Abstract
Introduction
Thyrotoxicosis is an endocrine disorder with prominent cardiovascular manifestations. Thyroid hormone acts through genomic and non-genomic mechanisms to regulate cardiac function. Echocardiography is a useful, non-invasive, easily accessible, and affordable tool for studying the structural and physiological function of the heart.
Aim
We studied thyrotoxicosis patients in a Nigerian Teaching Hospital and employed trans-thoracic echocardiography to find out if there were abnormalities in the hearts of these patients.
Methods
Fifty adult thyrotoxicosis patients diagnosed with clinical and thyroid function tests in the medical out-patient unit of the hospital were recruited and we performed transthoracic echocardiography with a Sonos 2000 HP machine.
Results
We documented the presence of abnormalities in the following proportion of thyrotoxicosis patients: left ventricular enhanced systolic function in 30%, enhanced diastolic function in 34%, diastolic dysfunction in 34%, heart failure with preserved ejection fraction in10%, heart failure with reduced ejection fraction in 6%, and left ventricular hypertrophy in 34%.
Conclusion
Echocardiography was useful in the stratification of cardiac function abnormalities and is indispensable as a guide in the choice of therapeutic options in patients with thyrocardiac disease. The finding of left ventricular enhanced systolic and diastolic functions signify early echocardiographic detectable cardiac abnormalities in thyrotoxicosis, and the clinical management includes the use of anti-thyroid drugs and β-adrenoceptor blockade. Diastolic dysfunction in thyrotoxicosis patients asymptomatic for cardiac disease should be treated with anti-thyroid drugs, and β-adrenoceptor blockade. The judicious application of clinical therapeutics will guide the use of anti-thyroid drugs, diuretics, digoxin, angiotensin inhibitors, and β-adrenoceptor blockade in the successful management of thyrotoxicosis patients with heart failure and reduced, preserved, or increased ejection fraction: parameters which are derived from echocardiography.
Introduction
Thyrotoxicosis is the syndrome resulting from an excess of circulating free thyroxine and free triiodothyronine.Citation1 When thyrotoxicosis is associated with thyroid gland over-activity, hyperthyroidism is said to occur. However, thyrotoxicosis can occur without hyperthyroidism when stored hormone is released from a damaged thyroid gland (eg, sub-acute thyroiditis, post-partum thyroiditis, amiodarone-induced thyroiditis) or when excess thyroid hormone is taken.Citation1 In 1935 Robert Graves, an Irishman, and in 1940 Karl Adolph van Basedow, a German, separately described Basedow–Graves’ or Graves’ disease which is now responsible for 70%–80% of all cases of hyperthyroidism.Citation2
Thyrotoxicosis affects the normal functioning of many tissues including their growth, differentiation, metabolism, and oxygen consumption. It affects the cardiovascular system profoundly. The cardiovascular manifestations of thyrotoxicosis are due to direct cellular effects of thyroid hormones on the heart and indirect cellular effects resulting from interactions with the sympathetic nervous system, alterations in peripheral vascular smooth muscle (VSM), rennin–angiotensin–aldosterone system, and erythropoietin production.Citation3
Thyroid hormone modulates cardiac function through regulation of the expression of some structural and regulatory genes. Within the myocardial cells are the fast α-myosin heavy chain and the slow β-myosin heavy chain which mediate contraction. Thyroid hormone upregulates α-gene which has higher ATPase activity and contractile properties and decreases the expression of β-gene with lower contractile properties. Thyroid hormone also upregulates the rate of Ca2+ release and reuptake by the sarcoplasmic reticulum and downregulates the inhibitor, phospholamban. The increase in cytosolic calcium increases systolic contraction and the more rapid calcium reuptake enhances diastolic relaxation in the heart.Citation4–Citation6
The sodium potassium ATPase, the voltage-gated potassium channels, and the sodium calcium exchanger are ion channels that are activated in thyrotoxicosis and they coordinate the electrochemical responses of the myocardium during cardiac contraction and relaxation.Citation6–Citation8 The β-adrenergic receptors are thought to be stimulated in thyrotoxicosis, leading to an increase in the intracellular second messenger, cAMP, which in turn accelerates diastolic depolarization and increases heart rate. The natriuretic peptides are secreted by cardiac myocytesCitation9 and are said to be upregulated by thyroid hormones. The pacemaker-related genes, hyperpolarization-activated cyclic nucleotide-gated channels 2 and 4, are also transcriptionally regulated by thyroid hormone.Citation10,Citation11
It has been suggested that hyperthyroidism resembles a hyperadrenergic state; however, there is no evidence that thyroid hormone excess enhances the sensitivity of the heart to adrenergic stimulation.Citation11–Citation13 Indeed, the role of the sympathetic nervous system in the pathophysiology of hyperthyroidism is unclear.Citation12 The clinical spectrum of symptoms in thyrotoxicosis suggests a hyperadrenergic state strengthened by the fact that the administration of β-adrenoceptor antagonists dramatically ameliorates the clinical state.Citation14–Citation16 In spite of this known fact, the concentrations of catecholamines in both plasmaCitation17 and urineCitation18 are normal or low in hyperthyroidism.
