Abstract
Infection with hepatitis C virus (HCV) is highly prevalent in chronic kidney disease (CKD) patients, mainly in those on hemodialysis (HD). The seroprevalence of HCV in developing countries ranges between 7% and 40%. Risk factors for this infection in the CKD population include the number of blood transfusions, duration of end-stage renal disease (ESRD), and prevalence of HCV in HD. Chronic HCV infection in patients with ESRD is associated with an increase in morbidity and mortality in the pre and post kidney transplant periods. The increase in mortality is directly associated with liver complications and an elevated cardiovascular risk in HCV-infected patients on hemodialysis. Antiviral treatment may improve the prognosis of patients with HCV, and standard interferon remains the cornerstone of treatment. Treatment of HCV in patients with CKD is complex, but achieving a sustained viral response may decrease the frequency of complications after transplantation. It appears that HCV-infected patients who remain on maintenance dialysis are at increased risk of death compared with HCV patients undergoing renal transplantation.
Introduction
Hepatitis C virus (HCV) infection is highly prevalent in patients with chronic kidney disease (CKD) requiring renal replacement therapy, and is the most frequently recognized cause of liver injury in patients with CKD. The prevalence of anti-HCV in developing countries ranges between 7% and 40%.Citation1–Citation3 Risk factors for patients on hemodialysis (HD) for acquiring HCV infection include number of previous blood transfusions,Citation4 duration of end-stage renal disease (ESRD),Citation5 prevalence of HCV in HD units,Citation2 a history of previous transplantCitation6 and patient age.Citation2,Citation7
Chronic HCV infection in patients with ESRD leads to an increase in morbidity and mortality in the pre and post renal transplant period.Citation8–Citation11 The increase in mortality is directly associated with liver complications, although HCV-related liver disease tends to be mostly asymptomatic in patients on long-term dialysis.Citation8,Citation11 Furthermore, patients undergoing kidney transplantation are at risk of developing graft nephropathy and diabetes mellitus secondary to HCV infection.Citation12–Citation15
Antiviral treatment may improve the prognosis of patients with HCV after kidney transplantation; however, despite the knowledge of the mechanisms of HCV transmission in HD units, experience in the treatment of HCV infection in patients with ESRD and in kidney transplant recipients is still limited.Citation16
Transmission and prevalence
In most regions, HCV infection is significantly more common in people with kidney disease when compared with the general population.Citation17 The estimated prevalence of HCV infection in HD patients is 3%–20% in the USA and Western Europe.Citation17 In 2002, approximately 8% of patients on chronic HD in the USA were seropositive for HCV.Citation18 Moreover, the seroprevalence in HD patients in other regions is significantly higher; for example, a study conducted in Egypt reported a higher prevalence (80%) in patients on chronic HD.Citation19
Inappropriate infection control practices, such as incorrect parenteral drug delivery, poor equipment sterilization, or both, have been documented during some outbreaks.Citation17 Therefore, guidelines exist for the prevention of HCV transmission in HD units, emphasizing infection control measures such as correct handling of parenteral medications, disinfection of HD machines and handwashing.Citation20,Citation21 HCV infection should be routinely sought in patients on chronic HD by determining anti-HCV antibody titers. In case of patients with negative results, the test should be repeated every 6–12 months.Citation21
Natural history of HCV infection in patients on dialysis
HCV infection does not usually present with acute symptoms, and disease progression is a long-term process. Mostly, patients are diagnosed with HCV infection after blood screening; no symptoms or elevations of liver enzyme levels are specific of the disease. Spontaneous clearance of HCV RNA has been documented in 1% of untreated patients on HD.Citation22
The impact of ESRD on the progression of liver injury secondary to HCV infection is difficult to assess due to the high mortality rate in patients in chronic HD units, and because assessing the degree of liver disease by biopsy is challenging due to the associated comorbidities, such as platelet disorders, prevailing in this population.
