Abstract
Many recent innovations have been made in developing new antiplatelet and anticoagulant drugs in the last few years, with a total of nine new antithrombotic drugs approved by the Food and Drug Administration after the year 2000. This has revolutionized the medical therapy given to manage acute coronary syndrome and support cardiac catheterization. The concept of dual antiplatelet therapy has been emphasized, and clopidogrel has emerged as the most-popular second antiplatelet drug after aspirin. Newer P2Y12 inhibitors like prasugrel and ticagrelor have been extensively studied and compared to clopidogrel. The role of glycoprotein (Gp) IIb/IIIa inhibitors is being redefined. Other alternatives to unfractionated heparin have become available, of which enoxaparin and bivalirudin have been studied the most. Apart from these, many more drugs with novel therapeutic targets are being studied and are currently under development. In this review, current evidence on these drugs is presented and analyzed in a way that would facilitate decision making for the clinician. For this analysis, various high-impact clinical trials, pharmacological studies, meta-analyses, and reviews were accessed through the MEDLINE database. Adopting a unique interdisciplinary approach, an attempt has been made to integrate pharmacological and clinical evidence to better understand and appreciate the pros and cons of each of these classes of drugs.
Introduction
The pharmacology of hemostasis and thrombosis has been rapidly evolving, with six new drugs approved by the Food and Drug Administration (FDA) after 2009. The evolution of antithrombotic drugs has been gradual in the 20th century, with aspirin, unfractionated heparin (UFH), and warfarin being the only ones available for the most part of the century. The last decade of the century saw some brisk developments with the introduction of thienopyridines (ticlopidine in 1991 and clopidogrel in 1997) and low-molecular-weight heparins (LMWHs) (enoxaparin in 1993 and dalteparin in 1994). Toward the end of the decade, the glycoprotein (Gp) IIb/IIIa inhibitors were introduced. The dawn of the 21st century marked an explosion of new discoveries with drugs like synthetic pentasaccharides (fondaparinux), direct thrombin inhibitors, and direct Xa inhibitors.Citation1 These drugs are listed in .
Table 1 FDA-approved antiplatelet and anticoagulant drugs listed in order of their year of approval
With this wide selection of therapeutic options at our disposal, it is only natural to expect that a lot of research has been done in defining and contrasting the safety and efficacy profiles of these drugs. This discussion focuses on analyzing the various therapeutic options currently available to support percutaneous coronary intervention (PCI) and for managing acute coronary syndrome (ACS). The latest American College of Cardiology Foundation/American Heart Association (ACC/AHA) guidelines have been referred to for the purpose of the discussion (unless otherwise specified).Citation2
Methods
The MEDLINE database was primarily explored via PubMed to search and access clinical trials, studies, meta-analyses, and reviews relevant to our discussion. The registry Clinical-Trials.gov was also referred to while analyzing the various clinical trials.
Since the discussion is broad-based, no uniform inclusion/exclusion criteria were defined in selecting the studies to be included in this review. An attempt has been made to select studies with the highest impact in terms of their reputation as well as their general influence on the trends in interventional pharmacology. A total of 30 clinical trials have been discussed and/or mentioned in this review. No original meta-analysis has been performed in this review. Any caveat, potential bias, or limitation, if present, has been mentioned along with the discussion of the individual studies.
Overview of the pharmacology of currently available drugs
Antiplatelets and anticoagulants affect the two main limbs of hemostasis: platelet reactivity and the coagulation cascade. Platelets and clotting factors form an interdependent, intricately interlinked, and almost sequential effector mechanism of hemostasis. Platelet adhesion, activation, and aggregation are the seminal steps of hemostasis. Although the coagulation cascade develops on a scaffolding of the platelet plug, platelet activation and aggregation are strongly facilitated by clotting factors: most prominently thrombin. Thrombin is a protein with multiple physiological roles involving various systems. This exemplifies the fact that multiple physiological pathways including the coagulation cascade, fibrinolytic pathway, kinin pathway, and the complement pathway are all interlinked.
Arterial and venous thrombi have traditionally been considered to be unique in terms of pathophysiology and clot content. Arterial thrombi are formed secondary to some form of endothelial dysfunction (eg, atherosclerotic plaque) in an environment where the shear stress is high. Such an environment leads to the formation of a platelet-rich clot with minimal activation of the coagulation cascade: sometimes referred to as the white thrombus. On the other hand, venous clots form in undamaged veins where the blood flow and shear stress are low. This allows adequate activation of the coagulation cascade that causes entrapment of red blood cells in the mesh, thereby forming a fibrin and red-cell rich red thrombus.Citation3
Based on this background, it is easy to comprehend why antiplatelet therapy is predominantly used to prevent arterial thrombosis (eg, ACS) and not venous thrombosis (eg, deep venous thrombosis). However, these distinctions are not at all absolute, and experimental evidence supports the use of adjunctive antiplatelet therapy for the management of venous thrombosis, thereby reinforcing the importance of undeniable crosstalk between the two systems.Citation4
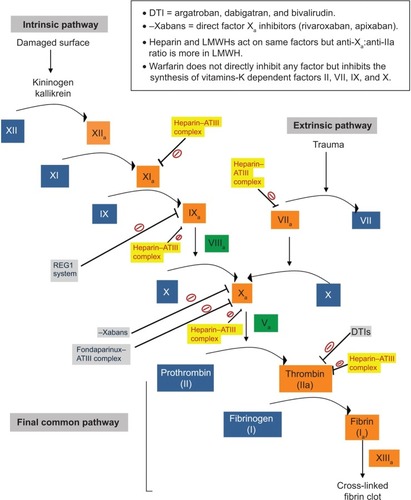
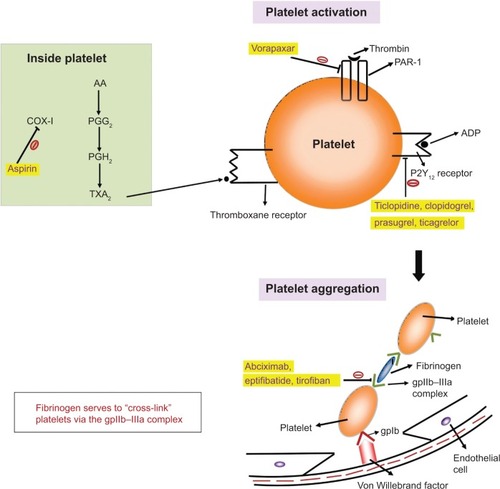
Each of these classes of drugs exploits a unique step in the normal hemostatic processes, which is illustrated in and . Other special characteristics of the pharmacology of individual drugs are discussed in the respective sections.
Figure 1 An overview of the mechanism of action of antiplatelet drugs, showing their effects on various steps of platelet activation and aggregation.
Current literature on the new drugs
Extensive research has been done to test the efficacy and safety profiles of the newer drugs and compare them with the current standard of treatment. The current understanding of the various characteristics of the available drugs and some of the pressing issues in interventional pharmacology is being discussed.
Although the role of aspirin in ACS is paramount and a class I indication as per the current guidelines,Citation2 the choice of second antiplatelet drug after aspirin has been evolving. P2Y12 inhibitors are a diverse class of drugs comprising thienopyridines (clopidogrel and prasugrel) and ticagrelor. A P2Y12 inhibitor is given along with aspirin for the acute management of ACS as well as for maintenance therapy post-PCI, to provide a more complete antiplatelet effect.