Thyrotoxicosis increases endothelial nitric oxide production via the triiodothyronine (T3)-mediated effects of thyroid receptor on the protein kinase pathway.Citation19–Citation21 Nitric oxide synthesized in endothelial cells then acts in a paracrine manner on adjacent VSM cells to facilitate vascular relaxation. Relaxation of VSM leads to decreased peripheral resistance and pressure, increased blood return to the heart, and increased blood volume which increases cardiac output (CO). Increased vascularity and angiogenesis reported in thyrotoxicosis may also lead to increased blood volume and CO.Citation22
The other factors that increase CO include activation of renin–angiotensin–aldosterone system, increased red cell mass, as well as increased blood volume: all these contribute to systemic hypertension seen in thyrotoxicosis patients.Citation23 Hypertension contributes in causing ventricular hypertrophy and myocardial remodeling in patients with thyrotoxicosis.Citation23 Diastolic and mean arterial blood pressures are reduced because of peripheral vasodilatation that occurs in thyrotoxicosis.Citation23 Pulmonary arterial hypertension also occurs in hyperthyroidism and it has been attributed to pulmonary vascular endothelial dysfunction, damage due to autoimmune process, increased metabolism of intrinsic pulmonary vasodilators, and high CO state.Citation24 In some cases, pulmonary hypertension may result in right heart failure.Citation24
Another effect of thyrotoxicosis on the vasculature is its anti-atherosclerotic effects through blood vessel dilatation and production of vasodilatory molecules. In contrast, in people with hypothyroidism, atherosclerosis has been attributed to hypercholesterolemia, hypertension, and impaired endothelial function, leading to increased cardiovascular risk. Treatment of hypothyroidism with thyroid hormone replacement restores euthyroidism and reverses the associated risk ratio.Citation25
Echocardiography is a very useful, non-invasive, easily accessible, and perhaps an affordable tool for studying the structure and physiological function of the heart. Assessment of ventricular function, particularly the left ventricle, is one of the commonest and most important applications of echocardiography. Echocardiographic examination of left ventricular (LV) function is useful in assessment of the effect of thyrotoxicosis on the heart.
The presence of LV dysfunction is a reliable prognostic indicator in all forms of cardiac disease. Indeed, echocardiographic findings may alter the course of management and provide opportunities for appropriate therapeutic intervention.
Objective
We studied patients who had thyrotoxicosis and assessed its effect on LV function. Echocardiographic study of these patients was done with a view to stratifying them according to the pattern of LV dysfunction.
Methods
We recruited 50 subjects with thyrotoxicosis who were aged 15 years and above and of both sexes, over a period of 1 year. The patients were recruited consecutively as they attended the medical out-patient unit of the University of Nigeria Teaching Hospital, Enugu after clinical and thyroid function assessments. Written consent was signed by the subjects and controls. The University of Nigeria Teaching Hospital Ethical committee approved the study and it was carried out in accordance with the Declaration of Helsinki ethical principles for medical research involving human subjects.Citation26
Echocardiography was done with a Sonos 2000 HP machine (Hewlett Packard, the Netherlands, Amsterdam) to assess LV systolic and diastolic functions. Transthoracic echocardiographic examinations were performed in all participants with 3.5 MHz transducer according to the recommendations of the American Society of Echocardiography.Citation27 The American Society of Echocardiography recommends that measurements should be taken from trailing edge to leading edge. All the measurements were done with the picture frozen on the screen and with in-built calipers of the echocardiographic equipment. The mean measurements were taken from three consecutive cycles. The measurements were initially taken by two examiners. Thereafter, all the measurements were taken by the same echocardiographer. Electrocardiography was also done in all the participants.
Thyroid function tests were done with kits from Syntron Bioresearch Inc., Carlsbad, CA, USA. These kits had a correlation coefficient of 0.09869 when compared with a standard kit made by Abbott Laboratories (North Chicago, IL, USA). The Syntron kits have the following intra-assay coefficient of variation: serum-free T3 − (6.8%), thyroid-stimulating hormone (TSH) − (4.3%), total free T3 − (4.4%), total tetraiodothyronine − (7.2%).Citation28
Exclusion criteria included patients with:
pre-existing hypertension (blood pressure ≥140/90 mmHg, or were on antihypertensive drugs before the onset of thyrotoxicosis);
diabetes mellitus (fasting blood glucose >6.1 mmol/L);
coronary artery disease (detected by electrocardio graphy);
anemia (hemogram <12 gm/L);
chronic alcohol consumption;
history of smoking, intake of illicit drugs, intake of herbal drugs, cardiotoxic drug consumption, pregnant state.
The controls were 50 age- and sex-matched subjects who do not have thyrotoxicosis and/or any co-morbidity.
Definitions
A serum-free T3 >4.2 pg/L and a concomitant suppressed TSH of >0.5 μU/mL was used to diagnose thyrotoxicosis. The following parameters were defined as follows:Citation27–Citation32
LV systolic dysfunction was present if there was any one of the following: i) LV ejection fraction <50%, ii) fractional shortening (FS) <30%, iii) cardiac index <2.8 L/min/m2, iv) CO <4 L/min, v) mean velocity of circumferential fiber (VCF) shortening <1.02 circumferences/sec, and vi) peak aortic systolic velocity <72 cm/sec;
LV diastolic dysfunction was present if there was any one of the following: LV E/A (early diastolic velocity/velocity with atrial contraction) <1.1, isovolumic relaxation time >90 msec, E wave deceleration time >210 msec, peak filling rate <5);
enhanced (meaning increased myocardial contractility) systolic functionCitation33 was present if there was any one of the following: ejection fraction >75%, FS >42%, CO >7 L/min, peak aortic systolic velocity >120 cm/sec;
enhanced (meaning increased) diastolic functionCitation34 was present if there was documentation of any one of the following: E wave velocity >72 cm/sec, A wave velocity >59 cm/sec, isovolumic relaxation time <76 msec, E wave deceleration time <179 msec in the thyrotoxicosis patients and age- and sex-matched control subjects;
left ventricular mass index (LVMI)Citation27 normal value: 43–96 g/m2 (for women) and 49–116 g/m2 (for men);
relative wall thicknessCitation27 of >0.43 indicates concentric hypertrophy; and <0.43 indicates eccentric hypertrophy.