Transient elastography is a noninvasive tool designed to assess the severity of hepatic fibrosis in terms of organ stiffness; it has been evaluated in HCV-infected patients on HD and compared with liver biopsy. Transient elastography appeared to be superior to other noninvasive methods (aspartate aminotransferase/platelet ratio index), and could potentially decrease the need for staging liver biopsies in HD patients with HCV infection.Citation23
Fabrizi et al established that the relative risk of death in patients with ESRD and HCV infection (all-cause mortality), was 1.35 (95% confidence interval [CI] 1.25–1.47); liver disease was the most frequent cause of death in this group of patients on HD.Citation24
A study of the natural course of HVC infection in HD patients was conducted by Okuda and YokosukaCitation25 who compared 189 patients with chronic HVC infection on HD (cases) and patients with chronic HCV infection and no ESRD (controls); the patients were age-matched and followed for 4–23 years. No cases progressed to cirrhosis, while 25% of patients in the control group developed cirrhosis (P<0.0001). Although overall mortality increases with HCV infection in ESRD patients, disease progression and development of liver failure appear to be slower and/or less likely in uremic patients.Citation26,Citation27
A recent meta-analysis of 14 observational studies demonstrated an independent and significant impact of HCV on mortality among patients on long-term dialysis, with an adjusted relative risk of 1.35 (95% CI 1.25–1.47); cardiovascular mortality was 1.26 (95% CI 1.10–1.45) in HCV-positive, being a major cause of death.Citation28
The course of the infection is not thoroughly understood, but some hypotheses argue that the viral load decreases in ESRD patients on HD when compared with nonuremic controls; however, not all the studies support this theory.Citation29,Citation30 Passage of viral particles into the dialysate fluid, trapping of the virus on the surface of the dialyzer membrane, and the significant amount of cytokines with antiviral properties indirectly produced by the host are the main areas of speculation in the tentative explanation of the effect of HD on HCV viremia.Citation31
The evidence for a relationship between HCV and survival among patients on peritoneal dialysis is more limited, the incidence of liver disease-related mortality is higher in patients with HCV infection than in HCV-negative cases and may be related to impaired nutritional status.Citation32
HCV infection in kidney transplant recipients
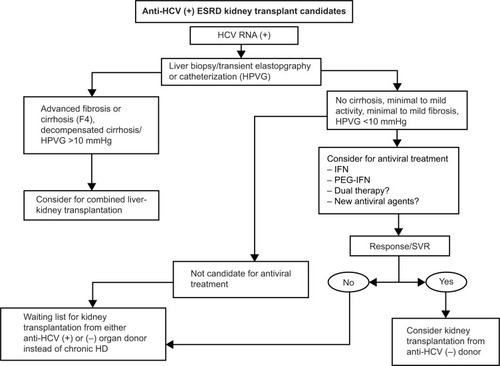
The management of kidney transplantation in HCV-positive patients remains a challenge because, aside from renal failure, liver disease must be taken into account. Various studies have revealed that, overall patient and graft survival are significantly shortened. Mortality resulting from liver disease as well as the risk of developing hepatocellular carcinoma are increased in HCV-positive kidney transplant recipients.Citation33,Citation34 Good survival rates have been obtained in these patients, especially if they have minimal or well-controlled liver disease.Citation35 In patients undergoing renal transplantation in the setting of established cirrhosis and a hepatic portal venous gradient (HPVG) below 10 mmHg, a combined liver-kidney transplantation may be unnecessary and a kidney transplant alone may be safely performed.Citation36,Citation37
The impact of immunosuppression on the clinical course and progression of HCV infection in kidney transplant recipients is not completely elucidated; frequently, HCV RNA levels increase after transplantation, which may be related to a decrease in viral clearance.Citation38 Further, the role of immunosupression on the progression of fibrosis in cases of HCV infection is uncertain in kidney transplant recipients. Several studies have found that the patterns of fibrosis progression are stable and may even improve after treatment;Citation39,Citation40 in fact, cyclosporine inhibits the replication of HCV in vitro.Citation41
There are case reports of fibrosing cholestatic hepatitis after kidney transplant related to immunosuppression. This entity is difficult to treat and is associated with high morbidity and mortality rates, and its treatment is associated with risk of graft rejection.Citation42,Citation43 Survival of patients with fibrosing cholestatic hepatitis improves with early initiation of PEGylated interferon (IFN)-α2a and ribavirin therapy, strict monitoring by biopsy and HCV load determinations, and replacement of tacrolimus with cyclosporine.Citation44 Aside from the liver complications associated with HCV infection, kidney transplant patients can develop immune complex glomerulonephritis and renal interstitial fibrosis and tubular atrophy (previously known as chronic allograft nephropathy).Citation45
Despite the potential development of complications and progression of HCV infection in renal post-transplant patients, HCV infection is not considered a contraindication for kidney transplantation because survival after transplantation is markedly higher than that of HCV patients who remain on chronic HD.Citation46,Citation47
Treatment of HCV infection in CKD patients in different settings
More information on the treatment of HCV infection in patients with CKD has recently become available. As in most patients with hepatitis C, the decision to initiate therapy is largely based on the stage of the liver disease, the expectation of a sustained viral response (SVR) and associated comorbidities. HCV patients on HD who are kidney transplant candidates should be treated more aggressively since viral eradication before kidney transplantation fosters a decrease of both hepatic and renal complications.