Clopidogrel: optimum loading dose
Various trials have shown the benefit of adding clopidogrel to aspirin in ACS patients, and it has emerged as the most common choice for the second antiplatelet agent after aspirin.Citation5,Citation6 Many studies have been done to determine the optimum loading dose of clopidogrel for patients undergoing PCI. The most prominent among these was the CURRENT/OASIS7 trial enrolling ACS patients planned to undergo PCI.Citation7 Incidence of primary outcomes (cardiovascular death, myocardial infarction [MI], or stroke at 30 days) was similar in both double-dose (600 mg) and standard-dose groups (300 mg) (4.2% versus 4.4%; hazard ratio [HR] =0.94; 95% confidence interval [CI]: 0.83–1.06; P=0.30). In the subset of patients undergoing PCI, the incidence of primary events was lower with double-dose clopidogrel (3.9% versus 4.5%; HR=0.85; 95% CI: 0.74–0.99; P=0.39). Post hoc analysis revealed a reduction in definite stent thrombosis (ST) in the double-dose group (1.6% versus 2.3%; HR=0.68; 95% CI: 0.55–0.85; P<0.001). Incidence of major bleeding was slightly higher in the double-dose group (2.5% versus 2.0%; HR=1.24; 95% CI: 1.05–1.46; P=0.01). Overall, the results were not entirely conclusive.
However, there have been other trials and meta-analyses done to address this issue, and double-dose regimen has invariably been proven to be superior.Citation8,Citation9 Siller-Matula et al performed a meta-analysis studying the efficacy of the two clopidogrel doses in patients undergoing PCI, at 1 month after start of therapy.Citation10 It showed a 34% relative risk (RR) reduction of major adverse cardiac events (MACE) in the double-dose group (RR=0.66; 95% CI: 0.52–0.84; P<0.001) with no increase in risk of major bleeding in the group (RR=0.91; 95% CI: 0.73–1.15; P=0.44).
von Beckerath et al in their pharmacological study enrolling 60 patients studied three different doses of clopidogrel: 300 mg, 600 mg, and 900 mg.Citation11 Compared to 300 mg, the 600 mg dose was shown to achieve a higher plasma concentration (P≤0.03) and an enhanced inhibition of adenosine diphosphate (ADP)-induced platelet aggregation 4 hours after drug administration (P=0.01 and P=0.004 for 5 and 20 μmol/L ADP, respectively). However, intestinal absorption was proposed to act as a bottleneck in doses higher than 600 mg. The 900 mg dosing failed to increase plasma concentration (P≥0.38) or inhibit ADP-induced platelet aggregation 4 hours after drug administration (P=0.59 and P=0.39 for 5 and 20 μmol/L ADP, respectively).
In a later study with a larger sample size (N=103) called the ALBION trial, Montalescot et al studied the pharmacology of different clopidogrel doses up to 24 hours after administration.Citation12 They noted a uniform increment in the efficacy of higher doses (600 mg and 900 mg) of clopidogrel compared to the standard dose (300 mg). Thus, when compared to 600 mg, the 900 mg dose was found to have a superior pharmacology. Higher doses had a faster onset as well as a greater maximal inhibition of platelet activity. The rates of hypo-responders were also decreased proportionately with higher doses (P=0.20 and P=0.007 for 5 and 20 μmol/L ADP, respectively).
Clopidogrel versus newer P2Y12 inhibitors
Two new P2Y12 inhibitors have been approved by the FDA recently: prasugrel in 2009 and ticagrelor in 2011. The newer drugs offer definite benefits over clopidogrel.
Pitfalls of clopidogrel
When compared to the newer P2Y12 inhibitors, the antiplatelet action of clopidogrel has been described to be slow, modest, and variable. Pharmacological studies comparing these drugs have shown a delayed onset of activity and higher on-treatment platelet reactivity with clopidogrel. Another issue with clopidogrel is that of resistance and interpatient variability. Serebruany et al did an ex vivo platelet-function study recruiting both healthy volunteers and post-PCI, heart failure, and stroke patients.Citation13 They demonstrated that the variability of response to clopidogrel follows a normal distribution, with the percentage of hypo-respondents and hyper-respondents (±2 standard deviations) in their study being 4.2% and 4.8%, respectively.
Various mechanisms have been proposed to explain clopidogrel resistance such as drug interactions, P2Y12 receptor variability, etc, but the one which has been studied the most is unfavorable metabolism. Both clopidogrel and prasugrel are prodrugs that have to be converted to their active metabolites. Hagihara et al in their study showed that 90% of clopidogrel was converted into an inactive acid metabolite.Citation14 Interpatient variability also exists in the activity of microsomal enzymes responsible for the formation of its active metabolite, with CYP2C19 polymorphisms being studied the most.Citation15 This explains the moderate efficiency and high variability of response to clopidogrel when compared to other drugs.
Clopidogrel versus prasugrel
The most prominent trial comparing these two drugs was the TRITON–TIMI 38 enrolling ACS patients undergoing PCI.Citation16 The prasugrel group had a significantly reduced incidence of primary outcome (cardiovascular death, nonfatal MI, or nonfatal stroke): 4.7% versus 5.6% in clopidogrel group (HR=0.82; 95% CI: 0.73–0.93; P<0.002).
The increased efficacy of prasugrel was at the expense of increased risk of bleeding. The incidence of non-coronary artery bypass grafting (CABG)-related TIMI (Thrombolysis In Myocardial Infarction) major bleed was 2.4% with prasugrel versus 1.8% with clopidogrel (HR=1.32; 95% CI: 1.03–1.68; P=0.03). The prasugrel group had an increased incidence of both “life threatening” (1.4% versus 0.9%; HR=1.52; 95% CI: 1.08–2.13; P=0.01) and “fatal” bleeding (0.4% versus 0.1%; HR=4.19; 95% CI: 1.58–11.11; P=0.002). Even though few patients underwent CABG, the incidence of CABG-related bleeding was also found to be higher in the prasugrel group: (13.4% versus 3.2%; HR=4.73; 95% CI: 1.90–11.82; P<0.001). Post hoc analysis revealed three high-risk groups: patients with a history of stroke/transient ischemic attack had a net harm from prasugrel, while elderly (>75 years) and patients with body weight less than 60 kg had no net clinical benefit.
Many studies have shown the pharmacological superiority of prasugrel over clopidogrel. Brandt et al measured inhibition of platelet aggregation after administering prasugrel in healthy subjects, compared it to that of clopidogrel up to 24 hours, and found it to be significantly higher (P<0.1).Citation17 The peak inhibition of platelet aggregation for prasugrel was also higher (P<0.001). The antiplatelet action of prasugrel was shown to be more rapid; time taken to reach ≥20% inhibition of platelet aggregation was 30 minutes for prasugrel and 1.5 hours for clopidogrel. This factor may have been responsible for a reduction in the rate of “early” MI (before day 3) in the TRITON–TIMI 38 trial (4.7% with prasugrel versus 5.6% with clopidogrel; P=0.01). The anti-platelet activity of prasugrel is also more consistent. Jernberg et al studying aspirin-treated patients with coronary artery disease documented no nonresponders with prasugrel as compared to 45% in the clopidogrel group (P=0.0007).Citation18
Clopidogrel versus ticagrelor
Ticagrelor is the latest addition to the group of P2Y12 inhibitors. It is chemically unique and is the first member of a new class of drugs known as cyclopentyl-triazolo-pyrimidines. The major trial designed to analyze the safety and efficacy of ticagrelor and compare it with clopidogrel was the PLATO trial enrolling 18,624 ACS patients in 43 countries.Citation19 The incidence of primary endpoint (death from vascular causes, MI, or stroke) at 12 months was significantly lower in the ticagrelor group (9.8% versus 11.7%; HR=0.84; 95% CI; 0.77–0.92; P<0.001). On further statistical analysis, these benefits were found to be both short-term (0–30 days) and long-term (31–360 days), and in both ST segment elevation MI (STEMI) and unstable angina/non-STEMI (NSTEMI) patients; this was irrespective of whether PCI was done or not.
There was no statistically significant increase in the incidence of total major bleeding in the ticagrelor group by both study criteria (11.6% versus 11.2%; HR=1.04; 95% CI: 0.95–1.13; P=0.43) and TIMI criteria (7.9% versus 7.7%; HR=1.03; 95% CI: 0.93–1.15; P=0.57). On a subset analysis, no increase in CABG-related major bleeding was found in the ticagrelor group by either criterion. However, a statistically significant increase in the incidence of non-CABG-related major bleeding was found in the ticagrelor group (4.5% versus 3.8%; HR=1.19; 95% CI: 1.02–1.38; P=0.03 by study criteria; and 2.8% versus 2.2%; HR=1.25; 95% CI: 1.03–1.53; P=0.03 by TIMI criteria).