Statistics
Continuous variables were expressed as mean ± 1 standard error of mean. Statistical comparisons were performed using SPSS software (version 15.0; SPSS Inc., Chicago, IL, USA). P<0.05 was considered significant.
Results
Thyrotoxicosis patients were leaner, and when their blood pressure was compared with controls there was no significant difference (). Palpitation was the commonest symptom, enlarged thyroid gland was present in more than 50% of the patients, and Graves’ disease was documented in 40% of the subjects ().
Table 1 Anthropometric data and blood pressure of patients and controls
Table 2 Frequencies of symptoms in thyrotoxicosis patients
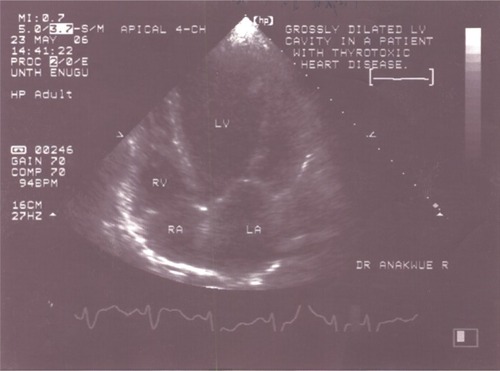
Echocardiographic measurements showed that the mean intra-observer agreement was 97% (k=0.86) and the mean inter-observer agreement was 92% (k=0.78). This indicated that intra-observer and inter-observer variability were negligible. Out of all 50 patients studied, eight (16%) had heart failure. Five of these patients had systolic heart failure with reduced ejection fraction: a typical echocardiogram is depicted in . The other three patients had heart failure with preserved ejection fraction. No patient had the so-called high-output heart failure, ie, heart failure with increased ejection fraction.
The left ventricle was dilated in 28% of the patients. The LV end diastolic diameter was significantly higher in the thyrotoxicosis patients than in the controls (5.35 cm 0.76 standard deviation [SD] versus [vs] 5.0 cm 0.73 SD − P<0.05) (). When all the thyrotoxicosis subjects were compared with control, it showed that the index patients with thyrotoxicosis had significantly increased systolic function (P<0.01), supporting the hyperdynamic clinical state of the disease (). Enhanced systolic function depicted in was documented in 14 patients, and their systolic parameters showed significant difference when compared with that of the control − P<0.01 ().
Figure 2 Apical four chamber view with continuous wave Doppler showing increased aortic flow velocity in a patient with enhanced systolic function in thyrotoxicosis.
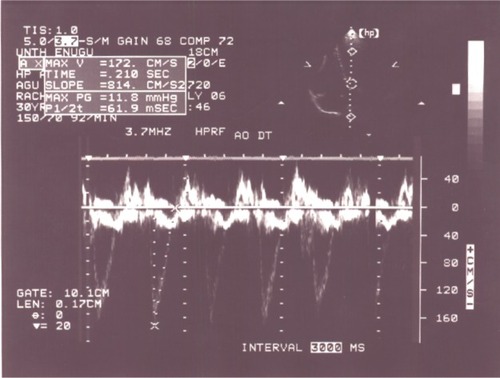
Table 3 Comparison of parameters of left ventricular systolic function between thyrotoxicosis patients and control
Table 4 Comparison of proportions of left ventricular enhanced systolic function parameters between thyrotoxicosis patients and control
Enhanced diastolic function depicted in was documented in 17 thyrotoxicosis patients and when parameters of diastolic function were compared with control, there was significant difference − P<0.01 (). It is obvious that some patients had combined enhanced systolic and enhanced diastolic functions.
Figure 3 Apical four chamber view with pulse wave Doppler showing increased mitral E and A wave velocities in keeping with enhanced diastolic function.
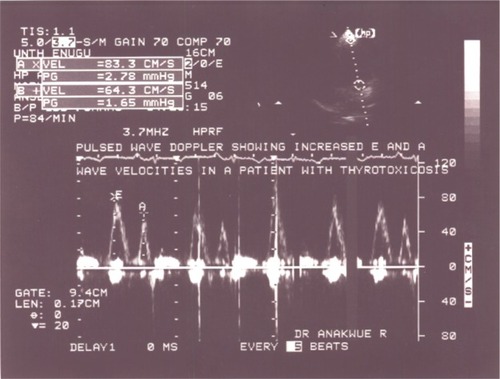
Table 5 Comparison of proportions of left ventricular enhanced diastolic function parameters of thyrotoxicosis patients and control
Diastolic dysfunction () was seen among 17 patients. shows that there was significant difference between thyrotoxicosis patients and controls when parameters of diastolic function were assessed − (P<0.01).
Figure 4 Pulse wave Doppler images demonstrating diastolic dysfunction.
