efficacy outcomes
Selection of appropriate outcome measures, whether clinically or as part of research trials, is often overlooked or undervalued. Clinically, patients often ask how their disease progression relates to the natural history of the disease. Longitudinal natural history trials provide access to the general trend of disease progression in patients with IBM; however, these may be less meaningful to an individual patient. These are no substitutes for a thorough evaluation of disease progression involving imaging, quantitative strength testing, as well as performance of functional tests, including walking, stair climbing, and balance tasks, during regularly scheduled clinic visits. Discussing an individual’s trajectory can help clinicians manage a patient’s expectations for the future in the presence of an advancing, progressive disease.
To effectively measure efficacy as part of clinical trials, outcome measure selection is crucial. This is particularly important in a slowly progressive disease such as IBM. Early clinical trials often attempt to measure differences in strength or function across groups in short-term trials, typically 48 weeks or less. In a disease like IBM, where initial ability may widely vary and where natural history studies report a range of disease progression, improper or careless choice of outcome may result in false negative outcomes. While the US Food and Drug Administration prefers use of a clinically meaningful, typically functional, outcome measure for product approval, guidelines for surrogate measures have been reported.Citation85 Where traditionally, an outcome such as timed walking would be chosen as the primary efficacy outcome for clinical trials, a surrogate measure such as strength or imaging may be a suitable alternative for short-term trials of innovative products.Citation28
Another important point to contemplate when selecting outcomes for clinical trials is the overall burden of testing on an individual throughout the course of a visit or the study. Often, clinical evaluation of a subject lasts between 1 and 3 hours for any given visit. In a disease such as IBM, with significant weakness often resulting in increased frequency of falls, the level of fatigue caused by assessments should be considered. For example, while the 6MWT has been successfully implemented in clinical trials involving products eventually achieving registration, it is important to evaluate all options available if a timed walking test is being chosen as a primary efficacy outcome. In IBM in particular, patients tend to walk at the same speed, which is often the fastest speed that minimizes falls.Citation96 Choice of the 2-minute walk test, included in the National Institutes of Health toolbox, may be a suitable alternative to capture the same information (distance walked on a timed test) while reducing the overall burden of testing.
Finally, a perfectly designed study with optimal safety and efficacy outcome selected has the potential to be derailed by variability in patient performance and clinical evaluator ratings. Reducing the burden of testing can minimize some variability of subject performance. However, more important is ensuring that subject motivation is considered. While it is near impossible to guarantee consistent motivation of a subject across visits or study sites, understanding and attempting to minimize the effect of motivation on subject performance will likely reduce variability associated with an individual’s performance.
Additionally, establishing clinical evaluator intra- and interrater reliability can assist in reducing variability of trial data. The more subjective an outcome measure, the more likely reliability issues are to arise. Establishing intra- and interrater reliability values on the outcomes included in a trial may assist in post hoc statistical analysis of study results. The process of determining evaluator reliability may in itself reduce some variability across sites in a multisite trial.
Conclusion
IBM is a slowly progressive disease with currently no effective pharmacologic treatment to slow or halt the disease process. While the underlying cause has yet to be determined, many trials are underway to treat major symptoms of the disease. The success of these trials is dependent on selection of appropriate outcome measures and proper clinical evaluator training. Implementation of an individualized exercise regimen or orthotic intervention may provide patients with a tangible option to temporarily reduce the progression of disease or reduce falls, although further research is needed to determine the full effects of these treatment options.
Disclosure
Dr Alfano currently serves as a clinical evaluator for the ongoing follistatin trial in IBM at the Research Institute at Nationwide Children’s Hospital. Dr Lowes also serves as a clinical evaluator for the ongoing clinical trial of the follistatin compound in IBM at The Research Institute at Nationwide Children’s Hospital. She also receives funding from Novartis Pharmaceuticals as principal investigator of a longitudinal natural history study in IBM. The authors report no other conflicts of interest in this work.
