Abstract
The management of gastroesophageal reflux disease (GERD) has been revolutionized with the development of proton pump inhibitors (PPIs). Unfortunately, due to the inherent pharmacokinetic and pharmacodynamic profiles of conventional PPIs, many patients continue to suffer from symptoms related to GERD despite appropriate use of PPIs. Dexlansoprazole MR is a PPI with a unique dual delayed-release delivery system that has been designed to address the unmet needs in GERD management. Specifically, dexlansoprazole MR addresses limitations with short plasma half-life and need for meal-associated dosing, characteristic of conventional PPIs. In addition, dexlansoprazole MR has been shown to be effective in several specific clinical situations. These include coadministration with clopidogrel, healing of all grades of erosive esophagitis, improvement in reflux-related quality of life, step down to once-per-day dosing, and treatment of Helicobacter pylori infections. Furthermore, dexlansoprazole MR has been found to induce symptom improvement in patients with nonerosive esophageal reflux disease, nocturnal heartburn and GERD-related sleep disturbance, and regurgitation. Overall, dexlansoprazole MR is a unique and useful tool in the management of GERD.
Definition of GERD
The definition of gastroesophageal reflux disease (GERD) has been an evolving one. Initially felt to be synonymous with tissue damage, it has been recognized that many patients with GERD experience a strong symptom-based component (including heartburn and regurgitation) in the absence of endoscopic and histologic evidence of injury. With the rise in prevalence of reflux disease, as well as varying manifestations of the disorder, a concise definition was felt to be necessary both to define the problem and to address management recommendations for patients and providers. In 2006, the Montreal consensus defined GERD as “a condition which develops when the reflux of stomach contents causes troublesome symptoms and/or complications.”Citation1 Using this unifying definition, treatment for patients with GERD should focus on management of both complications from reflux and significant symptoms relative to the disorder.
Epidemiology of GERD
A recently updated review on the epidemiology of GERD, incorporating studies from 1997 to 2011, found the prevalence of GERD in the USA to be 18.1%–27.8%.Citation2 Similarly, the prevalence of GERD in South America was 23%. Europe and the Middle East had a similar range of prevalence. In Europe, the prevalence was 8.8%–25.9%, while in the Middle East, the prevalence ranged from 8.7% to 33.3%. Interestingly, there was a tendency for GERD to be more prevalent in Northern Europe than Southern Europe. In contrast, East Asia and Australia demonstrated a lower prevalence, with the prevalence in East Asia ranging from 2.5% to 7.8% and that in Australia was 11.6%.Citation3
Risk factors and pathogenesis of GERD
There are many factors that contribute to the development of GERD. Obesity, particularly abdominal obesity, has been shown to significantly increase the risk of GERD presumably by elevating intra-abdominal pressure.Citation4 Aging may also play a role, as older patients often experience more severe esophageal mucosal damage.Citation5 Just as the definition of GERD has changed over time, the mechanisms underlying the pathogenesis of the disease have evolved. These include abnormalities in the function of the gastroesophageal junction, including the impact of transient lower esophageal sphincter relaxation, lower esophageal sphincter hypotension, and hiatal hernia, as well as decreased esophageal acid clearance and reflux related to the postprandial acid pocket.Citation4
Current management of GERD
Current approaches for the management of GERD focus on lifestyle interventions as well as pharmacologic therapy. Traditional recommendations for lifestyle modifications have included weight loss, head-of-bed elevation, and avoidance of meals 2–3 hours prior to sleep. Elimination of tobacco and alcohol, as well as dietary changes including avoidance of chocolate, caffeine, spicy foods, citrus, and carbonated beverages, has also been advocated.Citation6,Citation7
Recent guidelines published by the American College of Gastroenterology and the American Gastroenterological Association have recommended weight loss for overweight patients, as well as head-of-bed elevation and avoidance of meals prior to sleep for GERD patients with nocturnal symptoms, as there is evidence to support these measures.Citation6,Citation7 Unfortunately, there have been no studies that have shown clinical improvement in GERD patients with other lifestyle and dietary modifications, and patient-specific changes are recommended, rather than broadly advocating these measures for every GERD patient.