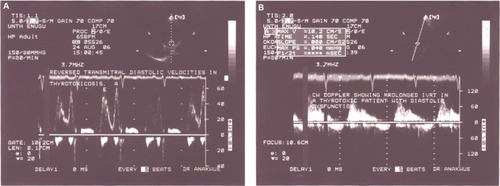
Table 6 Comparison of proportions of left ventricular diastolic dysfunction parameters of thyrotoxicosis patients and control
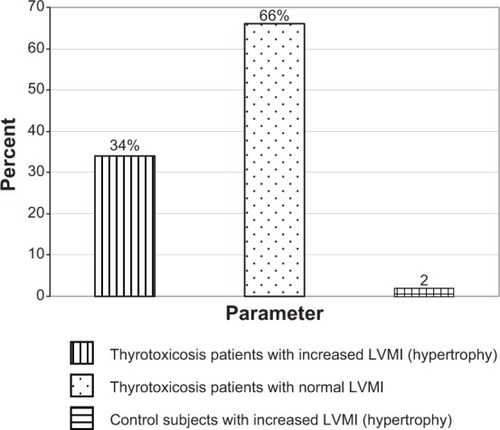
The inter-ventricular septal thickness and the posterior ventricular wall thickness were significantly higher in the thyrotoxicosis group than in the control: (0.95 cm vs 0.77 cm − P<0.05.) and (0.89 cm vs 0.79 cm − P<0.05), respectively. Four percent of the patients had inter-ventricular septal thickness >12 mm. The LVMI of subjects (127.03 g/m2) was also significantly higher than in the control (84.14 g/m2) at P<0.05, and the relative wall thickness was significantly higher in thyrotoxicosis patients than in the control (0.53 vs 0.33 − P<0.05). In effect, the thyrotoxicosis patients had eccentric hypertrophy. shows that 17 (34%) thyrotoxic patients had LV hypertrophy using LVMI. Thirty-three (66%) thyrotoxic patients had normal LVMI. Only one (2%) subject in the control had increased LVMI.
Figure 5 Bar chart showing the percentage of thyrotoxicosis patients with left ventricular hypertrophy using left ventricular mass index (LVMI).
summarizes the echocardiographic findings in the 50 thyrotoxicosis patients studied. shows the multivariate regression analysis between all the systolic and diastolic parameters and TSH and T3 of the patients with thyrotoxicosis and control. Both T3 and TSH models showed a good fit (R2=0.42, P<0.1, and P<0.05, respectively). T3 retained its independent positive association with the systolic and diastolic parameters. TSH also retained its independent negative association with the systolic and diastolic parameters.
Figure 6 The echocardiographic abnormalities seen in the study.
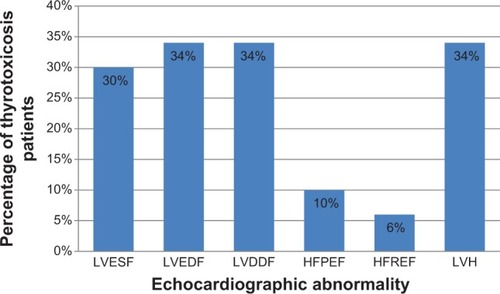
Table 7 Correlation of thyroid-stimulating hormone and free T3 with echocardiographic parameters
Discussion
Our study documented enhanced systolic and diastolic functions when the subjects were compared with controls ( and ), and these findings are in keeping with other reports which documented that patients who have thyrotoxicosis also have increased LV systolic and diastolic contractile functions, resulting from upregulation of contractile and calcium-regulatory proteins.Citation35–Citation37
Friedman et alCitation38 in the USA had reported enhanced systolic function by documenting the echocardiographic tracing of the septum and left posterior wall. Kral et alCitation39 in Czecholoslavakia studied 12 patients with hyperthyroidism and documented significant increase in mean VCF as well as cardiac index, stroke volume, and LV end diastolic volume. In Poland, Marcisz et al while studying hyperthyroidism, noted that it was associated with enhanced systolic function.Citation40 They demonstrated increased LV ejection function, FS, mean VCF, cardiac index and output-pressure index. So our study, which showed an increase of 18% and 19% in the LV ejection fraction and FS, respectively, in thyrotoxicosis patients when compared with control is not a new finding. Enhanced systolic function has also been documented in an animal study involving 103 cats with hyperthyroidism in which Bond et alCitation41 showed enhancement of indices of contractility: 21.4% in FS and 14.6% in mean VCF.