Treatment of chronic HCV in patients with ESRD
The risk of death in patients with HCV infection on dialysis is higher than in patients on dialysis without HCV.Citation48 It is recommended that HCV-positive patients who are candidates for kidney transplantation be treated with antiviral therapies. Tested therapeutic modalities for this group of patients include IFN alone or in combination with ribavirin. Infections due to HCV genotypes 1 and 4 are less responsive to IFN-based therapy and require treatment for 48 weeks ().
Figure 1 Proposed algorithm for evaluation and allocation of renal transplant candidates with HCV infection.
Monotherapy with standard IFN-α
There are reports demonstrating that use of IFN in patients awaiting kidney transplantation is associated with higher SVR rates as well as continuous biochemical improvement in the post-transplant period; also, HCV-infected patients who are renal transplant candidates and treated with IFN show lower rates of renal interstitial fibrosis and tubular atrophy.Citation49 This may be the result of the lower viral loads described in these patients, or a result of the incomplete removal of IFN during HD.
IFN therapy on HD patients is not well tolerated so high dropout rates are seen. Neurological (21%) and gastrointestinal (18%) adverse effects are reported.
At least two meta-analyses have shown that standard IFN therapy is associated with the achievement of SVR, although there is no evidence that this impacts the survival of patients with ESRD on HD. In patients with ESRD infected with HCV, conventional treatment with IFN monotherapy at a dose of 1–6 million units daily or three times a week for 12–48 weeks leads to SVR rates of 39%–41%, with treatment-related dropout rates of 26%–27%.Citation22,Citation50
Predictive factors associated with the response to IFN include: dosage (3 million units or above), duration of therapy (at least 6 months), low pretreatment viral load and liver histology (moderate injury).Citation22,Citation51–Citation53 Patients with decompensated liver disease are not candidates for treatment, the treatment should be stopped for those who do not achieve a negative viral load after 4–8 weeks of treatment, since the probability of SVR is remote.
In accordance with the 2008 KDIGO (Kidney Disease: Improving Global Outcomes) guidelines (),Citation20 the standard IFN dosage should be adjusted if the glomerular filtration rate is below 15 mL/min/1.73 m2; recommended for the management of HCV patients who are kidney transplant candidates, those on HD, and those with stage 5 CKD.
Table 1 Summary by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative of KDIGO guidelines for the prevention, diagnosis, evaluation and treatment of hepatitis C in patients with chronic renal failure 2008Citation85
PEGylated IFN monotherapy
There is no reported difference in the clearance of PEGylated IFN monotherapy (PEG-IFN-α2a) between patients with normal renal function and those with significantly decreased renal function (glomerular filtration rate >100 mL/min versus 20–40 mL/min). PEG-IFN pharmacokinetics may vary during the HD process. The largest series of HCV patients treated with PEG-IFN reported SVR rate of 14.1% and a high rate of adverse events (83%).Citation54 Recently, Wang et al prospectively evaluated the efficacy and tolerability of low doses of standard IFN-α2b (1.5×106 U three times per week) and PEG-IFN-α2a (67.5 μg once a week) in HCV-positive HD patients; SVR with PEG-IFN was obtained in 91.7% of cases, with a dropout rate of 8.3%. With standard IFN, SVR rate of 71.4% was achieved, with a dropout rate of 28.6%. Anemia was the most frequent side effect, observed in 55.5% of cases and erythropoietin therapy was required.Citation55 Two meta-analyses showed SVR and treatment-related dropout rates of 31%–37% and 23%–28%, respectively, which is comparable with standard IFN therapy.Citation22,Citation56
A study directly comparing treatment with standard IFN vs PEG-IFN demonstrated superior efficacy and safety of PEG-IFN.Citation57 Similarly, there were some predictors of SVR with PEG-IFN therapy, including low pretreatment levels of HCV RNA and a rapid virologic response.Citation57 In HCV-infected patients with CKD stages 3, 4 and 5 awaiting dialysis therapy, monotherapy of PEG-IFN with doses adjusted according to kidney function is suggested in accordance with the 2008 KDIGO guidelines ().Citation20
Combination therapy of standard IFN plus ribavirin
The elimination rate of ribavirin in patients with impaired renal function is decreased, and a small fraction of the drug is removed by HD. Ribavirin is contraindicated in patients with ESRD infected with HCV, because of the potential risk of life-threatening hemolytic anemia. However, some studies have shown that using a low dose of ribavirin (200–400 mg three times a week) in order to obtain serum levels of 10–15 μmol/L, combined with standard IFN plus high doses of erythropoietin, could be acceptable in these patients.