Current American College of Gastroenterology and American Gastroenterological Association guidelines advocate the use of proton pump inhibitors (PPIs) in both treatment and maintenance of healing of erosive esophagitis. Patients with nonerosive disease are advised to use either H2 receptor antagonists or PPIs for symptom relief.Citation6,Citation7
Unmet needs in GERD management
Clearly, the management of GERD has improved significantly with the development of PPIs. However, many patients still suffer from continued symptoms despite PPI therapy due to the inherent pharmacokinetic and pharmacodynamic limitations of conventional PPIs.Citation8–Citation11 For example, in a survey of 1,013 GERD patients, 35% of patients taking once-daily PPI therapy reported that their current PPI regimen failed to provide complete symptom relief. In a community-based survey of 617 GERD patients, 42% of those taking once-daily therapy supplemented their PPI with other medication – either over-the-counter or prescription medication, and 22% took PPI twice daily.Citation9 This trend is not exclusive to a Western population. Data from the GERD in Asia Pacific Survey evaluated 450 GERD patients and found that 45% of respondents reported limited improvement in nocturnal symptoms, and 49% had continued symptoms that required additional therapy.Citation12
Review of pharmacology, mode of action, and pharmacokinetics of dexlansoprazole MR
PPIs are weak bases that become trapped in acidic environments, undergo acid-catalyzed rearrangement, and subsequently irreversibly bind to and inhibit the active proton pump of the parietal cell.Citation13 Acid secretion is restored when new proton pumps are converted from their inactive state to their active form.Citation10 Conventional PPIs do not entirely eliminate acid secretion; however, single doses of these agents typically inhibit 70%–80% of active pumps.Citation10 While the development of PPIs revolutionized the management of acid-related disorders, unfortunately, there are inherent limitations with traditional PPIs, and many patients suffer from continued symptoms despite PPI therapy as noted above. The drawbacks of conventional PPI therapy primarily involve limitations in the underlying pharmacokinetic and pharmacodynamic properties of the drugs.Citation11 This is manifested in the need for mealtime dosing and the short half-lives of these drugs. Dexlansoprazole MR has been designed to effectively address these issues by prolonging PPI pharmacokinetic and pharmacodynamic profiles.Citation14
Problem #1: conventional PPIs have a short half-life, often resulting in breakthrough symptoms and necessitating more frequent dosing
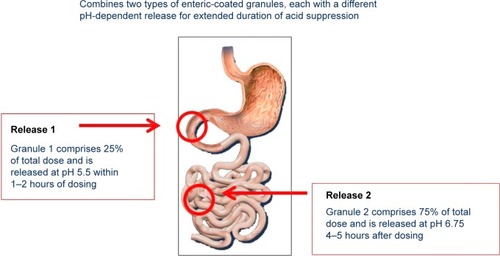
Dexlansoprazole is the R-enantiomer of lansoprazole.Citation15 It is highly protein bound and undergoes elimination via hepatic biotransformation. The R-enantiomer is subsequently associated with decreased clearance compared to the S-enantiomer; however, this is not the sole mechanism that extends the effect of dexlansoprazole MR, as the elimination half-life of dexlansoprazole is similar to other PPIs (approximately 1–2 hours).Citation14,Citation16–Citation18 It is the delivery system, rather than the inherently slower hepatic clearance, that prolongs the plasma residence time of dexlansoprazole MR. The dual delayed-release technology incorporates two distinct sets of enteric-coated granules that are designed to offer two distinct, pH-dependent releases of drug. After ingestion, the gelatin capsule containing the granules dissolves in the stomach, and the first set of granules (~25% of the drug dose) is released into the proximal duodenum (pH 5.5). This results in an early rise in plasma concentration (1–2 hours), similar to other PPIs. The remaining granules (75%) are designed to be released in the distal small intestine (pH 6.75), resulting in a second concentration peak at 4–5 hours after ingestion. This delivery system provides a prolonged duration of acid suppression and helps to limit the necessity for more than once-daily dosing ().Citation14,Citation17–Citation19 A randomized, open-label, two-period crossover study compared the pharmacodynamic effects of single-dose dexlansoprazole MR 60 mg and esomeprazole 40 mg on 24-hour intragastric pH in healthy adult subjects. Results showed the average 24-hour intragastric pH following a single dose of dexlansoprazole MR was higher compared to a single dose of esomeprazole (58% versus 48%; P=0.0003) The most profound difference was noted in the second half of the day, presumably due to the longer duration of action of dexlansoprazole MR.Citation20
Figure 1 Dexlansoprazole dual delayed-release technology releases drug in two phases.