Mintz et alCitation37 had documented the presence of enhanced diastolic function in nine newly diagnosed and untreated hyperthyroid patients, which normalized following attainment of euthyroid state. They concluded that their findings of enhanced cardiac diastolic performance did not support the hypothesis that thyrotoxicosis was associated with compromised LV function and suggested the possibility that the cardiac symptoms that accompany hyperthyroidism may be due to non-cardiac mechanisms.Citation37 This conclusion can be said to be valid only for patients with enhanced function, because enhanced diastolic function is an early state of thyrocardiac disease and is unlikely to be associated with any cardiac abnormality. Enhanced diastolic function results from enhanced myocardial relaxation, which is due to increased calcium reuptake by the sarcoplasmic reticulum.Citation37
The management of enhanced systolic and diastolic functions is achieved by rendering the patient euthyroid through the use of anti-thyroid therapy. The judicious use of anti-thyroid drugs in addition to β-adrenoceptor blockade will lead to decreased calcium release and reuptake from the sarcoplasmic reticulum, which reverses the enhanced systolic and diastolic functions, respectively. β blockade with propranolol decreases end systolic volume, whereas anti-thyroid therapy with carbimazole reduces end diastolic volume.Citation42 In other words, while β-adrenoceptor blockade depresses myocardial contractility, anti-thyroid therapy reverses the primary hemodynamic disturbance responsible for the increase in stroke volume.Citation42
β-adrenoceptor blockade has been used to modify the severity of hyperadrenergic symptoms of thyrotoxicosis and to treat tachycardia. Many β-adrenoceptors have been used, but propranolol and the longer-acting atenolol are more popular.Citation43 Propranolol has two roles in the treatment of hyperthyroidism, determined by the different isomers of propranolol. L-propranolol causes β-blockade, thus treating the symptoms associated with hyperthyroidism such as tremor, palpitations, anxiety, and heat intolerance.Citation43 D-propranolol inhibits thyroxine deiodinase, thereby blocking the conversion of tetraiodothyronine to T3, providing some though minimal therapeutic effect.Citation44 Atenolol is a selective beta blocker and so less likely than propranolol to cause bronchoconstriction in patients who have bronchial disease.Citation44
Impaired diastolic dysfunction was found in at least 28% of the thyrotoxicosis patients in our study (). These patients with diastolic dysfunction had no symptoms arising from the cardiovascular system. But it has been documented that the finding of diastolic dysfunction in an asymptomatic patient is a risk factor for the future development of heart failure, and the early identification of such patients provides a window of opportunity to prevent progression of what appears to be a preclinical heart disease.Citation45–Citation47
In a study in India, which involved 43 patients with newly diagnosed hyperthyroidism and 45 healthy participants, Jing et alCitation48 reported that the patients had impaired LV diastolic function. Diastolic dysfunction can also occur in sub-clinical hyperthyroidism. Smit et al in the Netherlands showed in a randomized placebo-controlled study that reversible diastolic dysfunction can occur after long-term exogenous subclinical hyperthyroidism.Citation49 They were concerned that even isolated diastolic dysfunction may be associated with increased mortality.Citation49 Yue et al in the People’s Republic of China also documented that LV diastolic dysfunction can occur in patients who present with heart failure.Citation50 In that study, out of the 6% of patients who had heart failure, half of them had heart failure with preserved ejection fraction. They concluded that diastolic dysfunction may play an important role in the pathogenesis of heart failure in thyrotoxicosis.Citation50 We also documented diastolic dysfunction as being a cause of heart failure with preserved ejection fraction in this study ().
In effect, thyrotoxic heart failure may present with preserved, reduced, or increased ejection fraction (high-output heart failure).Citation32,Citation51 In our earlier study, we reported that 10% of the patients with thyrotoxicosis had systolic dysfunction with preserved ejection fraction.Citation52 Similar findings have been found in other studies.Citation53,Citation54 This is an indication that echocardiography can be used to classify cardiac dysfunction in patients with thyrotoxicosis, including the determination of the ejection fraction, and these data are indispensable in planning a therapeutic regimen.
Patients who have diastolic dysfunction can only be detected using echocardiography, and this invariably influences their management. Therapeutic management of patients with diastolic dysfunction may differ from those with systolic dysfunction. Patients with isolated diastolic dysfunction will respond to anti-thyroid drugs (to render them euthyroid), β-blockers, and diuretics in moderate doses.Citation50 Inotropes which are useful in patients with systolic dysfunction will not be required in patients with isolated diastolic dysfunction.Citation50
In thyrotoxicosis patients who have heart failure with preserved or increased ejection fraction, inotropes are of doubtful therapeutic effect.Citation51 Anti-hyperthyroid drugs, judicious use of diuretics, and some β-adrenoceptor blockers are more useful in managing this group of patients.Citation55 The use of vasodilators like angiotensin-converting inhibitors, angiotensin receptor blockers, and β-blockers with vasodilatory properties (eg, carvedilol, nebivolol) may aggravate the clinical condition in heart failure with increased ejection fraction because of associated decreased peripheral vascular resistance.Citation51
Heart failure with reduced ejection fraction may be found in patients with thyrotoxicosis in spite of the fact that it is a hypermetabolic condition. Some predisposing conditions include pre-existing hypertension, ischemic heart disease, and mitral valve disease, which may be found in Graves’ and Hashimoto’s diseases.Citation56,Citation57 The pathophysiology of thyrotoxic cardiac disease with reduced ejection fraction may include direct damage due to autoimmune myocarditis, congestive circulation secondary to excess sodium, and fluid retention related to hyperthyroidism, upregulation of the renin–angiotensin–aldosterone system, sustained tachycardia, and/or atrial fibrillation.Citation41 Some of the arrhythmias, particularly atrial fibrillation, predisposes to increased mortality in thyrotoxicosis patients and worsens prognosis when associated with heart failure.Citation58,Citation59 It should be emphasized that atrial fibrillation is associated with increased mortality in thyrotoxicosis and so it should be treated appropriately whenever it occurs.Citation60 β-adrenoceptor blockade, digoxin, calcium channel blockers, and anticoagulants are all useful in the management of atrial fibrillation associated with thyrotoxicosis.