There is no information available on the appropriate ribavirin dosage or its adverse effects in patients with HCV who are on dialysis. There are some studies involving small numbers of patients treated with combination therapy (IFN + ribavirin) and different ribavirin doses, yielding different SVR (17%–66%) and discontinuation (0%–33%) rates.Citation58–Citation60
A meta-analysis by Fabrizi et alCitation61 reported SVR rate of 56% (95% CI 28–85) and a dropout rate of 25% (95% CI 10–40). The most frequent side effects requiring interruption of treatment were anemia (26%) and heart failure (9%). Despite this evidence, ribavirin is not recommended for routine use in the management of HCV patients with a glomerular filtration rate below 50 mL/min unless a new large-scale study confirms its safety profile.
Combined antiviral therapy of PEG-IFN plus ribavirin
There is little information of the use of double therapy in HCV patients with CKD. In a prospective study of HCV patients on chronic HD (young patients awaiting kidney transplantation), cases were treated for 24–48 weeks depending on genotype; the SVR rate was 97.5%, 74% of patients required treatment with erythropoietin, and 31% required reduction of the ribavirin dosage. These patients also required higher erythropoietin doses (10,000–40,000 IU/week) to maintain an adequate dose of ribavirin during treatment to achieve adequate viral suppression. In this study, like in other settings, patients with HCV genotypes 2 and 3 had higher SVR rates compared with genotypes 1 and 4.Citation62
Patients who relapsed after initial treatment with IFN monotherapy were evaluated in a study by Djordjeviƈ et al in which four patients relapsed after 12 weeks of conventional therapy were considered for a second treatment consisting of standard IFN administered for another 24 weeks, although all patients had viral suppression, none achieved SVR.Citation63 In a second study conducted in 2009, 35 patients relapsing at week 24 were retreated with double standard therapy of IFN or PEG-IFN and ribavirin for 48 weeks (genotype 1) and 24 weeks (genotype 2), the average SVR was 60%, albeit higher in patients with genotype 2 (80% versus 52%), most patients required combined treatment with erythropoietin. Independent predictors of SVR were pretreatment viral load and rapid virologic response.Citation64
Recently, 12 HCV-positive (kidney and liver) transplant recipients treated with PEG-IFN-α plus ribavirin were studied; no acute rejection was observed, renal function remained stable during and after discontinuing treatment, and there was no allograft dysfunction. Two patients had a partial virologic response and four had SVR; these data suggest that combination therapy does not increase the risk of acute kidney graft rejection after liver-kidney transplantation.Citation65
Combined antiviral treatment with PEG-IFN and ribavirin is suggested in HCV-infected patients with CKD grades 1 or 2, as in the non CKD population. Ribavirin doses should be titrated according to patient tolerance and in accordance with the 2008 KDIGO guidelines ().Citation20
Acute HCV therapy in patients with ESRD
HCV progresses to chronic infection in 90% of uremic patients. Monitoring aminotransferase levels in the HD population may facilitate the detection of acute viral infections. In terms of the management of acute HCV infection, some studies have evaluated therapy based on IFN-α for 12 weeks and reported that 67% of patients with acute hepatitis C achieved SVR compared with 0% of without treatment patients.Citation66
A recent meta-analysisCitation67 determined that virologic response after antiviral therapy was more common than spontaneous viral clearance in dialysis patients with acute hepatitis; also, IFN-based treatment of acute hepatitis C in dialysis populations yielded SVR in 50% of cases.