Treatment with ticagrelor, like aspirin, was associated with a statistically significant reduction in all-cause mortality (4.5% versus 5.9%; HR=0.78, 95% CI: 0.69–0.89; P<0.001). This observation can be attributed to better efficacy, backed up by a superior pharmacological profile, without a concomitant increase in the rate of overall major bleeding. This definite survival benefit with ticagrelor is in stark contrast to the absence of such benefit with the other antiplatelet drugs like prasugrel and Gp IIb/IIIa inhibitors.
The encouraging results in clinical trials have been backed up by favorable pharmacological data. As opposed to thienopyridines, ticagrelor is a reversible P2Y12 inhibitor: a factor that may have contributed to its better safety profile. Gurbel et al studied the pharmacology of ticagrelor in their two studies.Citation20,Citation21 The ONSET/OFFSET study enrolled patients with stable coronary artery disease on aspirin therapy, and compared the pharmacology of ticagrelor with that of clopi-dogrel.Citation20 The antiplatelet action of ticagrelor was found to be rapid (41% inhibition of platelet aggregation at 30 minutes versus 8% with clopidogrel; P<0.0001) and more potent (90% of patients had >70% inhibition of platelet aggregation at 2 hours versus 16% with clopidogrel; P<0.0001). Despite the greater antiplatelet effect, inhibition of platelet aggregation at 24 hours after the last dose was equal in both ticagrelor- and clopidogrel-treated patients, thereby implying faster offset of action. The RESPOND study, recruiting a similar patient population, compared the antiplatelet action of ticagrelor in clopidogrel responders and nonresponders.Citation21 The antiplatelet effect of ticagrelor was found to be same in both these groups. Inhibition of platelet aggregation in nonresponders treated with ticagrelor was higher than those treated with clopidogrel, at all times (P<0.05).
Armstrong et al explored another aspect of ticagrelor pharmacology in which it was shown to inhibit cellular uptake of adenosine via equilibrative nucleoside transporter 1.Citation22 This potentially increases the local concentration of adenosine, which is a known inhibitor of platelet aggregation. This multipronged effect of ticagrelor on adenosine and ADP pharmacology may have contributed to the superior efficacy observed in clinical trials.
Prasugrel versus ticagrelor
As discussed previously, both prasugrel and ticagrelor have been found to have superior clinical efficacy as compared to clopidogrel, with a significantly increased risk of bleeding events with prasugrel. However, till date, no head-to-head comparison has been made between these two newer P2Y12 inhibitors. A multicenter, randomized trial, ISAR REACT 5, is underway to compare prasugrel and ticagrelor in ACS patients with a planned invasive strategy and can help define the best option for P2Y12 inhibition.Citation23
In a pharmacodynamic study by Alexopoulos et al enrolling clopidogrel hypo-responders 24 hours post-PCI, ticagre-lor was found to be superior to prasugrel.Citation24 The primary endpoint of platelet reactivity was found to be significantly lower with ticagrelor (32.9 platelet reactivity units; 95% CI: 18.7–47.2) as compared to prasugrel (101.3 platelet reactivity units; 95% CI: 86.8–115.7).
Timing of administering the second antiplatelet
As mentioned before, dual antiplatelet therapy with aspirin and a P2Y12 inhibitor has become the standard of care, with the 2013 ACC/AHA guidelines for STEMI recommending the use of a P2Y12 inhibitor (clopidogrel, prasugrel, or ticagrelor) as a class I indication.Citation25 However, no explicit recommendations have been made with respect to the timing of the second antiplatelet drug after aspirin. Administration of a loading dose of a P2Y12 inhibitor both before and during primary PCI has been approved. Administration of early dual antiplatelet therapy (which is commonly practiced) has a logistic drawback in patients where the coronary anatomy upon catheterization is found to be non-amenable to PCI, thereby requiring emergency CABG. Various studies have underlined the increased risk of postoperative bleeding with preoperative clopidogrel use.Citation26,Citation27 The previous guidelines recommended a minimum time period of 5 days after stopping the second antiplatelet drug (a P2Y12 inhibitor) before considering emergency CABG.Citation28 The current guidelines have revised the minimum recommended time period to 24 hours.Citation25
Kapetanakis et al proposed withholding the administration of clopidogrel until an angiogram is performed (thereby revealing the coronary anatomy) and the need for CABG is assessed.Citation29 However, it has been argued that this strategy will deny optimum antiplatelet therapy in the majority of the patients who do not require surgery, especially in view of the slower onset of antiplatelet action of clopidogrel. In the CIPAMI trial, early clopidogrel administration in STEMI patients was found to have a lower incidence of composite endpoint (death, reinfarction, urgent target-vessel revascu-larization) as compared to clopidogrel administration after angiogram (3% versus 7%; P=0.09).Citation30 Another proposed strategy is to withhold the dual antiplatelet therapy before angiography, only in patients where the likelihood of CABG is high. A risk score to predict the need for CABG was designed by Sadanandan et alCitation31 although its clinical applicability has been questioned.Citation32
As the newer P2Y12 inhibitors prasugrel and ticagrelor have a superior pharmacological profile compared to clopidogrel, this property can be exploited to devise novel therapeutic strategies. Marchini et al presented an algorithm in which dual antiplatelet therapy was withheld in patients requiring emergent cardiac catheterization if the anticipated delay from presentation to catheterization was less than 6 hours.Citation33 Prasugrel was administered only after confirming the coronary anatomy during angiography and assessing the need for CABG. The underlying rationale is that, because of the faster and more-potent antiplatelet action of prasugrel, this strategy will achieve an enhanced platelet inhibition within a similar or potentially faster timeframe compared to preprocedural clopidogrel therapy. Ticagrelor can potentially fit in this treatment strategy as well.
Gp IIb/IIIa inhibitors
Most of the earlier studies done on Gp IIb/IIIa inhibitors were in the pre-dual antiplatelet-therapy era and demonstrated a clinical benefit at a somewhat increased risk of bleeding.Citation34,Citation35 The FINESSE (Facilitated Intervention With Enhanced Reperfusion Speed to Stop Events) study enrolled STEMI patients undergoing PCI and randomized them to receive abciximab plus reteplase (combination facilitated PCI), abciximab (facilitated PCI), or placebo (primary PCI) before the procedure.Citation36 There was no statistically significant clinical benefit with either facilitated or combination facilitated PCI, and each of these was associated with an increased risk of bleeding.
The results from the limited studies in which clopidogrel pretreatment was given showed questionable benefit and an increased bleeding risk.Citation37 Arguments have thus been made to restrict the use of Gp IIb/IIIa inhibitors as a bailout strategy in face of intraprocedural complications during PCI (eg, distal embolization, no reflow phenomenon, etc). The same has been adopted in the European Society of Cardiology (ESC) guidelines.Citation38
Of special interest is the meta-analysis performed by De Luca et al evaluating STEMI patients undergoing primary PCI.Citation37 The analysis showed no mortality benefit after 30 days (2.8% versus 2.9%; P=0.75) and an increased risk of major bleeding (4.1% versus 2.7%; P=0.0004) with Gp IIb/IIIa inhibitors. However, a significant relationship between patient risk profile and mortality benefit from Gp IIb/IIIa inhibitors was observed (P=0.008), with higher-risk patients benefitting the most.
This notion is in line with the findings of the ISAR-REACT 2 trial in which clinical benefits of abciximab were observed in high-risk NSTEMI patients with elevated troponin levels.Citation39 In these patients, the primary endpoint (death, MI, or urgent target-vessel revascularization within 30 days) in the abciximab group was 13.1% compared with 18.3% in the placebo group (RR=0.71; 95% CI: 0.54–0.95; P=0.02). This benefit was not observed in patients without an elevated troponin level. This drives the rationale of restricting routine Gp IIb/IIIa inhibitor use to high-risk patients (eg, large anterior wall MI and/or large clot burden).