Problem #2: for maximum efficacy, typical PPIs must be dosed prior to meals
Parietal cell proton pumps are activated with meals, and the majority of pumps are inserted with the morning meal. Typical PPIs inhibit stimulated acid secretion, and are recommended to be taken prior to food consumption, particularly 30–60 minutes prior to the morning meal, in order to maximize acid suppression. This requirement can be burdensome for many patients and can lead to issues with continued symptoms. On the other hand, due to its prolonged duration of action, dexlansoprazole MR inhibits both basal and stimulated gastric acid secretion, and is effective regardless of food consumption.Citation21 Using a randomized, open-label crossover study design to evaluate the impact of food on the pharmacodynamics of dexlansoprazole MR, fasting subjects were given placebo 30 minutes before, 5 minutes before, or 30 minutes after a high-fat breakfast on day 1, and on day 3 were given dexlansoprazole MR for each crossover period. Dexlansoprazole plasma concentrations were measured on day 3 and on days 1 and 3. Twenty-four-hour intragastric pH was also measured. No clinically meaningful differences in pharmacokinetic or pharmacodynamic measures between any of the periods were observed. The investigators concluded that food as well as timing of intake of dexlansoprazole MR dosing relative to a meal did not impact the drugs ability to inhibit acid.Citation22 An additional randomized, open-label, four-way crossover study evaluated 48 healthy subjects receiving dexlansoprazole MR 60 mg once daily 30 minutes before breakfast, lunch, dinner, or an evening snack. There were no clinically meaningful differences in mean 24-hour intragastric pH, or in the percentage of time 24-hour intra-gastric pH which was greater than 4, implying the lack of effect of mealtime during the day on acid control.Citation23
Safety, efficacy, and tolerability of dexlansoprazole MR
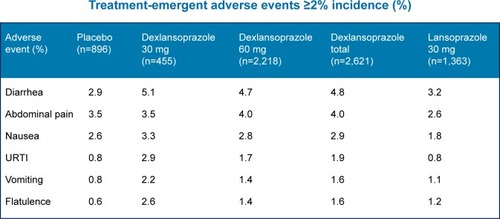
Dexlansoprazole MR is well tolerated, and has been shown to have a safety profile similar to lansoprazole. Data from 4,270 patients from six controlled studies and a 12-month safety study showed fewer adverse events per 100-patient months in the dexlansoprazole MR group compared to the lansoprazole and placebo groups (15.64–18.75 versus 21.06 and 24.49, respectively).Citation20 Additionally, fewer patients receiving dexlansoprazole MR discontinued therapy, when compared to placebo (P<0.05).Citation24 No concerning histologic findings were noted on gastric biopsies.Citation24 The most frequent adverse event was diarrhea, followed by abdominal pain, nausea, upper respiratory tract infection, vomiting, and flatulence.Citation17 outlines the most common adverse events seen in patients during the dexlansoprazole MR clinical trials. Additionally, there are several potential risks associated with PPIs as a class. These include risk of Clostridium difficile and other enteric infections, possible increased risk of community-acquired pneumonia in short-term users, and an unclear association between bone fracture and PPI use.Citation7
Figure 2 Most common adverse events: dexlansoprazole vs lansoprazole.
Abbreviation: URTI, upper respiratory tract infection.