Treatment of chronic HCV in kidney transplant candidates
Evidence regarding standard IFN therapy in HD patients shows that 38% of patients achieved SVR; of these, 76% were transplanted and received immunosuppressive therapy with antithymocyte globulin, and viremia was absent in 100% of patients 22.5 months after transplantation.Citation68
The use of IFN before renal transplantation may decrease the occurrence of de novo or recurrent glomerulonephritis. An additional benefit of pretransplant antiviral therapy is a decreased incidence of renal interstitial fibrosis and tubular atrophy, since HCV infection has been implicated in its pathogenesis. Antiviral therapy can also decrease the incidence of post-transplant diabetes mellitus in the graft recipient.
HPVG has recently been proposed as a parameter to determine whether well compensated cirrhotic patient can be considered for block transplantation or only kidney transplantation. The consensus suggests that patients with a HPVG <10 mmHg should be candidates only for renal transplant.Citation34,Citation69
A systematic review and meta-analysis suggest that HCV patients who remain on HD have 2.19 times greater risk of death when compared with those who undergo kidney transplantation; eight patients need to be transplanted to prevent one death, particularly in HCV patients aged 45 years or older.Citation47
Patients with chronic hepatitis C should be treated with standard therapy or PEG-IFN. If there is no early viral response, treatment should be discontinued and patients should be referred to a specialist in hepatology for a second evaluation. Moreover, if an early viral response is obtained, waiting is recommended for at least 28 days after administration of IFN prior to kidney transplantation.Citation20–Citation34
Treatment of patients with HCV after kidney transplantation
It has been postulated that the immune stimulating effects of IFN can promote allograft rejection in patients who receive a kidney transplant. Hassan et al recently evaluated 12 HCV-positive liver-kidney transplant recipients treated with PEG-IFN-α plus ribavirin. No acute rejection was observed, renal function remained stable during and after discontinuing treatment, there was no allograft dysfunction, two patients had a partial viral response, and four had SVR. These data suggest that combination therapy did not foster a higher risk of acute kidney graft rejection after liver-kidney transplantation.Citation70
Recently, Wei et alCitation71 conducted an updated meta-analysis to evaluate IFN-based antiviral therapy in HCV infection after renal transplantation. The overall comparative SVR rates in PEG-IFN-based and standard IFN-based therapy were 40.6% and 20.9%, respectively. The most frequent side effect requiring discontinuation of treatment was graft dysfunction (occurring in 45% of cases), demonstrating the limited safety and efficacy of IFN-based antiviral therapy for HCV infection after kidney transplantation.
Based on the KDIGO guidelines (),Citation20 IFN therapy should be considered in patients at high risk of graft loss, like fibrosing cholestatic hepatitis or threatening life vasculitis. If immunosuppressive agents are used with new antiviral drugs such as protease inhibitors, like telaprevir and boceprevir, the risk of drug toxicity is increased.Citation72
Kidney donor with positive serology for HCV
Several studies have established that HCV-positive transplant recipients receiving organs from HCV-positive donors suffer from higher rates of liver disease but not lower survival rates when compared with patients who receive organs from HCV-negative donors.Citation73 HCV can be transmitted from an infected donor to the recipient, and there are factors that influence the transmission of HCV infection, such as viral load.
Only 29% of HCV-positive recipients are transplanted with HCV-positive kidneys. The kidneys are discarded 2.5 times more often due to the sense that HCV-positive kidneys may adversely compromise recipient liver function. Despite the slightly increased risk, a national study has suggested that there is likely to be a survival benefit in most HCV-positive patients transplanted from an HCV-positive kidney compared with waiting for an HCV-negative organ.Citation74 Recently, Kucirka et al analyzed 6,250 patients with HCV who had undergone a kidney transplant and were captured in the United Network for Organ Sharing (UNOS) database. They recorded the liver-related outcomes and found that 1% of the HCV-positive recipients eventually enter to the liver transplant waiting list over a 13-year study period. Those who received HCV-positive kidneys had a 2.6-fold higher hazards ratio of enrolling in the liver transplant list (P≤0.0001). They concluded that transplantation of an HCV-positive kidney may decrease the recipient’s time on the list by over a year, which is a better option than waiting for an HCV-negative kidney, due to the high risk of kidney-related mortality while awaiting transplantation.Citation75
Morales et alCitation76 compared the outcomes in kidney transplant recipients (HCV-positive) who received a graft from an HCV-positive donor with those of patients (HCV positive) who received a kidney graft from an HCV-negative donor. They found no significant difference in patient survival at 5 and 10 years (84.8% at 5 years and 72.7% at 10 years versus 86.6% and 76.7%, respectively). Decompensated liver disease rates were also not significantly different between the two groups.