While some studies have shown benefit of intracoronary administration of abciximab,Citation40,Citation41 others have shown no benefit,Citation42 and intracoronary Gp IIb/IIIa-inhibitor therapy remains a class IIb indication.Citation43 A meta-analysis comparing the three Gp IIb/IIIa inhibitors in patients undergoing PCI for STEMI showed no difference in their efficacy and risk profiles.Citation44 Although all three approved Gp IIb/IIIa inhibitors have been shown to have comparable benefits, abciximab has been studied the most and is hence commonly used.
Protease-activated receptor 1 inhibitor: vorapaxar
The most recent addition to antiplatelet drugs is vorapaxar, approved in May 2014. Protease-activated receptor 1 inhibition prevents thrombin-mediated platelet activation. It has been proposed that adding a platelet-activated receptor 1 inhibitor to the current standard of dual antiplatelet therapy may provide a more comprehensive platelet inhibition. This approval was based on the findings of the TRA 2°P-TIMI 50 trial, which enrolled patients with a history of ACS on maintenance antiplatelet therapy.Citation45 As compared to dual antiplatelet therapy, maintenance therapy with vorapaxar resulted in some reduction in primary outcomes of cardiovascular death, MI, or stroke (8.1% versus 9.7%; HR=0.80; 95% CI: 0.72–0.89; P<0.0001) at the expense of an increased risk of bleeding (3.4% versus 2.1%; HR=1.61; 95% CI: 1.31–1.97; P<0.0001).
LMWHs versus UFH
LMWHs boast certain practical advantages over UFH. Because of its favorable pharmacology, weight-adjusted enoxaparin has been shown to provide more stable and reliable anticoagulation without the need of laboratory monitoring.Citation46 Enoxaparin, the most widely used LMWH, has been compared to heparin in various studies. The most prominent amongst these was the SYNERGY trial, which enrolled high-risk NSTEMI patients.Citation47 The incidence of primary composite endpoint of all-cause death or nonfatal MI during the first 30 days was comparable in enoxaparin and heparin groups (14.0% versus 14.5%; odds ratio =0.96; 95% CI: 0.86–1.06; P=0.40). Also, no differences in ischemic events during PCI were observed between the two groups. A statistically significant increase in TIMI major bleeding was observed with enoxaparin (9.1% versus 7.6%; P=0.008), but GUSTO (Global Utilization of Streptokinase and t-PA for Occluded Arteries) severe bleeding rates were comparable in the two groups (2.7% versus 2.2%; P=0.08).
Cohen et al in their study showed that prerandomization anticoagulant therapy or switching from one anticoagulant to another at the time of randomization could have potentially influenced the endpoint analysis of the SYNERGY trial.Citation48 In the subgroup analysis, it was found that patients receiving “consistent enoxaparin therapy” experienced fewer deaths or MI when compared to “consistent heparin therapy” (13.3% versus 15.9%; HR=0.82; 95% CI: 0.72–0.94; P=0.004; adjusted P=0.041). However, an increased incidence of GUSTO severe bleeding was observed with consistent enoxaparin therapy (2.9% versus 2.1%; P=0.0465).
The more recently concluded ATOLL trial enrolling STEMI patients undergoing primary PCI showed encouraging results.Citation49 Intravenous (IV) enoxaparin was found to reduce the primary endpoint (30-day incidence of death, complication of MI, procedure failure, or major bleeding) when compared to UFH (28% versus 34%; RR=0.83; 95% CI: 0.68–1.01; P=0.06), with no difference in the occurrence of major bleeding (5% versus 5%; P=0.79). Another salient trial that deserves mention is the STEEPLE trial enrolling patients undergoing elective PCI, which interestingly showed a decreased incidence of non-CABG related bleeding in the first 48 hours with enoxaparin therapy compared to heparin (5.9% versus 8.5%; 95% CI: −4.7 to −0.6; P=0.01).Citation50
Based on the current data, enoxaparin seems to be more effective than UFH at the expense of a modest and questionable increase in the risk of bleeding. The other FDA-approved LMWHs dalteparin and tinzaparin have not been studied extensively for ACS. In the limited evidence available, both have been found to be clinically comparable or inferior to enoxaparin.Citation51,Citation52 Although dalteparin has been approved for use in unstable angina/NSTEMI, the data on its efficacy profile is not substantial. Tinzaparin has no role in ACS currently.
Bivalirudin versus UFH
Bivalirudin has emerged as the third viable option for anti-coagulation in ACS. However, arguments on its safety and efficacy profile have been marked by intense controversy and debate. The most prominent amongst the earlier trials analyzing bivalirudin was the ACUITY trial published in 2004.Citation53 In this trial, patients with STEMI were enrolled and randomized to receive UFH/enoxaparin plus a Gp IIb/IIIa inhibitor, bivalirudin plus a Gp IIb/IIIa inhibitor, or bivalirudin alone. Bivalirudin monotherapy was found to be clinically noninferior to heparin plus Gp IIb/IIIa-inhibitor therapy. Moreover, a strong evidence of significantly lower risk of major bleeding was observed in the bivalirudin group as compared to the heparin plus Gp IIb/IIIa group (4% versus 7%; RR=0.52; 95% CI: 0.40–0.66; P<0.0001). However, it is critical to note that when the bivalirudin plus Gp IIb/IIIa-inhibitor group was compared to the heparin plus Gp IIb/IIIa-inhibitor group, both these groups were found to have similar rates of composite ischemic endpoint (9% versus 8%; P=0.16) and bleeding (8% versus 7%; P=0.32).
The findings of clinical noninferiority and reduced bleeding risk with bivalirudin therapy versus heparin plus Gp IIb/IIIa-inhibitor therapy in the ACUITY trial was in line with the results obtained from the subsequent trials: ISAR-REACT 4 (enrolling NSTEMI patients undergoing PCI) and HORIZONS-AMI. The HORIZONS-AMI trial enrolled high-risk STEMI patients undergoing PCI.Citation54 Bivalirudin when compared with heparin plus Gp IIb/IIIa-inhibitor therapy showed similar rates of MACE (5.4% versus 5.5%; P=0.95) but a decreased risk of major bleeding (4.9% versus 8.3%; RR=0.60; 95% CI: 0.46–0.77; P<0.001). A reduction in death from cardiac causes at 30 days and all-cause mortality was also observed in the bivalirudin group owing to a significantly reduced risk of major bleeding. Of special note, however, was the finding of increased rate of acute ST in the bivalirudin group in the first 24 hours (1.3% versus 0.3%; P<0.001), but no significant increase in ST was shown to be present at 30 days (2.5% versus 1.9%; P=0.30).
It is imperative to note that in all clinical trials on bivalirudin discussed so far, the reduced bleeding risk was observed when it was compared to heparin plus routine Gp IIb/IIIa-inhibitor therapy. A series of trials with modified study design, all published after 2013, have introduced a whole new perspective on the current standing of bivalirudin. In the EUROMAX trial, STEMI patients undergoing PCI were enrolled and randomized to either test or control groups.Citation55 The test group received bivalirudin, with Gp IIb/IIIa-inhibitor use restricted to bailout; and the control group received heparin/enoxaparin, with optional use of Gp IIb/IIIa inhibitors at the physician’s discretion. Overall, the rate of Gp IIb/IIIa-inhibitor use was differential: 11.5% in the bivalirudin group and 69.1% in the control group.