Dexlansoprazole MR and clopidogrel
There is continued debate regarding the potential of PPIs to reduce the effectiveness of clopidogrel. A recent randomized, open-label, two-period crossover study examined the effects of four different PPIs (including dexlansoprazole MR, lansoprazole, omeprazole, and esomeprazole) on the pharmacokinetics and pharmacodynamics of clopidogrel.Citation25 All PPIs were found to decrease the peak plasma concentration of the active metabolite of clopidogrel; however, dexlansoprazole MR was found to have the least effect. Additionally, dexlansoprazole MR did not significantly decrease the area under the curve for clopidogrel active metabolites, and more importantly, did not significantly interfere with the action of clopidogrel on platelet function. The study concluded that among PPIs, dexlansoprazole MR and lansoprazole could be selected to minimize any effect of the PPI–clopidogrel interaction.Citation25
Dexlansoprazole MR and erosive esophagitis
Erosive esophagitis is a significant complication of GERD. PPIs have been shown to be the most effective treatment for healing erosive esophagitis, and have been shown to maintain healing.Citation26 In two identical, double-blind, randomized controlled trials, the role of dexlansoprazole MR in healing erosive esophagitis was examined. Patients from 188 US centers and 118 non-US centers with endoscopically confirmed erosive esophagitis (at least 30% of participants had moderate or severe LA Grade C or D esophagitis) were randomized to 8 weeks of one of three treatment groups: dexlansoprazole MR 60 mg or 90 mg or lansoprazole 30 mg. The proportion of patients who demonstrated complete endoscopic healing of erosive esophagitis over 8 weeks was the primary efficacy endpoint. Life table analysis in the intention-to-treat population indicated that dexlansoprazole MR healed 92%–95% of patients versus 86%–92% healed by lansoprazole. Individual study results using more conservative statistical methods, crude rate analysis, demonstrated that esophagitis healing rates for both 60 mg and 90 mg dexlansoprazole MR doses at week 8 were superior to lansoprazole in one study, while 60 mg dexlansoprazole MR was non-inferior, and 90 mg was superior to lansoprazole in the other study.Citation26 In an integrated analysis, healing of moderate-to-severe (LA Grades C and D) esophagitis was significantly greater with dexlansoprazole MR 90 mg than lansoprazole. All treatment groups had good symptom control and tolerated study medications.Citation26
Dexlansoprazole MR has not only been shown to be effective in providing initial symptom relief and healing of erosive esophagitis, but it has also been shown to provide continued symptom relief and maintain endoscopic healing in patients with erosive esophagitis. A Phase III, randomized, double-blind, multicenter, placebo-controlled, 6-month trial evaluated the efficacy of dexlansoprazole MR 30 mg and 60 mg daily in maintaining healed erosive esophagitis and providing symptom relief. Patients who had participated in one of the two esophagitis healing trials and achieved complete mucosal healing were eligible to participate. The primary efficacy endpoint was the percentage of patients who maintained healed esophagitis for 6 months as assessed by endoscopy. Dexlansoprazole MR was significantly more effective in maintaining healed erosive esophagitis than placebo. The cumulative rates of maintaining healing over 6 months using the intention-to-treat population and time-to-event (life table) analysis were 74.9% and 82.5% in the dexlansoprazole MR 30 mg and 60 mg groups, respectively, compared with 27% in the placebo group. Additionally, both dexlansoprazole MR 30 mg and 60 mg were highly effective in relieving patient symptoms. Median percentages of 24-hour heartburn-free days during the 6-month trial were 96% and 91% in patients receiving dexlansoprazole MR 30 mg and 60 mg, respectively, compared to 29% with placebo.Citation21
Dexlansoprazole MR and nonerosive esophageal reflux disease