Patients with systolic dysfunction with reduced ejection fraction and tachycardia will benefit from treatments aimed at slowing the heart rate or controlling the ventricular response in atrial fibrillation. This appears to improve LV function even before initiation of anti-thyroid therapy.Citation57 This class of patients with dilated, poorly contracting hearts will also benefit from inotropes, in conjunction with classic forms of treatment for congestive heart failure. However, larger than usual doses of inotropes such as digoxin may be required. A relative resistance to digoxin may be present, due both to increased renal clearance,Citation61 increased biliary excretion,Citation62 and the increased number of Na/K ATPase units in the cardiac muscle.Citation63
There may be no clinical trials in support of the use of β-adrenoceptor blockers in thyrotoxic heart failure, but they are effective in alleviating the attendant symptoms of hyperthyroidism and also in reducing heart rate. However, invasive monitoring in hyperthyroid patients with cardiac failureCitation64 has demonstrated depressed myocardial function in response to β-adrenoceptor blockade, demonstrated by decreased stroke volume and increased pulmonary artery diastolic pressure.
If thyrotoxic heart failure is truly congestive with associated fluid retention, β-adrenoceptor blockers, which are really negatively inotropic, should be stepped down until the patient is hemodynamically stable. This is in spite of the fact that β-adrenoceptor blockers are indicated in the current guidelines for management of heart failure. A practical guideline for the use of β-adrenoceptor blockers in heart failure in thyrotoxicosis may be to optimize the reduction of fluid retention before the introduction of β blockers that have been found to be useful in previous clinical trials on heart failure therapeutics, namely carvedilol (COPERNICUS),Citation65 metoprolol (MERIT-HF),Citation66 and bisoprolol (CIBIS II).Citation67 The ultra-short acting β-adrenoceptor blockers esmolol, propranolol, and atenolol have been used in cases of heart failure associated with thyrotoxicosis as a therapeutic trial, but this practice is not widely accepted because there may be worsening of heart function.Citation68–Citation71
Eccentric hypertrophy was documented in this work, but concentric hypertrophy has also been seen in some thyrotoxicosis patients.Citation72 LV hypertrophy has also been documented in patients receiving thyroxine in the absence of significant changes in heart rate and blood pressure, suggesting that there is a direct trophic effect of thyroid hormone on the myocardium.Citation72,Citation73 There have been reports from Asia and Germany, documenting the presence of LV hypertrophy in thyrotoxicosis patients.Citation74,Citation75 LV hypertrophy has been associated with cardiovascular events and should therefore be treated. Thyrotoxicosis patients who have LV hypertrophy should receive anti-thyroid drugs as well as beta-blockers and calcium blockers, which are known to reverse ventricular remodeling.Citation76
In summary, thyrotoxicosis is a treatable cause of heart failure and so diagnosis is important and rewarding.Citation77 Heart failure in people with thyrotoxicosis who have increased or preserved ejection fraction, almost always resolves after euthyroid state is established, but when there is associated reduced ejection fraction, complete resolution of the heart failure may be less predictable.Citation33,Citation78 summarizes the definitive treatment of thyrotoxic cardiac disease as well as the management of the associated hemodynamic changes and complications.
Table 8 Summary of drug treatment of thyrotoxic cardiac disease and management of associated hemodynamic changes and complications
Conclusion
Echocardiography is useful in the stratification of cardiac function abnormalities and is indispensable in the management of patients with thyrotoxicosis. The finding of LV-enhanced systolic and diastolic functions signify early echocardiographic detectable cardiac abnormalities in thyrotoxicosis, and the clinical management is with anti-thyroid drugs and β-adrenoceptor blockade. Diastolic dysfunction in thyrotoxicosis patients asymptomatic for cardiac disease should be treated with anti-thyroid drugs and β-adrenoceptor blockade.
Thyrocardiac patients with heart failure will require echocardiography to determine their ejection fraction, as this may influence a specific therapeutic regimen. A sound knowledge of clinical therapeutics and its application is indispensable in the use of anti-thyroid drugs, β-adrenoceptor blockade, diuretics, and digoxin, and in the management of thyrotoxicosis patients with heart failure who have increased, preserved, or reduced ejection fraction.
Disclosure
The authors have no conflicts of interest to disclose.
References
- GordonHWLeonardSLEllenWSThe heart in endocrine and nutritional disordersBraunwaldEHeart Disease5th edPhiladelphiaW B Saunders199718901894
- WeetmanAPGrave’s disease 1835–2002Horm Res200359Suppl 111411812638522
- KahalyGJDillmannWHThyroid hormone action in the heartEndocrine Rev200526570472815632316
- KissEJakabGKraniasEGEdesIThyroid hormone-induced alterations in phospholamban protein expression: regulatory effects on sarcoplasmic reticulum Ca2+ transport and myocardial relaxationCirc Res19947522452518033338