References
- PaltielADIngvarssonELeeDKDemographic and clinical features of inclusion body myositis in north AmericaMuscle Nerve Epub12302014
- FlacheneckerPEpidemiology of neuroimmunological diseasesJ Neurol2006253Suppl 5V2V816998750
- CorteseAMachadoPMorrowJLongitudinal observational study of sporadic inclusion body myositis: implications for clinical trialsNeuromuscul Disord20132340441223489664
- LynnSJSawyersSMMollerPWO’DonnellJLChapmanPTAdult-onset inflammatory myopathy: North Canterbury experience 1989–2001Intern Med J200535317017315737137
- MeyerAMeyerNSchaefferMGottenbergJEGenyBSibiliaJIncidence and prevalence of inflammatory myopathies: a systematic reviewRheumatology (Oxford)2015541506325065005
- WilsonFCYtterbergSRSt SauverJLReedAMEpidemiology of sporadic inclusion body myositis and polymyositis in Olmsted County, MinnesotaJ Rheumatol200835344544718203321
- GreenbergSABiomarkers of inclusion body myositisCurr Opin Rheumatol201325675376224067380
- HerbertMKStammen-VogelzangsJVerbeekMMDisease specificity of autoantibodies to cytosolic 5′-nucleotidase 1A in sporadic inclusion body myositis versus known autoimmune diseasesAnn Rheum Dis Epub2242015
- JoshiPRVetterkeMHauburgerATacikPStoltenburgGHanischFFunctional relevance of mitochondrial abnormalities in sporadic inclusion body myositisJ Clin Neurosci2014211959196325311418
- LloydTEChristopher-StineLPinal-FernandezICytosolic 5′-nucleotidase 1A is a common target of circulating autoantibodies in several autoimmune diseasesArthritis Care Res Epub4172015
- MorosettiRGliubizziCSancriccaCTWEAK in inclusion-body myositis muscle: possible pathogenic role of a cytokine inhibiting myogenesisAm J Pathol20121801603161322314077
- MoussaCEFuQKumarPTransgenic expression of β-APP in fast-twitch skeletal muscle leads to calcium dyshomeostasis and IBM-like pathologyFASEB J200620122165216716940437
- PinkusJLAmatoAATaylorJPGreenbergSAAbnormal distribution of heterogeneous nuclear ribonucleoproteins in sporadic inclusion body myositisNeuromuscul Disord20142461161624857366
- RygielKAMillerJGradyJPRochaMCTaylorRWTurnbullDMMitochondrial and inflammatory changes in sporadic inclusion body myositisNeuropathol Appl Neurobiol201541328830324750247
- GriggsRCAskanasVDiMauroSInclusion body myositis and myopathiesAnn Neurol1995387057137486861
- Hilton-JonesDMillerAPartonMHoltonJSewryCHannaMGInclusion body myositis: MRC Centre for Neuromuscular Diseases, IBM workshop, London, 13 June 2008Neuromuscul Disord20102014214720074951
- RoseMRGroupEIW188th ENMC International workshop: inclusion body myositis, 2–4 December 2011, Naarden, The NetherlandsNeuromuscul Disord2013231044105524268584
- LazarouINGuernePAClassification, diagnosis, and management of idiopathic inflammatory myopathiesJ Rheumatol20134055056423504386
- MachadoPMDimachkieMMBarohnRJSporadic inclusion body myositis: new insights and potential therapyCurr Opin Neurol20142759159825159931
- MastagliaFLNeedhamMInclusion body myositis: a review of clinical and genetic aspects, diagnostic criteria and therapeutic approachesJ Clin Neurosci20152261325510538
- NeedhamMCorbettADayTChristiansenFFabianVMastagliaFLPrevalence of sporadic inclusion body myositis and factors contributing to delayed diagnosisJ Clin Neurosci2008151350135318815046
- CoxFMTitulaerMJSontJKWintzenARVerschuurenJJBadrisingUAA 12-year follow-up in sporadic inclusion body myositis: an end stage with major disabilitiesBrain20111343167317521908393
- ErikssonMLindbergCHand function in 45 patients with sporadic inclusion body myositisOccup Ther Int20121910811622495756
- BenvenisteOGuiguetMFreebodyJLong-term observational study of sporadic inclusion body myositisBrain20111343176318421994327
- LindbergCOldforsAPrognosis and prognostic factors in sporadic inclusion body myositisActa Neurol Scand201212535335821916852
- AllenbachYBenvenisteODecostreVQuadriceps strength is a sensitive marker of disease progression in sporadic inclusion body myositisNeuromuscul Disord20122298098622738680