Nonerosive esophageal reflux disease (NERD) is a term used to describe symptoms suggestive of GERD in patients with no endoscopic evidence of esophagitis.Citation18 Treatment of patients with NERD can be challenging, as many patients may not respond to traditional PPI therapy. A randomized, double-blind, placebo-controlled study compared a 4-week course of dexlansoprazole MR 30 mg and 60 mg with placebo in patients with NERD. Patients enrolled in the study were required to have symptoms of heartburn for at least 6 months prior to enrollment, as well as symptoms of heartburn for ≥4 out of 7 days prior to randomization. These patients were also required to have no evidence of esophageal erosions on endoscopy. The primary endpoint of the study was the percentage of 24-hour heartburn-free days. Results showed a significant difference in symptom relief (as assessed by 24-hour heartburn-free days) in patients receiving dexlansoprazole MR versus those receiving placebo (54.9% and 50% versus 18.5%, respectively; P>0.00001).Citation18 Additionally, a significantly greater percentage in the dexlansoprazole MR groups (30 mg and 60 mg) achieved sustained resolution of heartburn by the end of treatment compared with placebo (59% and 42% versus 14% respectively; P<00001).Citation18
A systematic review and indirect comparison of randomized controlled trials comparing the effectiveness of dexlansoprazole MR and esomeprazole in patients with NERD suggested significantly better symptom control in NERD patients treated with dexlansoprazole MR 30 mg versus esomeprazole 20 mg or 40 mg.Citation27
Dexlansoprazole MR and body mass index
A higher body mass index (BMI) is a strong and independent risk factor for the development of GERD, as well as for complications of the disease, such as erosive esophagitis, Barrett’s esophagus, and esophageal adenocarcinoma.Citation28 Only a small number of studies have investigated the effect of BMI on response to PPI therapy. Results from these studies have been conflicting, with some studies showing a positive impact of BMI on response to PPI therapy and other showing no effect or a negative effect of BMI on PPI therapy.Citation28 A 2012 study of both NERD patients and patients with erosive esophagitis confirmed the association between a higher BMI and severity of esophagitis as well as increasing GERD symptoms, measured by a symptom diary.Citation28 NERD patients with higher BMI treated with dexlansoprazole MR tended to receive more benefit in symptom relief when compared to those with a lower BMI. This benefit was significantly more pronounced in patients with erosive esophagitis treated with dexlansoprazole MR. Moreover, healing rates in patients treated with dexlansoprazole but not lansoprazole were higher in obese patients compared with those with a BMI <30 kg/m2.Citation28 The study concluded that both NERD patients and erosive esophagitis patients were more symptomatic with increasing BMI. While this was a trend in patients with NERD, this was statistically significant for patients with erosive esophagitis. While all patients benefited from dexlansoprazole MR treatment, those with a higher BMI appeared to have the greatest benefit.Citation28
Dexlansoprazole MR, nocturnal heartburn, and GERD-related sleep disturbances
Many patients with GERD suffer from nocturnal symptoms and associated sleep disturbance. In fact, up to 89% of patients with GERD suffer from nocturnal symptoms.Citation29 Dexlansoprazole MR has been found to be effective in reducing these nighttime symptoms. A prospective, randomized, double-blind, placebo-controlled study evaluated the effect of dexlansoprazole 30 mg for relief of nocturnal symptoms over a 4-week period in patients with three or more nights of moderate-to-severe heartburn per week, prior to randomization. The secondary endpoint of the study was to assess the efficacy of dexlansoprazole MR for improvement of GERD-related sleep disturbance. Dexlansoprazole MR 30 mg was significantly better than placebo in decreasing nocturnal symptoms, with 73.1 heartburn-free nights in the dexlansoprazole group versus 35.7 heartburn-free nights in the placebo group (P<0.0001).Citation29 Additionally, a greater percentage of patients in the dexlansoprazole group (47.5%) had relief of nocturnal heartburn symptoms over the last week of the study period compared to placebo (19.6%; P<0.001).Citation29 Finally, dexlansoprazole was found to be effective at reducing GERD-related sleep disturbance (69.7% in the dexlansoprazole group versus 47.9% in the placebo group; P<0.001).Citation29