- KleinIOjamaaKThyroid hormone and the cardiovascular systemN Engl J Med2001344750150911172193
- DillmannWHCellular action of thyroid hormone on the heartThyroid200212644745212165105
- DanziSKleinIThyroid hormone and the cardiovascular systemMinerva Endocrinol200429313915015282446
- LadensonPWShermanSIBaughmanKLRayPEFeldmanAMReversible alterations in myocardial gene expression in a young man with dilated cardiomyopathy and hypothyroidismProc Natl Acad Sci U S A19928912525152551376915
- LewickiJAProtterAAPhysiological studies of the natriuretic peptide familyLaraghJHBrennerBMHypertension: Pathophysiology, Diagnosis and ManagementNew YorkRaven Press199510291053
- ShiWWymoreRYuHDistribution and prevalence of hyperpolarization-activated cation channel (HCN) mRNA expression in cardiac tissuesCirc Res1999851e1e610400919
- PachuckiJBurmeisterLALarsenPRThyroid hormone regulates hyperpolarization-activated cyclic nucleotide-gated channel (HCN2) mRNA in the rat heartCirc Res199985649850310488052
- HoitBDKhourySFShaoYGabelMLiggetSBWalshRAEffects of thyroid hormone on cardiac b-adrenergic responsiveness in conscious baboonsCirculation19979625925989244231
- LeveyGSCatecholamine sensitivity, thyroid hormone and the heartAm J Med197150413420
- KleinIEndocrine disorders and cardiovascular diseaseZipesDPLibbyPBonowRBraunwaldEBraunwald’s Heart Disease: A Textbook of Cardiovascular Medicine7th edPhiladelphiaW.B. Saunders200520512065
- VentrellaSMKleinIBeta-adrenergic receptor blocking drugs in the management of hyperthyroidismThe Endocrinologist199445391399
- LeveyGSKleinICatecholamine-thyroid hormone interactions and the cardiovascular manifestations of hyperthyroidismAm J Med19908866426462189309
- CoulombePDussaultJHWalkerPPlasma catecholamine concentration in hyperthyroidism and hypothyroidismMetabolism1976259973978958003
- BaylissRIEdwardsOMUrinary excretion of free catecholamines in Graves’ diseaseJ Endocrinol19714911671735108819
- DavisPJDavisFBNongenomic actions of thyroid hormone on the heartThyroid200212645946612165107
- HiroiYKimHHYingHRapid nongenomic actions of thyroid hormoneProc Natl Acad Sci U S A200610338141041410916966610
- ParkKWDaiHBOjamaaKLowensteinEKleinISellkeFWDirect vasomotor effect of thyroid hormones on rat skeletal muscle resistance arteriesAnesth Analg19978547347389322448
- NapoliRBiondiBGuardasoleVImpact of hyperthyroidism and its correction on vascular reactivity in humansCirculation2001104253076308011748103
- WoeberKAThyrotoxicosis and the heartN Engl J Med199232794981603141
- ArmigliatoMPaoliniRAggioSHyperthyroidism as a cause of pulmonary arterial hypertension: a prospective studyAngiology200657560060617067983
- IchikiTThyroid hormone and atherosclerosisVascul Pharmacol2010523–415115619808101
- World Health Organization [homepage on the Internet]World Medical Association Declaration of Helsinki. Ethical Principles for Medical Research Involving Human SubjectsBulletin of the World Health Organization2001 Available from: www.who.int/bulletin/archives/79(4)373.pdfAccessed October 15, 2014
- IlercilAO’GradyMJRomanMJReference values for echocardiographic measurements in urban and rural populations of differing ethnicity: the Strong Heart StudyJ Am Soc Echocardiogr200114660161111391289
- Syntron Bioresearch IncLaboratory Instruction ManuscriptCarlsbad, CA, USA1998 Catalog numbers10062210
- FeigenbaumHEchocardiography5th edPhiladelphiaLippincott Williams and Wilkins1994
- SchillerNBShahPMCrawfordMRecommendations for quantification of the left ventricle by two-dimensional echocardiographyJ Am Soc Echocardiogr198925362364
- FazioSPalmieriEALombardiGBiondiBEffects of Thyroid Hormone on the Cardiovascular SystemRecent Prog Horm Res200459315014749496
- MehtaPADubreySWHigh output heart failureQJM2009102423524118990720
- SangsterJKPancieraDLAbbottJACardiovascular Effects of Thyroid DiseaseCompend Contin Educ Vet2013357E523677842
- ShapiroSMBersotinMMLaksMMIn search of the Holy Grail: the study of diastolic ventricular functions by use of Doppler echocardiographyJ Am Coll Cardiol1991177151715192033183
- BuccinoRASpannJFJrPoolPESonnenblickEHBraunwaldEInfluence of the thyroid state on the intrinsic contractile properties and the energy stores of the myocardiumJ Clin Invest19674610166916826061742
- FeldmanTBorowKMSarneDHNeumannALangRMMyocardial mechanics in hyperthyroidism: importance of left ventricular loading conditions, heart rate and contractile stateJ Am Coll Cardiol1986759679743958379
- MintzGPizzarelloRKleinIEnhanced left ventricular diastolic function in hyperthyroidism: noninvasive assessment and response to treatmentJ Clin Endocrinol Metab19917311461502045465
- FriedmanMJOkadaRDEwyGAHellmanDJLeft ventricular systolic and diastolic function in hyperthyroidismAm Heart J19821046130313087148648
- KralJHradecJLimanovaJHeart in thyroid diseasesCor Vasa19923421081141304451
- MarciszCKucharzEJJonderkoGWojewódkaJThe Systolic function of the Left Ventricle of heart in patients with hyperthyroidism during therapyPol Arch Med Wewn2001105213113811505747
- BondBRFoxPRPetersonMESkavarilRVEchocardiographic findings in 103 cats with hyperthyroidismJ Am Vet Med Assoc198819211154615492970449
- MerillonJPPassaPHChastreJWolfAGourgonRLeft ventricular function and hyperthyroidismBr Heart J19814621371437272124