The incidence of primary composite outcome of death or non-CABG related major bleeding at 30 days was lower in the bivalirudin group (5.1% versus 8.5%; RR=0.60; 95% CI: 0.43–0.82; P=0.001), as was the risk of major bleeding (2.6% versus 6.0%; RR=0.43; 95% CI: 0.28–0.66; P<0.001). However, the incidence of MACE was similar in both bivalirudin and heparin groups (65% versus 61%; RR=1.09; 95% CI: 0.77–1.52; P=0.64). As noted in the HORIZONS-AMI trial, the risk of acute ST was significantly higher with bivalirudin (1.1% versus 0.2%; RR=6.11; 95% CI: 1.37–27.24; P=0.007). All three P2Y12 inhibitors were used alternatively in a similar proportion in both groups. Overall, the results of EUROMAX were positive and consistent with those of the previous trials.
In the BRAVE-4 trial, STEMI patients undergoing primary PCI were randomized to receive either prasugrel plus bivalirudin or clopidogrel plus heparin.Citation56 Notably, the use of Gp IIb/IIIa inhibitors was reserved to bailout in both groups (3.0% in the prasugrel plus bivalirudin group versus 6.1% in the clopidogrel plus heparin group). The primary composite endpoint (death, MI, unplanned revascularization, ST, stroke, or bleeding at 30 days) was similar in both the groups (15.6% in the prasugrel plus bivalirudin group versus 14.5% in the clopidogrel plus heparin group; RR=1.09; one-sided 97.5% CI: 0–1.79; P=0.680). Bleeding according to HORIZONS-AMI definition was also similar (14.1% in the prasugrel plus bivalirudin group versus 12.0% in the clopidogrel plus heparin group; RR=1.18; 95% CI: 0.74–1.88; P=0.543). Thus, prasugrel plus bivalirudin therapy failed to show any safety or efficacy benefit in the BRAVE-4 trial. The trial was however stopped prematurely owing to slow recruitment, having enrolled only 548 of the 1,240 patients originally planned, and was thus underpowered to test its primary hypothesis.
The most recently published HEAT-PPCI trial recruited STEMI patients undergoing primary PCI randomized to receive either bivalirudin or heparin, with the use of Gp IIb/IIIa inhibitors restricted to bailout in both groups.Citation57 It was the first major trial making a head-to-head comparison between bivalirudin and heparin with a nondifferential Gp IIb/IIIa-inhibitor use in the two groups (13% in the bivalirudin group versus 15% in the heparin group). Use of the various P2Y12 inhibitors was also proportional in both groups, with ticagrelor being used the most (61%). The incidence of primary composite outcome (all-cause mortality, cerebrovascular accident, reinfarction, or unplanned target lesion revascularization) was higher in the bivalirudin group compared to the heparin group (8.7% versus 5.7%; RR=1.52; 95% CI: 1.09–2.13; P=0.01). Notably, the incidence of major bleeding was similar in both groups (3.5% in the bivalirudin group versus 3.1 % in the heparin group; RR=1.15; 95% CI: 0.70–1.89; P=0.59). Similar to the HORIZONS-AMI and EUROMAX trials, the incidence of ST was found to be significantly higher with bivalirudin (3.4% versus 0.9%; RR=3.91; 95% CI: 1.6–9.5; P=0.001).
In light of this new information, the role of bivalirudin is being revisited. Although the earlier trials showed a decreased risk of bleeding with bivalirudin, the latest data argues that this finding may have been attributed to differential use of Gp IIb/IIIa inhibitors in the two trial groups. Furthermore, there is substantial evidence of increased risk of ST with bivalirudin as compared to heparin. An overview of the current evidence on bivalirudin is presented in .
Table 2 Summary of the findings from some of the salient trials on bivalirudin
Fondaparinux: current status
Although trials evaluating fondaparinux have shown clinical benefit, the major drawback has been the substantially increased risk of catheter-related thrombosis. Hence, the current guidelines recommend against using fondaparinux monotherapy to support PCI (class III indication).Citation43 For instance, in the OASIS 6 trial enrolling patients with STEMI, the rate of guiding catheter-related thrombosis was significantly higher in patients receiving fondaparinux (0 versus 22; P<0.001).Citation58
The oral anticoagulants (rivaroxaban, apixaban, dabigatran)
There is no role of long-term use of oral anticoagulants (like rivaroxaban, apixaban, dabigatran) as maintenance therapy for ACS as per the current guidelines, but their utility has been explored.Citation59 Most promising has been the results of the ATLAS ACS 2-TIMI 51 trial enrolling stabilized ACS patients within 7 days of hospitalization.Citation60 The patients were randomized to either rivaroxaban therapy (2.5 mg or 5.0 mg), or placebo. Incidence of composite primary endpoint of death from cardiovascular causes, MI, or stroke in these three groups was 9.1%, 8.8%, and 10.7%, respectively (HR for rivaroxaban combined =0.84; 95% CI: 0.75–0.96; P=0.008). The incidence of TIMI major bleeding not associated with CABG was 1.8%, 2.4%, and 0.6%, respectively (HR for rivaroxaban combined =3.96; 95% CI: 2.46–6.38; P<0.001). Thus, the better efficacy outcomes were at a cost of increased risk of bleeding. FDA approval was declined based on concerns over missing follow-up safety data and methodological issues with the trial.Citation61
Results of the trials evaluating the other oral anticoagulants apixaban and dabigatran have not been encouraging. The APPRAISE-2 trial recruited patients with recent ACS (within the previous 7 days) who were randomized to receive either apixaban or placebo as maintenance therapy.Citation62 The trial was terminated prematurely because of strong evidence of increased risk of TIMI major bleeding in the apixaban group (1.3% versus 0.5%; HR=2.59; 95% CI: 1.50–4.46; P=0.001) and no reduction in ischemic events (7.5% versus 7.9%; HR=0.95; 95% CI: 0.80–1.11; P=0.51).
The RE-DEEM trial also recruited patients with recent ACS and randomized them to receive various doses of dabigatran (50 mg, 75 mg, 110 mg, 150 mg) or placebo.Citation63 A dose-dependent increase in major or minor bleeding was observed with dabigatran (HR for 150 mg group: 4.27; 95% CI: 1.86–9.81). There was no significant reduction in the rate of ischemic events with dabigatran (3.8% with placebo, 4.6% with 50 mg dose, 4.9% with 75 mg dose, 3.0% with 110 mg dose, and 3.5% with 150 mg dose).
Patients on long-term anticoagulation undergoing PCI: current status of the “triple therapy”
Antithrombotic therapy in patients on long-term anticoagulation undergoing PCI (eg, patients with atrial fibrillation) presents with a unique therapeutic challenge. Conventionally, patients are treated with dual antiplatelet therapy in addition to an oral anticoagulant as maintenance therapy after PCI (also known as triple oral antithrombotic therapy). This approach has been marked by the dilemma of increased bleeding risk with triple therapy versus ischemic risk without it, as reviewed by Reed and Cannon.Citation64
The first major trial addressing this issue was the WOEST trial completed recently, which enrolled patients on warfarin undergoing PCI and randomized them to receive either triple therapy or clopidogrel plus warfarin.Citation65 The primary endpoint of bleeding within 1 year of PCI was significantly lower in the double therapy group compared to the triple therapy group (19.4% versus 44.4%; HR=0.36; 95% CI: 0.26–0.50; P<0.0001). The secondary endpoint of death, MI, stroke, systemic embolization, and target-vessel revascularization was somewhat lower in the double therapy group (11.3% versus 17.7%; HR=0.60; 95% CI: 0.38–0.94; P=0.025). In a subgroup analysis, uninterrupted oral anticoagulation therapy during PCI was not associated with an increase in bleeding or major adverse cardiac and cerebrovascular events compared to the conventional heparin-bridging therapy.Citation66
In spite of these new developments, more evidence is needed to form definite conclusions in this regard. Of note, the most recent ACC/AHA guidelines on atrial fibrillation has assigned a class IIb recommendation to the use of clopidogrel plus oral anticoagulants (without aspirin) for the maintenance therapy of atrial fibrillation patients who have undergone coronary revascularization.Citation67 Conclusion of some of the ongoing trials will provide more evidence in this regard.Citation68
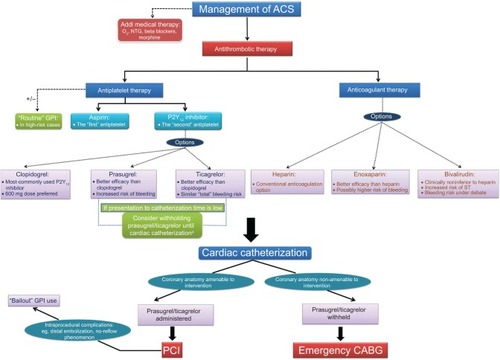
A decision tree depicting the current trends in interventional pharmacology is illustrated in .