Dexlansoprazole MR and step down to once-per-day therapy
Many patients with GERD have symptoms that are not well controlled on once-daily PPI therapy. A 2009 survey of 619 patients with GERD found that 22.2% of GERD patients were using a bid PPI regimen due to uncontrolled GERD symptoms, or persistent nocturnal symptoms.Citation9 The ability to decrease dosing, while maintaining symptom control, would be beneficial for many reasons, including convenience, compliance, and cost. A multicenter, single-blind study evaluated the efficacy of dexlansoprazole MR 30 mg once daily in maintaining control of heartburn in patients whose symptoms were previously well controlled on twice-daily PPI. The study found that 88% of patients maintained symptom control after stepping down from twice-daily therapy to once-daily dexlansoprazole MR 30 mg.Citation30
Dexlansoprazole MR and regurgitation
Regurgitation is a significant symptom manifestation of GERD, and unfortunately, is often not adequately treated with traditional PPIs. In fact, regurgitation may be an important factor in patients who experience incomplete response to PPI treatment.Citation31 Symptoms of regurgitation were recently evaluated in a post hoc analysis of NERD and erosive esophagitis patients receiving dexlansoprazole MR, placebo, or lansoprazole. This study utilized the PAGI-SYM (Patient Assessment of Upper Gastrointestinal-Symptom Severity) questionnaire to assess the effectiveness of PPI therapy on symptoms of regurgitation. NERD patients with at least mild symptoms of regurgitation received dexlansoprazole MR 30 mg, 60 mg, or placebo for 4 weeks. At weeks 2 and 4, both dexlansoprazole MR 30 mg and dexlansoprazole 60 mg provided a statistically significant improvement in regurgitation symptoms compared with placebo. Patients with erosive esophagitis and at least mild regurgitation received either dexlansoprazole MR 60 mg or lansoprazole 30 mg. There was a statistically significant improvement in regurgitation symptoms in the week 4 dexlansoprazole group compared with lansoprazole. Both groups had similar improvement in regurgitation symptoms at week 8.Citation32
Dexlansoprazole MR and quality of life
Dexlansoprazole MR has been shown to improve several health-related quality of life measures in GERD patients. A prospective, randomized, double-blind, placebo-controlled parallel group study comparing dexlansoprazole MR 30 mg to placebo found a significantly greater decrease in overall impairment of work productivity and impairment of regular activities in the dexlansoprazole MR group, as well as reduced number of impaired work hours when compared to placebo.Citation29
A study of 178 patients with well-controlled GERD symptoms on twice-daily PPI who were subsequently switched to masked dexlansoprazole MR 30 mg daily and placebo for 6 weeks observed a statistically significant improvement in quality of life scores (P=0.16) (PAGI-QOL – Patient Assessment of Upper Gastrointestinal Disorders-Quality of Life) in patients with well-controlled symptoms on dexlansoprazole MR 30 mg.Citation30
Dexlansoprazole MR and Helicobacter pylori
There are very limited data on the use of dexlansoprazole MR in the treatment of H. pylori infections. A recent prospective, open-label, single-center pilot study of 13 patients evaluated the efficacy of dual therapy (amoxicillin 1 g bid and dexlansoprazole MR 120 mg bid) in the treatment of H. pylori infections. The authors theorized that maintaining a nonacidic environment with the use of dexlansoprazole MR would prevent bacteria from converting to a non-replicative state, and improve eradication rates. The results, however, were disappointing, and the study was stopped early due to a high failure rate – the eradication rate was only 53%.Citation33 Whether treatment with more frequent doses of amoxicillin, as has been used in other studies, would have resulted in better cure of infection remains to be seen.Citation34 Although further studies are needed to define the role of dexlansoprazole MR in the management of H. pylori, presumably, it should be as effective as other PPIs when used as a part of traditional treatment regimens.