- HogrelJYAllenbachYCanalAFour-year longitudinal study of clinical and functional endpoints in sporadic inclusion body myositis: implications for therapeutic trialsNeuromuscul Disord20142460461024857365
- LowesLPAlfanoLViolletLKnee extensor strength exhibits potential to predict function in sporadic inclusion-body myositisMuscle Nerve20124516316822246869
- RoseMRMcDermottMPThorntonCAPalenskiCMartensWBGriggsRCA prospective natural history study of inclusion body myositis: implications for clinical trialsNeurology20015754855011502935
- JacksonCEBarohnRJGronsethGPandyaSHerbelinLMuscle Study GroupInclusion body myositis functional rating scale: a reliable and valid measure of disease severityMuscle Nerve20083747347618236463
- LotzBPEngelAGNishinoHStevensJCLitchyWJInclusion body myositis. Observations in 40 patientsBrain1989112Pt 37277472543478
- OhTHBrumfieldKAHoskinTLKasperbauerJLBasfordJRDysphagia in inclusion body myositis: clinical features, management, and clinical outcomeAm J Phys Med Rehabil20088788388918936555
- KoEHRubinADDysphagia due to inclusion body myositis: case presentation and review of the literatureAnn Otol Rhinol Laryngol201412360560824634148
- BalloPChiodiLCameliMDilated cardiomyopathy and inclusion body myositisNeurol Sci20123336737021922313
- FinstererJStollbergerCKovacsGGSehnalELeft ventricular hypertrabeculation/noncompaction coincidentally found in sporadic inclusion body myositisInt J Cardiol201316861061223462629
- InamoriYHiguchiIInoueTInclusion body myositis coexisting with hypertrophic cardiomyopathy: an autopsy studyNeuromuscul Disord20122274775422560514
- UtzWSchmidtSSchulz-MengerJLuftFSpulerSCardiac involvement in sporadic inclusion-body myositisCirculation201012170670820142463
- Della MarcaGSancriccaCLosurdoASleep disordered breathing in a cohort of patients with sporadic inclusion body myositisClin Neurophysiol20131241615162123583020
- Rodriguez CruzPMNeedhamMHollingsworthPMastagliaFLHillmanDRSleep disordered breathing and subclinical impairment of respiratory function are common in sporadic inclusion body myositisNeuromuscul Disord2014241036104125227894
- BradySSquierWHilton-JonesDClinical assessment determines the diagnosis of inclusion body myositis independently of pathological featuresJ Neurol Neurosurg Psychiatry2013841240124623864699
- SayersMEChouSMCalabreseLHInclusion body myositis: analysis of 32 casesJ Rheumatol199219138513891331441
- LloydTEMammenALAmatoAAWeissMDNeedhamMGreenbergSAEvaluation and construction of diagnostic criteria for inclusion body myositisNeurology20148342643324975859
- ChahinNEngelAGCorrelation of muscle biopsy, clinical course, and outcome in PM and sporadic IBMNeurology20087041842417881720
- GirolamoFLiaAAmatiAOverexpression of autophagic proteins in the skeletal muscle of sporadic inclusion body myositisNeuropathol Appl Neurobiol20133973674923452291
- GreenbergSATheories of the pathogenesis of inclusion body myositisCurr Rheumatol Rep20101222122820425523
- HinikerADanielsBHLeeHSMargetaMComparative utility of LC3, p62 and TDP-43 immunohistochemistry in differentiation of inclusion body myositis from polymyositis and related inflammatory myopathiesActa Neuropathol Commun201312924252466
- WeihlCCPestronkASporadic inclusion body myositis: possible pathogenesis inferred from biomarkersCurr Opin Neurol20102348248820664349
- BenvenisteOStenzelWHilton-JonesDSandriMBoyerOvan EngelenBGAmyloid deposits and inflammatory infiltrates in sporadic inclusion body myositis: the inflammatory egg comes before the degenerative chickenActa Neuropathol201512961162425579751
- De BleeckerJLDe PaepeBAronicaE205th ENMC International workshop: pathology diagnosis of idiopathic inflammatory myopathies part II 28–30 March 2014, Naarden, The NetherlandsNeuromuscul Disord20152526827225572016
- GreenbergSACytoplasmic 5′-nucleotidase autoantibodies in inclusion body myositis: isotypes and diagnostic utilityMuscle Nerve20145048849224752512
- LarmanHBSalajeghehMNazarenoRCytosolic 5′-nucleotidase 1A autoimmunity in sporadic inclusion body myositisAnn Neurol20137340841823596012