Figure 3 A decision tree depicting the various therapeutic options available for the management of ACS, and highlighting their unique characteristics based on the current evidence.
Abbreviations: ACS, acute coronary syndrome; Addl, additional; CABG, coronary artery bypass grafting; GPI, glycoprotein IIb/IIIa inhibitor; NTG, nitroglycerine; O2, oxygen; PCI, percutaneous coronary intervention; ST, stent thrombosis.
Future directions
The pharmacology of hemostasis and thrombosis is an ever-evolving field, and many more novel antiplatelet and anticoagulant drugs are being developed and tested. A brief overview of some of the important drugs under trial is presented below.
Upcoming antiplatelet drugs
IV P2Y12 inhibitors
Among the most important upcoming antiplatelet drugs are the reversible IV P2Y12 inhibitors: cangrelor and elinogrel. The earlier Phase III trials CHAMPION PLATFORM and CHAMPION PCI failed to show clinical benefit of cangrelor use during PCI.Citation69,Citation70 However, encouraging results were obtained in the most recent CHAMPION PHOENIX trial, which enrolled patients undergoing urgent or elective PCI randomized to receive cangrelor or clopidogrel before PCI.Citation71 The cangrelor group then received clopidogrel after the infusion was complete. The rate of primary composite endpoint of death, MI, ischemia-driven revascularization, or ST at 48 hours was lower in the cangrelor group (4.7% versus 5.9%; RR=0.78; 95% CI: 0.66–0.93; P=0.005). There was no significant increase in severe bleeding with cangrelor (0.16% versus 0.11%; odds ratio =1.50; 95% CI: 0.53–4.22; P=0.44).
The contradictory findings of the CHAMPION trials have been attributed to different definitions of periprocedural MI. A reduction in the rate of periprocedural MI accounted for most of the benefits of cangrelor observed in the CHAMPION PHOENIX trial (3.8% versus 4.7%; RR=0.80; 95% CI: 0.67–0.97; P=0.02), whereas the same outcome was responsible for its failure in the previous trials. It has been argued that the definition of periprocedural MI in the previous studies did not allow for discrimination between reinfarction and biomarker-positive ACS: an issue that was supposedly addressed in the CHAMPION PHOENIX trial.Citation71 The FDA however declined to approve cangrelor because of inconclusive evidence.Citation72
Elinogrel is the only P2Y12 inhibitor available for both oral and IV routes of administration. This gives it a unique pharmacological advantage over cangrelor, where an IV dose during PCI can be followed by an oral dose for a smooth transition of the antiplatelet effect. IV administration of cangrelor has to be followed by administration of another oral P2Y12 inhibitor (like clopidogrel). This in turn raises the issue of drug interaction, as studied by Steinhubl et al.Citation73 However, the results of the Phase II trial INNOVATE-PCI were equivocal, with similar efficacy endpoints and no increase in TIMI major/minor bleeding observed with elinogrel.Citation74 No Phase III trials have been planned as of now.
An important clinical benefit of these IV P2Y12 inhibitors is their quick onset of action and a short half-life. They can have a unique advantage for use in patients undergoing emergency CABG. Due to their quick offset of action, surgery can be safely performed within a few hours of stopping the drug. The BRIDGE trial enrolled patients undergoing planned CABG, and tested the use of cangrelor 1–6 hours before surgery.Citation75 Platelet reactivity throughout the treatment period was lower with cangrelor than placebo (platelet reactivity units <240 throughout infusion in 98.8% versus 19.0% of patients; RR=5.2; 95% CI: 3.3–8.1; P<0.001). After discontinuation of cangrelor 1–6 hours before surgery, platelet reactivity was found to be similar in both groups (mean ±1 standard deviation platelet reactivity unit values: 279.7±106.5 in cangrelor group versus 297.8±67.3 in placebo group; P=0.212). Rates of CABG-related bleeding were similar with cangrelor and placebo groups (11.8% versus 10.4%; RR=1.1; 95% CI: 0.5–2.5; P=0.763). Thus, the findings of the trial supported this idea.
Upcoming anticoagulant drugs
Engineered LMWH
M118 (also referred to as “adomiparin sodium” in some texts) is a novel LMWH with the desirable attributes of both enoxaparin and UFH. It has shown potent activity against both factors Xa and IIa, and is thereby easily monitored by a point-of-care assay. It has predictable pharmacokinetics with both subcutaneous and IV routes and can be easily reversed by protamine sulfate. M118 is being tested for use in ACS and has so far successfully completed a Phase II trial called the EMINENCE trial (enrolling patients undergoing elective PCI).Citation76 M118 was found to be clinically noninferior to heparin at preventing PCI-related complications, and the adverse event profiles of M118 and UFH were found to be comparable.
RNA aptamers
RNA aptamers are single-stranded oligonucleotide sequences that bind to certain protein sequences with high specificity. Since these are nucleotides, their complementary sequences can be engineered and used as very effective antidotes. One such anticoagulation system being tested for use with PCI is the REG 1 system, which inhibits factor IXa. In a successfully completed Phase II trial, REVERSAL-PCI, the REG 1 system was compared with UFH.Citation77 Anticoagulation was partially reversed after PCI and fully reversed 4 hours later. This was in an attempt to achieve adequate intraprocedural anticoagulation, with rapid and effective reversal after the PCI. This system is currently being studied in a Phase III trial.Citation78 This novel strategy of the REG 1 system is certainly a hallmark of the innovations being made in this field.
A list of all the trials investigated and discussed in this review is presented in .
Table 3 List of clinical trials in order of their mention in the review
Conclusion
There should be no doubt in saying that these are the most exciting times in this part of the world of pharmacotherapeutics. With advances in the understanding of basic mechanisms of hemostasis and thrombosis, ingenious molecular targets are being exploited, and many of these theoretical hypotheses have been translated into success in clinical trials. It has become imperative for the clinician to be well versed with all the current evidence available and adapt that knowledge in making day-to-day decisions. The need for individualization of therapy is being stressed. A couple of decades ago, the clinician did not have much to choose from; now, there is a plethora of options available. A sound knowledge of the current evidence on these drugs can help make rational decisions.
The most-recently approved P2Y12 inhibitor ticagrelor seems to be superior to other P2Y12 inhibitors as it has been shown to be more effective than clopidogrel with no increase in total bleeding risk (although an increase in the risk of non-CABG-related bleeding was observed). However, we need to wait for the conclusion of the ongoing trial to make a more direct comparison between prasugrel and ticagrelor. Routine use of Gp IIb/IIIa inhibitors can be made in high-risk patients, while their use in other patients should be reserved to bailout. Enoxaparin has been found to be more efficacious but somewhat less safe than heparin, whereas the role of bivalirudin is being debated.
With this myriad of options available and more on their way, these innovations should be celebrated as an opportunity to practice better medicine.
Disclosure
The authors report no conflicts of interest in this work.