Comparison of dexlansoprazole MR and other PPIs
While there are no direct comparisons of dexlansoprazole MR with PPIs other than lansoprazole, an indirect comparison of randomized controlled trials of dexlansoprazole in the treatment of erosive esophagitis healing, maintenance of healed erosive esophagitis, and treatment of NERD suggested better treatment effect of dexlansoprazole MR in symptom control in NERD. However, there was no significant difference in erosive esophagitis outcomes.Citation27
Conclusion – dexlansoprazole MR’s place in therapy
Dexlansoprazole MR is a PPI with a unique delivery system that extends its duration of action and has been shown to be effective for management of many aspects of GERD symptoms. Its distinctive pharmacokinetic and pharmacodynamic properties make it a useful tool in the armamentarium of GERD therapy. Dexlansoprazole MR can be considered for patients with varying manifestations of GERD, including those with a new diagnosis of GERD, erosive esophagitis, or need for maintenance of healing of erosive esophagitis, as well as symptomatic nonerosive GERD. Additionally, patients who require 24-hour symptom relief, experience nocturnal symptoms, have incomplete response to once-daily conventional PPI therapy, are obese, use clopidogrel, or require dosing flexibility are all excellent candidates for treatment with dexlansoprazole MR ().
Table 1 Patients for whom dexlansoprazole MR is appropriate
Disclosure
Dr Peura is a consultant and speaker for Takeda and a consultant for Pfizer. The authors report no other conflicts of interest in this work.
References
- VakilNvan ZantenSVKahrilasPDentJJonesRGlobal ConsensusGThe Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensusAm J Gastroenterol200610119001920 quiz 4316928254
- El-SeragHBSweetSWinchesterCCDentJUpdate on the epidemiology of gastro-oesophageal reflux disease: a systematic reviewGut20146387188023853213
- DentJEl-SeragHBWallanderMAJohanssonSEpidemiology of gastro-oesophageal reflux disease: a systematic reviewGut20055471071715831922
- BoeckxstaensGEl-SeragHBSmoutAJKahrilasPJSymptomatic reflux disease: the present, the past and the futureGut2014631185119324607936
- BecherADentJSystematic review: ageing and gastro-oesophageal reflux disease symptoms, oesophageal function and reflux oesophagitisAliment Pharmacol Ther20113344245421138458
- KahrilasPJShaheenNJVaeziMFAmerican Gastroenterological Association Medical Position Statement on the management of gastroe-sophageal reflux diseaseGastroenterology2008135138313911391e1e518789939
- KatzPOGersonLBVelaMFGuidelines for the diagnosis and management of gastroesophageal reflux diseaseAm J Gastroenterol2013108308328 quiz 2923419381
- CheyWDModyRRIzatEPatient and physician satisfaction with proton pump inhibitors (PPIs): are there opportunities for improvement?Dig Dis Sci2010553415342220397047
- CheyWDModyRRWuEQTreatment patterns and symptom control in patients with GERD: US community-based surveyCurr Med Res Opin2009251869187819530980
- KatzPOScheimanJMBarkunANReview article: acid-related disease – what are the unmet clinical needs?Aliment Pharmacol Ther200623suppl 292216700899
- ShinJMKimNPharmacokinetics and pharmacodynamics of the proton pump inhibitorsJ Neurogastroenterol Motil201319253523350044
- GohKLChoiMGHsuWPUnmet treatment needs of gastroe-sophageal reflux disease in Asia: gastroesophageal reflux disease in Asia Pacific SurveyJ Gastroenterol Hepatol2014291969197524990817
- SchubertMLPeuraDAControl of gastric acid secretion in health and diseaseGastroenterology20081341842186018474247
- BehmBWPeuraDADexlansoprazole MR for the management of gastroesophageal reflux diseaseExpert Rev Gastroenterol Hepatol2011543944521780890
- KatsukiHYagiHArimoriKDetermination of R(+)- and S(−)- lansoprazole using chiral stationary-phase liquid chromatography and their enantioselective pharmacokinetics in humansPharm Res1996136116158710755
- AbelCDesiletsARWillettKDexlansoprazole in the treatment of esophagitis and gastroesophageal reflux diseaseAnn Pharmacother20104487187720371754