- LuXPengQWangGDiscovery of new biomarkers of idiopathic inflammatory myopathyClin Chim Acta201544411712525681646
- PlukHvan HoeveBJvan DoorenSHAutoantibodies to cytosolic 5′-nucleotidase 1A in inclusion body myositisAnn Neurol20137339740723460448
- MastagliaFLSporadic inclusion body myositis: variability in prevalence and phenotype and influence of the MHCActa Myol200928667120128139
- MastagliaFLNeedhamMScottASporadic inclusion body myositis: HLA-DRB1 allele interactions influence disease risk and clinical phenotypeNeuromuscul Disord20091976376519720533
- Rojana-udomsartAJamesICastleyAHigh-resolution HLA-DRB1 genotyping in an Australian inclusion body myositis (s-IBM) cohort: an analysis of disease-associated alleles and diplotypesJ Neuroimmunol2012250778222633068
- ScottAPLaingNGMastagliaFRecombination mapping of the susceptibility region for sporadic inclusion body myositis within the major histocompatibility complexJ Neuroimmunol2011235778321543121
- Rojana-udomsartAMitrpantCJamesIAnalysis of HLA-DRB3 alleles and supertypical genotypes in the MHC Class II region in sporadic inclusion body myositisJ Neuroimmunol201325417417723010279
- GangQBettencourtCMachadoPMThe effects of an intronic polymorphism in TOMM40 and APOE genotypes in sporadic inclusion body myositisNeurobiol Aging2015361766.e1e325670332
- TascaGMonforteMDe FinoCKleyRARicciEMirabellaMMRI pattern recognition in sporadic inclusion body myositisMuscle Nerve Epub2532015
- DionECherinPPayanCMagnetic resonance imaging criteria for distinguishing between inclusion body myositis and polymyositisJ Rheumatol2002291897190612233884
- PhillipsBACalaLAThickbroomGWMelsomAZilkoPJMastagliaFLPatterns of muscle involvement in inclusion body myositis: clinical and magnetic resonance imaging studyMuscle Nerve2001241526153411745956
- CoxFMReijnierseMvan RijswijkCSWintzenARVerschuurenJJBadrisingUAMagnetic resonance imaging of skeletal muscles in sporadic inclusion body myositisRheumatology (Oxford)2011501153116121288962
- HinikerADanielsBHLeeHSMargetaMComparative utility of LC3, p62 and TDP-43 immunohistochemistry in differentiation of inclusion body myositis from polymyositis and related inflammatory myopathiesActa Neuropathol Commun201312924252466
- ArnardottirSAlexandersonHLundbergIEBorgKSporadic inclusion body myositis: pilot study on the effects of a home exercise program on muscle function, histopathology and inflammatory reactionJ Rehabil Med2003351313512610846
- BarohnRJAmatoAASahenkZKisselJTMendellJRInclusion body myositis: explanation for poor response to immunosuppressive therapyNeurology199545130213047617187
- HengstmanGJTer LaakHJvan EngelenBGvan VenrooijBGAnti-Jo-1 positive inclusion body myositis with a marked and sustained clinical improvement after oral prednisoneJ Neurol Neurosurg Psychiatry20017070611336039
- LindbergCPerssonLIBjorkanderJOldforsAInclusion body myositis: clinical, morphological, physiological and laboratory findings in 18 casesActa Neurol Scand1994891231318191875
- QuartuccioLDe MarchiGScottCAFerraccioliGBeltramiCADe VitaSTreatment of inclusion body myositis with cyclosporin-A or tacrolimus: successful long-term management in patients with earlier active disease and concomitant autoimmune featuresClin Exp Rheumatol20072524625117543149
- CherinPPelletierSTeixeiraAIntravenous immunoglobulin for dysphagia of inclusion body myositisNeurology20025832611805271
- DalakasMCSoniesBDambrosiaJSekulECuplerESivakumarKTreatment of inclusion-body myositis with IVIg: a double-blind, placebo-controlled studyNeurology1997487127169065553
- DoblougCWalle-HansenRGranJTMolbergOLong-term follow-up of sporadic inclusion body myositis treated with intravenous immunoglobulin: a retrospective study of 16 patientsClin Exp Rheumatol20123083884222935197
- RecherMSahrbacherUBremerJArndtBSteinerUFontanaATreatment of inclusion body myositis: is low-dose intravenous immunoglobulin the solution?Rheumatol Int20123246947220044785