References
- FDA approved Drug Products [webpage on the Internet]Silver SpringU.S. Food and Drug Administration Available from: http://www.accessdata.fda.gov/Scripts/cder/drugsatfda/index.cfmAccessed May 21, 2014
- ACC/AHA Joint GuidelinesDallasAmerican Heart Association2014 Available from: http://my.americanheart.org/professional/StatementsGuidelines/ByTopic/TopicsA-C/ACCAHA-Joint-Guidelines_UCM_321694_Article.jspAccessed July 27, 2014
- LijferingWMFlintermanLEVandenbrouckeJPRosendaalFRCannegieterSCRelationship between venous and arterial thrombosis: a review of the literature from a causal perspectiveSemin Thromb Hemost201137888589622198853
- SpbieszcykPFishbeinMCGoldhaberSZAcute pulmonary embolism. don’t ignore the plateletCirculation20021061748174912356622
- YusufSZhaoFMehtaSREffects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevationN Engl J Med2001345749450211519503
- ChenZMJiangLXChenYPAddition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trialLancet200536694971607162116271642
- CURRENT-OASIS 7 InvestigatorsMehtaSRBassandJPDose comparisons of clopidogrel and aspirin in acute coronary syndromesN Engl J Med20103631093094220818903
- PattiGColonnaGPasceriVPepeLLMontinaroADi SciascioGRandomized trial of high loading dose of clopidogrel for reduction of periprocedural myocardial infarction in patients undergoing coronary intervention: results from the ARMYDA-2 (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty) studyCirculation2005111162099210615750189
- DangasGMehranRGuagliumiGRole of clopidogrel loading dose in patients with ST-segment elevation myocardial infarction undergoing primary angioplasty: results from the HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trialJ Am Coll Cardiol200954151438144619796737
- Siller-MatulaJMHuberKChristGImpact of clopidogrel loading dose on clinical outcome in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysisHeart2011979810520736210
- von BeckerathNTaubertDPogatsa-MurrayGSchömigEKastratiASchömigAAbsorption, metabolization, and antiplatelet effects of 300-, 600-, and 900-mg loading doses of clopidogrel: results of the ISAR-CHOICE (Intracoronary Stenting and Antithrombotic Regimen: Choose Between 3 High Oral Doses for Immediate Clopidogrel Effect) TrialCirculation2005112192946295016260639
- MontalescotGSiderisGMeulemanCA randomized comparison of high clopidogrel loading doses in patients with non-ST-segment elevation acute coronary syndromes: the ALBION (Assessment of the Best Loading Dose of Clopidogrel to Blunt Platelet Activation, Inflammation and Ongoing Necrosis) trialJ Am Coll Cardiol200648593193816949482
- SerebruanyVLSteinhublSRBergerPBMalininAIBhattDLTopolEJVariability in platelet responsiveness to clopidogrel among 544 individualsJ Am Coll Cardiol200545224625115653023
- HagiharaKKazuiMKuriharaAA possible mechanism for the differences in efficiency and variability of active metabolite formation from thienopyridine antiplatelet agents, prasugrel and clopidogrelDrug Metab Dispos200937112145215219704027
- BrandtJTCloseSLIturriaSJCommon polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrelJ Thromb Haemost20075122429243617900275
- WiviottSDBraunwaldEMcCabeCHPrasugrel versus clopi-dogrel in patients with acute coronary syndromesN Engl J Med2007357202001201517982182
- BrandtJTPayneCDWiviottSDA comparison of prasugrel and clopidogrel loading doses on platelet function: magnitude of platelet inhibition is related to active metabolite formationAm Heart J2007153166.e9e1617174640
- JernbergTPayneCDWintersKJPrasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery diseaseEur Heart J200627101166117316621870
- WallentinLBeckerRCBudajATicagrelor versus clopidogrel in patients with acute coronary syndromesN Engl J Med2009361111045105719717846
- GurbelPABlidenKPButlerKRandomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET studyCirculation2009120252577258519923168
- GurbelPABlidenKPButlerKResponse to ticagrelor in clopi-dogrel nonresponders and responders and effect of switching therapies: the RESPOND studyCirculation2010121101188119920194878
- ArmstrongDSummersCEwartLNylanderSSidawayJEvan GiezenJJCharacterization of the adenosine pharmacology of ticagrelor reveals therapeutically relevant inhibition of equilibrative nucleoside transporter 1J Cardiovasc Pharmacol Ther201419220921924414167
- SchulzSAngiolilloDJAntoniucciDRandomized comparison of ticagrelor versus prasugrel in patients with acute coronary syndrome and planned invasive strategy – design and rationale of the iNtracoro-nary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment (ISAR-REACT) 5 trialJ Cardiovasc Transl Res2014719110024371012
- AlexopoulosDGalatiAXanthopoulouITicagrelor versus prasugrel in acute coronary syndrome patients with high on-clopidogrel platelet reactivity following percutaneous coronary intervention: a pharmacodynamic studyJ Am Coll Cardiol201260319319922789884
- O’GaraPTKushnerFGAscheimDD2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice GuidelinesCirculation2013127e362e42523247304
- YendeSWunderinkRGEffect of clopidogrel on bleeding after coronary artery bypass surgeryCrit Care Med200129122271227511801823
- HongoRHLeyJDickSEYeeRRThe effect of clopidogrel in combination with aspirin when given before coronary artery bypass graftingJ Am Coll Cardiol200240223123712106925
- AntmanEMHandMArmstrongPW2007 Focused Update of the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: developed in collaboration With the Canadian Cardiovascular Society endorsed by the American Academy of Family Physicians: 2007 Writing Group to Review New Evidence and Update the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction, Writing on Behalf of the 2004 Writing CommitteeCirculation2008117229632918071078
- KapetanakisEIMedlamDABoyceSWClopidogrel administration prior to coronary artery bypass grafting surgery: the cardiologist’s panacea or the surgeon’s headache?Eur Heart J200526657658315723815
- ZeymerUArntzHRMarkBEfficacy and safety of a high loading dose of clopidogrel administered prehospitally to improve primary percutaneous coronary intervention in acute myocardial infarction: the randomized CIPAMI trialClin Res Cardiol2012101430531222186968
- SadanandanSCannonCPGibsonCMA risk score to estimate the likelihood of coronary artery bypass surgery during the index hos-pitalization among patients with unstable angina and non-ST-segment elevation myocardial infarctionJ Am Coll Cardiol200444479980315312862
- FitchettDEikelboomJFremesSDual antiplatelet therapy in patients requiring urgent coronary artery bypass grafting surgery: a position statement of the Canadian Cardiovascular SocietyCan J Cardiol20092512683689 English; French19960127
- MarchiniJMorrowDResnicFAn algorithm for use of prasugrel (effient) in patients undergoing cardiac catheterization and percutaneous coronary interventionCrit Pathw Cardiol20109419219821119336
- MontalescotGBarraganPWittenbergOPlatelet glycoprotein IIb/IIIa inhibition with coronary stenting for acute myocardial infarctionN Engl J Med2001344251895190311419426
- AntoniucciDMiglioriniAParodiGAbciximab-supported infarct artery stent implantation for acute myocardial infarction and long-term survival: a prospective, multicenter, randomized trial comparing infarct artery stenting plus abciximab with stenting aloneCirculation2004109141704170615066943
- EllisSGTenderaMde BelderMAFacilitated PCI in patients with ST-elevation myocardial infarctionN Engl J Med2008358212205221718499565
- De LucaGNavareseEMarinoPRisk profile and benefits from Gp IIb-IIIa inhibitors among patients with ST-segment elevation myocardial infarction treated with primary angioplasty: a meta-regression analysis of randomized trialsEur Heart J200930222705271319875386
- Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC)StegPGJamesSKESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevationEur Heart J201233202569261922922416
- KastratiAMehilliJNeumannFJAbciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment: the ISAR-REACT 2 randomized trialJAMA2006295131531153816533938