- WittbrodtETBaumCPeuraDADelayed release dexlansoprazole in the treatment of GERD and erosive esophagitisClin Exp Gastroenterol2009211712821694835
- FassRCheyWDZakkoSFClinical trial: the effects of the proton pump inhibitor dexlansoprazole MR on daytime and nighttime heartburn in patients with non-erosive reflux diseaseAliment Pharmacol Ther2009291261127219392864
- VakilyMZhangWWuJAtkinsonSNMulfordDPharmacokinetics and pharmacodynamics of a known active PPI with a novel dual delayed release technology, dexlansoprazole MR: a combined analysis of randomized controlled clinical trialsCurr Med Res Opin20092562763819232037
- KukulkaMEisenbergCNudurupatiSComparator pH study to evaluate the single-dose pharmacodynamics of dual delayed-release dexlansoprazole 60 mg and delayed-release esomeprazole 40 mgClin Exp Gastroenterol2011421322022016582
- MetzDCHowdenCWPerezMCLarsenLO’NeilJAtkinsonSNClinical trial: dexlansoprazole MR, a proton pump inhibitor with dual delayed-release technology, effectively controls symptoms and prevents relapse in patients with healed erosive oesophagitisAliment Pharmacol Ther20092974275419210298
- LeeRDVakilyMMulfordDWuJAtkinsonSNClinical trial: the effect and timing of food on the pharmacokinetics and pharmacodynamics of dexlansoprazole MR, a novel dual delayed release formulation of a proton pump inhibitor – evidence for dosing flexibilityAliment Pharmacol Ther20092982483319243357
- LeeRDMulfordDWuJAtkinsonSNThe effect of time-of-day dosing on the pharmacokinetics and pharmacodynamics of dexlansoprazole MR: evidence for dosing flexibility with a dual delayed release proton pump inhibitorAliment Pharmacol Ther2010311001101120180789
- PeuraDAMetzDCDabholkarAHParisMMYuPAtkinsonSNSafety profile of dexlansoprazole MR, a proton pump inhibitor with a novel dual delayed release formulation: global clinical trial experienceAliment Pharmacol Ther2009301010102119735233
- FrelingerAL3rdLeeRDMulfordDJA randomized, 2-period, crossover design study to assess the effects of dexlansoprazole, lansoprazole, esomeprazole, and omeprazole on the steady-state pharmacokinetics and pharmacodynamics of clopidogrel in healthy volunteersJ Am Coll Cardiol2012591304131122464259
- SharmaPShaheenNJPerezMCClinical trials: healing of erosive oesophagitis with dexlansoprazole MR, a proton pump inhibitor with a novel dual delayed-release formulation – results from two randomized controlled studiesAliment Pharmacol Ther20092973174119183157
- WuMSTanSCXiongTIndirect comparison of randomised controlled trials: comparative efficacy of dexlansoprazole vs esomeprazole in the treatment of gastro-oesophageal reflux diseaseAliment Pharmacol Ther20133819020123718547
- PeuraDAPilmerBHuntBModyRPerezMCThe effects of increasing body mass index on heartburn severity, frequency and response to treatment with dexlansoprazole or lansoprazoleAliment Pharmacol Ther20133781081823451835
- FassRJohnsonDAOrrWCThe effect of dexlansoprazole MR on nocturnal heartburn and GERD-related sleep disturbances in patients with symptomatic GERDAm J Gastroenterol201110642143121224838
- FassRInadomiJHanCModyRO’NeilJPerezMCMaintenance of heartburn relief after step-down from twice-daily proton pump inhibitor to once-daily dexlansoprazole modified releaseClin Gastroenterol Hepatol20121024725322155561
- KahrilasPJHowdenCWHughesNResponse of regurgitation to proton pump inhibitor therapy in clinical trials of gastroesophageal reflux diseaseAm J Gastroenterol201110614191425 quiz 2621537361
- PeuraDAPilmerBHuntBModyRPerezMCDistinguishing the impact of dexlansoprazole on heartburn vs regurgitation in patients with gastro-oesophageal reflux diseaseAliment Pharmacol Ther2013381303131124118079
- AttumiTAGrahamDYHigh-dose extended-release lansoprazole (dexlansoprazole) and amoxicillin dual therapy for Helicobacter pylori infectionsHelicobacter20141931932224698653
- YangJCLinCJWangHLHigh-dose dual therapy is superior to standard first-line or rescue therapy for helicobacter pylori infectionClin Gastroenterol Hepatol201413589590525460556