- PatwaHSChaudhryVKatzbergHRae-GrantADSoYTEvidence-based guideline: intravenous immunoglobulin in the treatment of neuromuscular disorders: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of NeurologyNeurology2012781009101522454268
- BadrisingUAMaat-SchiemanMLFerrariMDComparison of weakness progression in inclusion body myositis during treatment with methotrexate or placeboAnn Neurol20025136937211891832
- VordenbaumenSNeuen-JacobERichterJSchneiderMInclusion body myositis in a patient with long standing rheumatoid arthritis treated with anti-TNFα and rituximabClin Rheumatol20102955555820108015
- BarohnRJHerbelinLKisselJTPilot trial of etanercept in the treatment of inclusion-body myositisNeurology200666S123S12416432140
- DastmalchiMGrundtmanCAlexandersonHA high incidence of disease flares in an open pilot study of infliximab in patients with refractory inflammatory myopathiesAnn Rheum Dis2008671670167718272672
- DalakasMCRakocevicGSchmidtJEffect of Alemtuzumab (CAMPATH 1-H) in patients with inclusion-body myositisBrain20091321536154419454532
- GreenbergSAComment on alemtuzumab and inclusion body myositisBrain2010133e135 author reply e619892769
- TerraccianoCNogalskaAEngelWKAskanasVIn AbetaPP-overexpressing cultured human muscle fibers proteasome inhibition enhances phosphorylation of AbetaPP751 and GSK3β activation: effects mitigated by lithium and apparently relevant to sporadic inclusion-body myositisJ Neurochem201011238939619878439
- KitazawaMTrinhDNLaFerlaFMInflammation induces tau pathology in inclusion body myositis model via glycogen synthase kinase-3βAnn Neurol200864152418318434
- DimachkieMMBarohnRJInclusion body myositisNeurol Clin201432629646vii25037082
- KosmidisMLAlexopoulosHTzioufasAGDalakasMCThe effect of anakinra, an IL1 receptor antagonist, in patients with sporadic inclusion body myositis (sIBM): a small pilot studyJ Neurol Sci201333412312523998706
- ZongMDorphCDastmalchiMAnakinra treatment in patients with refractory inflammatory myopathies and possible predictive response biomarkers: a mechanistic study with 12 months follow-upAnn Rheum Dis20147391392023625983
- MorosettiRGliubizziCBroccoliniASancriccaCMirabellaMMesoangioblasts of inclusion-body myositis: a twofold tool to study pathogenic mechanisms and enhance defective muscle regenerationActa Myol201130242821842589
- MorosettiRMirabellaMGliubizziCMyoD expression restores defective myogenic differentiation of human mesoangioblasts from inclusion-body myositis muscleProc Natl Acad Sci U S A2006103169951700017077152
- Northwestern UniversityStem cell transplantation in idiopathic inflammatory myopathy diseases Available from: https://clinicaltrials.gov/ct2/show/NCT00278564. ClinicalTrials.gov identifier: NCT00278564Accessed August 31, 2015
- Richard BarohnMDArimoclomol in sporadic inclusion body myositis Available from: https://clinicaltrials.gov/ct2/show/NCT00769860. ClinicalTrials.gov identifier: NCT00769860Accessed August 31, 2015
- LahoutiAHAmatoAAChristopher-StineLInclusion body myositis: updateCurr Opin Rheumatol20142669069625215417
- MendellJRRodino-KlapacLSahenkZGene therapy for muscular dystrophy: lessons learned and path forwardNeurosci Lett2012527909922609847
- MendellJRSahenkZMalikVA phase 1/2a follistatin gene therapy trial for becker muscular dystrophyMol Ther20152319220125322757
- Lach-TrifilieffEMinettiGCSheppardKAn antibody blocking activin type II receptors induces strong skeletal muscle hypertrophy and protects from atrophyMol Cell Biol20143460661824298022
- AmatoAASivakumarKGoyalNTreatment of sporadic inclusion body myositis with bimagrumabNeurology2014832239224625381300
- Novartis PharmaceuticalsEfficacy and safety of bimagrumab/BYM338 at 52 weeks on physical function, muscle strength, mobility in sIBM patients (RESILIENT) Available from: https://clinicaltrials.gov/ct2/show/NCT01925209. ClinicalTrials.gov identifier: NCT01925209Accessed August 31, 2015
- AlfanoLNLowesLPDvorchikIThe 2 min walk test is sufficient for evaluating walking abilities in sporadic inclusion body myositisNeuromuscul Disord20142422222624342281