Transplanting kidneys from HCV-positive organ donors into HCV-positive/RNA-negative recipients leads to greater viral reactivation than in those HCV-positive/RNA-positive recipient.Citation77 Thus, many transplant centers have adopted the policy of transplanting HCV-positive kidneys into HCV-positive/RNA-positive patients or those with active viremia.Citation78 The type of HCV genotype might not have a significant impact on survival in patients with ESRD, since survival in patients with mixed genotypes was similar to that of patients with a single genotype.Citation79
New therapies for HCV in patients with ESRD?
In May 2011, the US Food and Drug Administration (FDA) approved the NS3/4A protease inhibitors, boceprevir and telaprevir, for the treatment of HCV genotype 1, marking the beginning of an era of direct-acting antiviral agents. Protease inhibitors such as boceprevir and telaprevir in combination with IFN and ribavirin (triple therapy) have become another new management strategy for HCV genotype 1 infection, whereby up to 75% of previously untreated patients with HCV genotype 1 have achieved SVR. However, these new drugs have not been studied in patients with renal impairment. The protease inhibitor simeprevir has recently been licensed, others including faldaprevir, asunaprevir, vaniprevir, and ritonavir-boosted ABT-450 are currently in phase II or phase III studies. Boceprevir, telaprevir, and simeprevir are all metabolized in the liver, and renal clearance contributes minimally to the elimination of these drugs. Therefore, it is not expected that renal impairment will have an important influence on the pharmacokinetics of HCV protease inhibitors. No clinically significant difference in the pharmacokinetic parameters of boceprevir was observed between patients with ESRD and healthy subjects, so there appears to be no dose adjustment required.Citation80
Recently, Durmortier et al reported four ESRD patients with HCV (most commonly genotype 1b) who did not respond to a prior course of PEG-IFN and ribavirin; while awaiting kidney transplantation, they received a second-line antiviral regimen of PEG-IFN, ribavirin, and telaprevir. After 12 weeks of therapy, tolerance was acceptable and HCV-RNA became undetectable in three of the four patients. The dose of ribavirin ranged from 200 mg three times per week to 200 mg/day, and the severity of liver fibrosis ranged from grade 1 to grade 3.Citation81
The pharmacokinetic parameters for simeprevir were also not influenced by creatinine clearance, and no dose adjustments were necessary in patients with mild, moderate, or severe renal impairment, but there is no clear evidence on its safety and efficacy in patients with ESRD or in those on HD.Citation82
Oral nucleotide inhibitors of the HCV nonstructural protein 5B, such as sofosbuvir, have proven activity against all HCV genotypes. Sofosbuvir was approved by the FDA in 2013 for use in combination with ribavirin for the treatment of HCV genotypes 2 and 3 or in combination in PEG-IFN and ribavirin for infection in genotypes 1 and 4.Citation82 Sofosbuvir is eliminated by the kidneys, not require dose adjustment in early grades of CKD; however, there is no current dosing recommendation for patients with ESRD.Citation83
No adjustment of boceprevir dosage is required for patients with impaired renal function, but despite this observation, a paucity of studies evaluating standard combination therapy suggests that routine use of this combination should not be applied in the population with advanced renal failure.Citation84
With the development of sofosbuvir and the more recent drugs, there will be promising IFN-free and ribavirin-free therapy regimens, but unfortunately there are no clinical trials studying patients with HCV and associated CKD. However, we believe that, in the near future, regimens with higher success rates and less severe adverse effects, especially in this particular population of patients, will be available. To date, there are no studies on the efficacy and safety of these new agents in organ recipients, including kidney transplant recipients.