- KakkarAKMoustaphaAHanleyHGComparison of intracoro-nary vs intravenous administration of abciximab in coronary stentingCatheter Cardiovasc Interv2004611313414696156
- HansenPRIversenAAbdullaJImproved clinical outcomes with intracoronary compared to intravenous abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a systematic review and meta-analysisJ Invasive Cardiol201022627828220516508
- BertrandOFRodés-CabauJLaroseEIntracoronary compared to intravenous Abciximab and high-dose bolus compared to standard dose in patients with ST-segment elevation myocardial infarction undergoing transradial primary percutaneous coronary intervention: a two-by-two factorial placebo-controlled randomized studyAm J Cardiol2010105111520152720494655
- LevineGNBatesERBlankenshipJC2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and InterventionsCirculation2011124e574e65122064601
- GurmHSTamhaneUMeierPGrossmanPMChetcutiSBatesERA comparison of abciximab and small-molecule glycoprotein IIb/IIIa inhibitors in patients undergoing primary percutaneous coronary intervention: a meta-analysis of contemporary randomized controlled trialsCirc Cardiovasc Interv20092323023620031720
- SciricaBMBonacaMPBraunwaldEVorapaxar for secondary prevention of thrombotic events for patients with previous myocardial infarction: a prespecified subgroup analysis of the TRA 2°P-TIMI 50 trialLancet201238098501317132422932716
- FareedJHoppensteadtDWalengaJPharmacodynamic and pharmacokinetic properties of enoxaparin: implications for clinical practiceClin Pharmacokinet200342121043105712959635
- FergusonJJCaliffRMAntmanEMEnoxaparin vs unfraction-ated heparin in high-risk patients with non-ST-segment elevation acute coronary syndromes managed with an intended early invasive strategy: primary results of the SYNERGY randomized trialJAMA20042921455415238590
- CohenMMahaffeyKWPieperKA subgroup analysis of the impact of prerandomization antithrombin therapy on outcomes in the SYNERGY trial: enoxaparin versus unfractionated heparin in non-ST-segment elevation acute coronary syndromesJ Am Coll Cardiol20064871346135417010793
- MontalescotGZeymerUSilvainJIntravenous enoxaparin or unfractionated heparin in primary percutaneous coronary intervention for ST-elevation myocardial infarction: the international randomised open-label ATOLL trialLancet2011378979269370321856483
- MontalescotGGalloRWhiteHDEnoxaparin versus unfrac-tionated heparin in elective percutaneous coronary intervention 1-year results from the STEEPLE (SafeTy and efficacy of enoxaparin in per-cutaneous coronary intervention patients, an international randomized evaluation) trialJACC Cardiovasc Interv20092111083109119926048
- KleinWBuchwaldAHillisSEComparison of low-molecular-weight heparin with unfractionated heparin acutely and with placebo for 6 weeks in the management of unstable coronary artery disease. Fragmin in unstable coronary artery disease study (FRIC)Circulation199796161689236418
- KatsourasCMichalisLKPapamichaelNEnoxaparin versus tinzaparin in non-ST-segment elevation acute coronary syndromes: results of the enoxaparin versus tinzaparin (EVET) trial at 6 monthsAm Heart J2005150338539116169312
- StoneGWWhiteHDOhmanEMBivalirudin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a subgroup analysis from the Acute Catheterization and Urgent Intervention Triage strategy (ACUITY) trialLancet2007369956590791917368152
- StoneGWWitzenbichlerBGuagliumiGBivalirudin during primary PCI in acute myocardial infarctionN Engl J Med2008358212218223018499566
- StegPGvan ‘t HofAHammCWBivalirudin started during emergency transport for primary PCIN Engl J Med2013369232207221724171490
- SchulzSRichardtGLaugwitzKLPrasugrel plus bivalirudin vs clopidogrel plus heparin in patients with ST-segment elevation myocardial infarctionEur Heart J201435342285229424816809
- ShahzadAKempIMarsCUnfractionated heparin versus bivali-rudin in primary percutaneous coronary intervention (HEAT-PPCI): an open-label, single centre, randomised controlled trialLancet2014
- YusufSMehtaSRChrolaviciusSEffects of fondaparinux on mortality and reinfarction in patients with acute ST-segment elevation myocardial infarction: the OASIS-6 randomized trialJAMA2006295131519153016537725
- SinnaevePRAdriaenssensTHöchtlTHuberKNew oral anticoagulant agents after ACSEur Heart J Acute Cardiovasc Care201211879324062894
- MegaJLBraunwaldEWiviottSDRivaroxaban in patients with a recent acute coronary syndromeN Engl J Med2012366191922077192
- Rivaroxaban STEMI study hits print as FDA delivers setback: ATLAS ACS 2-TIMI 51 [article on the Internet]Medscape; WebMD LLC3052013 Available from: http://www.medscape.com/viewarticle/791479Accessed July 27, 2014
- AlexanderJHLopesRDJamesSApixaban with antiplatelet therapy after acute coronary syndromeN Engl J Med2011365869970821780946
- OldgrenJBudajAGrangerCBDabigatran vs placebo in patients with acute coronary syndromes on dual antiplatelet therapy: a randomized, double-blind, phase II trialEur Heart J201132222781278921551462
- ReedGWCannonCPTriple oral antithrombotic therapy in atrial fibrillation and coronary artery stentingClin Cardiol2013361058559423873635
- DewildeWJOirbansTVerheugtFWUse of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trialLancet201338198721107111523415013
- DewildeWJJanssenPWKelderJCUninterrupted oral anticoagulation versus bridging in patients with long-term oral anticoagulation during percutaneous coronary intervention: subgroup analysis from the WOEST trialEuroIntervention2014pii:20140202–08
- JanuaryCTWannLSAlpertJS2014AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm SocietyCirculation Epub2014410
- Janssen Scientific Affairs, LLCA Study Exploring Two Strategies of Rivaroxaban (JNJ39039039; BAY-59–7939) and One of Oral Vitamin K Antagonist in Patients With Atrial Fibrillation Who Undergo Percutaneous Coronary Intervention (PIONEER AF-PCI) Available from: http://clinicaltrials.gov/show/NCT01830543. NLM identifier: NCT01830543Accessed July 27, 2014
- BhattDLLincoffAMGibsonCMIntravenous platelet blockade with cangrelor during PCIN Engl J Med2009361242330234119915222
- HarringtonRAStoneGWMcNultySPlatelet inhibition with cangrelor in patients undergoing PCIN Engl J Med2009361242318232919915221
- BhattDLStoneGWMahaffeyKWEffect of platelet inhibition with cangrelor during PCI on ischemic eventsN Engl J Med2013368141303131323473369
- FDA Advisory Panel Votes No on Approving Cangrelor for PCI, Bridge Therapy [article on the Internet]Medscape; WebMD LLC2122014 Available from: http://www.medscape.com/viewarticle/820567Accessed July 27, 2014
- SteinhublSROhJJOestreichJHFerrarisSCharnigoRAkersWSTransitioning patients from cangrelor to clopidogrel: pharmacodynamic evidence of a competitive effectThromb Res2008121452753417631948
- WelshRCRaoSVZeymerUA randomized, double-blind, active-controlled phase 2 trial to evaluate a novel selective and reversible intravenous and oral P2Y12 inhibitor elinogrel versus clopidogrel in patients undergoing nonurgent percutaneous coronary intervention: the INNOVATE-PCI trialCirc Cardiovasc Interv20125333634622647518
- AngiolilloDJFirstenbergMSPriceMJBridging antiplatelet therapy with cangrelor in patients undergoing cardiac surgery: a randomized controlled trialJAMA2012307326527422253393
- RaoSVMelloniCMyles-DimauroSEvaluation of a new heparin agent in percutaneous coronary intervention: results of the phase 2 evaluation of M118 IN pErcutaNeous Coronary intErvention (EMINENCE) TrialCirculation2010121151713172120368520
- CohenMGPurdyDARossiJSFirst clinical application of an actively reversible direct factor IXa inhibitor as an anticoagulation strategy in patients undergoing percutaneous coronary interventionCirculation2010122661462220660806
- Regado Biosciences, IncA Study To Determine the Efficacy and Safety of REG1 Compared to Bivalirudin in Patients Undergoing PCI (Regulate) Available from: http://clinicaltrials.gov/show/NCT01848106. NLM identifier: NCT01848106Accessed July 27, 2014