- EberOBuchingerWLindnerWThe effect of D-versus L-propranolol in the treatment of hyperthyroidismClin Endocrinol (Oxf)19003233633722344697
- GeffnerDLHershmanJMβ-adrenoceptor blockade in treatment of hyperthyroidismAm J Med199293161681352658
- AurigemmaGPGottdienerJSShemanskiLGardinJKitzmanDPredictive valve of systolic and diastolic function for incident congestive heart failure in the elderly: the cardiovascular health studyAm J Coll Cardiol200137410421048
- RedfieldMMJacobsenSJBurnettJCJrMahoneyDWBaileyKRRodehefferRJBurden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemicJAMA2003289219420212517230
- GaaschWHDiagnosis and treatment of heart failure based on left ventricular systolic or diastolic dysfunctionJAMA199427116127612808151903
- JingXCLiuYHuangHLeft ventricular diastolic function of patients with newly diagnosed hyperthyroidismSichuan Da Xue Xue Bao Yi Xue Ban2012433462466 Chinese22812259
- SmitJWEustatia-RuttenCFCorssmitEPReversible Diastolic Dysfunction after Long-term exogenous subclinical hyperthyroidism: a randomized placebo-controlled studyJ Clin Endocrinolol Metab2005901160416047
- YueWS1ChongBHZhangXHHyperthyroidism-induced left ventricular diastolic dysfunction: implication in hyperthyroidism-related heart failureClin Endocrinol (Oxf)201174563664321470287
- AnandISFloreaVGHigh output cardiac failureCurr Treat Options Cardiovasc Med20013215115911242561
- AnakwueRCOnwubereBJAnisiubaBCIkehVOMbahAIkeSOCongestive heart failure in subjects with thyrotoxicosis in a black communityVasc Health Risk Manag2010647347720730063
- KolawoleBABalogunMOThyrotoxicosis and heart-a review of the literatureNiger J Med2001102505411705057
- DanbauchiSSAnumahFEAlhassanMAThyrocardiac Disease in Zaria: Clinical and Echocardiographic CharacteristicsEchocardiography Journal2004215
- ChoudhuryRPMacDermotJHeart failure in thyrotoxicosis, an approach to managementBr J Clin Pharmacol19984654214249833593
- IkramHThe nature and prognosis of thyrotoxic heart diseaseQ J Med19855421319283975344
- LeveyGSKleinICatecholamine-thyroid hormone interactions and the cardiovascular manifestations of hyperthyroidismAm J Med19908866426462189309
- SiuCWYeungCYLauCPKungAWTseHFIncidence, clinical characteristics and outcome of congestive heart failure as the initial presentation in patients with primary hyperthyroidismHeart200793448348717005710
- NiakaraABamaANebieLVSigns and outcome of 61 cases of thyrotoxic heart diseaseTrop Cardiol2004301182427
- OsmanFDaykinJSheppardMCGammageMDFranklnJAAtrial fibrillation predicts mortality in thyrotoxicosis. British Endocrinology Society meeting 2002Bioscientifica2002275278
- HuffmanDHKlaassenCDHartmanCRDigoxin in hyperthyroidismClin Pharmacol Ther1977225 Pt 1533538913019
- ShenfieldGMThompsonJHornDBPlasma and urinary digoxin in thyroid dysfunctionEur J Clin Pharmacol1977126437443598418
- ChaudhurySIsmail-BeigiFGickGGLevensonREdelmanISEffect of thyroid hormone on the abundance of Na,K adenosine triphosphate-subunit messenger ribonucleic acidMol Endocrinol19871183892842662
- IkramHHaemodynamic effects of beta adrenergic blockade in hyperthyroid patients with and without heart failureBr Med J19771607515051507871633
- PackerMCoatsAJFowlerMBEffect of Carvedilol on Survival in Severe Chronic Heart FailureN Engl J Med2001344221651165811386263
- No authors listedEffects of Metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL randomised Intervention Trial in Congestive Heart Failure (MERIT-HF)Lancet199935391692001200710376614
- No authors listedThe Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trialLancet1999353914691310023943
- IsleyWLDahlSGibbsHUse of esmolol in managing a thyrotoxic patient needing emergency surgeryAm J Med19908911221231973332
- RubenfeldSSilvermanVEWelchKMMalletteLEKohlerPOVariable plasma propranolol levels in thyrotoxicosisN Engl J Med19793007353354759897
- RoutledgePAShandDGClinical pharmacokinetics of propranololClin Pharmacokinet1979427390378502
- GeffnerDLSladekJHershmanJMPharmacokinetics and clinical effects of atenolol therapy of hyperthyroidismDrugs Exp Clin Res19901641671732076652
- BiondiBPalmieriEALombardiGFazioSEffects of thyroid hormone on cardiac function: the relative importance of heart rate, loading conditions, and myocardial contractility in the regulation of cardiac performance in human hyperthyroidismJ Clin Endocrinol Metab200287396897411889145
- DonatelliMAssennatoPAbbadiVCardiac changes in subclinical and overt hyperthyroid women: retrospective studyInt J Cardiol2003902–315916412957747
- ChingGWFranklynJAStallardTJCardiac hypertrophy as a result of long term thyroxine therapy and thyrotoxicosisHeart19967543633688705762
- DorrMWolffBRobinsonDMThe association of thyroid function with cardiac mass and left ventricular hypertrophyJ Clin Endocrinol Metab200590267367715522926
- KleinILeveyGSThe cardiovascular system in thyrotoxicosisBravermanLEUtigerRDWerner and Ingbar’s The Thyroid: a Fundamental and clinical Text8th edPhiladelphiaLippincott Williams and Wilkins2000596604
- UmpierrezGEChallapalliSPattersonCCongestive heart failure due to reversible cardiomyopathy in patients with hyperthyroidismAm J Med Sci19953103991027668312
- RiazKForkerADIsleyWLHamburgMSMcCulloughPAHyperthyroidism: a curable cause of congestive heart failure–three case reports and a review of literatureCongest Heart Fail200391404612556677