Conclusion
Despite the screening of blood products, nosocomial HCV transmission continues to occur in HD units. HCV infection decreases the survival of patients and grafts. Treatment for HCV in patients with CKD is complicated, but achieving SVR can decrease post-transplant complications. Kidney transplantation alone must be considered in patients with compensated HCV-positive with cirrhosis and a HPVG <10 mmHg. Patients with ESRD who remain on HD are at higher risk of death when compared with those who receive a kidney graft. Kidneys obtained from HCV-positive donors and transplanted into HCV-positive recipients may be useful in expanding the donor pool by increasing the rate of utilization of these kidneys. Antiviral treatment can improve renal function in patients with HCV-associated glomerulopathy. New antiviral therapies needs to be evaluated to confirm the role of treatment in the ESRD HCV-positive population.
Disclosure
The authors report no conflicts of interest in this work.
References
- FinelliLMillerJTokarsJArduimoMNational surveillance of dialysis-associated diseases in the United StatesSemin Dial200518526115663766
- FissellRBBragg-GreshamJLWoodsJDPatterns of hepatitis C prevalence and seroconversion in hemodialysis units from three continents: the DOPPSKidney Int2004652335234215149347
- JadoulMPoignetJLGeddesCThe changing epidemiology of hepatitis C virus (HCV) infection in haemodialysis: European multi-centre studyNephrol Dial Transplant20041990490915031348
- WreghittTBlood–borne virus infections in dialysis units – a reviewRev Med Virol1999910110910386337
- AmiriZShakibAToorchiMSeroprevalence of hepatitis C and risk factors in haemodialysis patients in Guilan, Islamic Republic of IranEast Mediterr Health J20051137237616602456
- SypsaVPsichogiouMKatsoulidouAIncidence and patterns of hepatitis C virus seroconversion in a cohort of hemodialysis patientsAm J Kidney Dis20054533434315685512
- Kalantar-ZadehKKilpatrickRDMcAllisterCJHepatitis C virus and death risk in hemodialysis patientsJ Am Soc Nephrol2007181584159317429053
- NakayamaEAkibaTMarumoFSatoCPrognosis of anti-hepatitis C virus antibody-positive patients on regular hemodialysis therapyJ Am Soc Nephrol2000111896190211004221
- FabriziFMartinPDixitVBunnapradistSDulaiGMeta-analysis: effect of hepatitis C virus infection on mortality in dialysisAliment Pharmacol Ther2004201271127715606388
- LegendreCGarrigueVLe BihanCHarmful long-term impact of hepatitis C virus infection in kidney transplant recipientsTransplantation1998656676709521201
- MathurinPMouquetCPoynardTImpact of hepatitis B and C virus on kidney transplantation outcomeHepatology1999292572639862875
- FabriziFPoordadFMartinPHepatitis C infection and the patient with end stage renal diseaseHepatology20023631012085342
- MartinPFabriziFHepatitis C virus and kidney diseaseJ Hepatol20084961362418662838
- FabriziFLamperticoPLunghiGManganoSAucellaFMartinPReview article: hepatitis C virus infection and type-2 diabetes mellitus in renal diseases and transplantationAliment Pharmacol Ther20052162363215771749
- SezerSOzdemirFAkcayAAratZBoyaciogluSHaberalMRenal transplantation offers a better survival in HCV-infected ESRD patientsClin Transplant20041861962315344970
- Al-FreahMZeinoZHeneghanMManagement of hepatitis C in patients with chronic kidney diseaseCurr Gastroenterol Rep201214788622161023
- PatelPThompsonNKallenAArduinoMEpidemiology, surveillance, and prevention of hepatitis C virus infections in hemodialysis patientsAm J Kidney Dis20105637137820570422
- TokarsJFrankMAlterMArduinoMNational surveillance of dialysis-associated diseases in the United States, 2000Semin Dial20021516217112100454
- GoharSKhalilRElaishNKhedrEAhmedMPrevalence of antibodies to hepatitis C virus in hemodialysis patients and renal transplant recipientsJ Egypt Public Health Assoc19957046548417214170
- Kidney Disease: Improving Global Outcomes (KDIGO)KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney diseaseKidney Int Suppl2008Suppl 109S1S99
- [No authors listed]Recommendations for preventing transmission of infections among chronic hemodialysis patientsMMWR Recomm Rep200150143
- GordonCEUhligKLauJSchmidCHLeveyASWongJBInterferon treatment in hemodialysis patients with chronic hepatitis C virus infection: a systematic review of the literature and meta-analysis of treatment and efficacy and harmsAm J Kidney Dis20085126327718215704
- LiuCHLiangCCHuangKWTransient elastography to assess hepatic fibrosis in hemodialysis chronic hepatitis C patientsClin J Am Soc Nephrol201161057106521393486
- FabriziFTakkoucheBLunghiGDixitVMessaPMartinPThe impact of hepatitis C virus infection on survival in dialysis patients: meta-analysis of observational studiesJ Viral Hepat20071469770317875004
- OkudaKYokosukaONatural history of chronic hepatitis C in patients on hemodialysis: case control study with 4–23 years of follow upWorld J Gastroenterol2004102209221215259067
- TrevizoliJEde Paula MenezesRRibeiro VelascoLFHepatitis C is less aggressive in hemodialysis patients than in nonuremic patientsClin J Am Soc Nephrol200831385139018650408
- FurusyoNHayashiJAriyamaIMaintenance hemodialysis decreases serum hepatitis C virus (HCV) RNA levels in hemodialysis patients with chronic HCV infectionAm J Gastroenterol20099549049610685756
- FabriziFDixitVMessaPImpact of hepatitis C on survival in dialysis patients: a link with cardiovascular mortality?J Viral Hepat20121960160722863263
- AzevedoHAVillela-NogueiraCAPerezRMSimilar HCV viral load levels and genotype distribution among end-stage renal disease patients on hemodialysis and HCV infected with normal renal functionJ Nephrol20072060961617918148
- FabriziFMessaPMartinPImpact of hemodialysis therapy on hepatitis C virus infection: a deeper insightInt J Artif Organs20093211119241358
- RostaingLPeresCTkaczukJEx vivo flow cytometry determination of intracytoplasmic expression of IL-2, IL-6, IFN-gamma and TNF alpha in monocytes and T-lymphocytes in chronic hemodialysis patientsAm J Nephrol200020182610644863
- WangSMLiuHChouCHuangCShihCChenWMortality in hepatitis C-positive patients treated with peritoneal dialysisPerit Dial Int20082818318718332455
- BloomRDLakeJREmerging issues in hepatitis C virus positive liver and kidney transplant recipientsAm J Transplant200662232223716869798
- MoralesJMAguadoJMHepatitis C and renal transplantationCurr Opin Organ Transplant20121760961523111646
- RothDReddyKRKupinWLong-term impact of HCV on clinical outcomes and liver histology in kidney recipientsAm J Transplant2004A478289
- ParameshASDavisJYMallikarjunCKidney transplantation alone in ESRD patients with hepatitis C cirrhosisTransplantation20129425025422790385
- CamposSParsikiaAZakiRFOrtizJAKidney transplantation alone in ESRD patients with hepatitis C cirrhosisTransplantation2012946566
- JustaSMintzRMintzMSerial measurements of hepatitis C viral load by real-time polymerase chain reaction among recipients of living-donor renal transplants: a short-term follow-up study from a single centerTransplant Proc2010423568357321094817
- RothDGaynorJReddyKEffect of kidney transplantation on outcomes among patients with hepatitis CJ Am Soc Nephrol2011221152116021546575
- AlricLDi-MartinoVSelvesJLong-term impact of renal transplantation on liver fibrosis during hepatitis C virus infectionGastroenterology20021231494149912404224
- ManuelOBaid-AgrawalSMoradpourDPascualMImmunosuppression in hepatitis C virus-infected patients after kidney transplantationContrib Nephrol2012769710722310785
- TothCPascualMChungRHepatitis C virus-associated fibrosing cholestatic hepatitis after renal transplantation: response to interferon-alpha therapyTransplantation199866125412589825826
- SiddiquiARAbbasZLuckNHExperience of fibrosing cholestatic hepatitis with hepatitis C virus in kidney transplant recipientsTransplant Proc20124472172422483477
- CimsitBAssisDCaldwellCSuccessful treatment of fibrosing cholestatic hepatitis after liver transplantationTransplant Proc20114390590821486625
- StokesMImmune complex glomerulonephritis in patients with hepatitis CSaudi J Kidney Dis Transpl20001139640418209331
- Vallet-PichardAFontaineHMalletVPolSViral hepatitis in solid organ transplantation other than liverJ Hepatol20115547448221241754
- IngsathitAKamanamoolNThakkinstianASumethkulVSurvival advantage of kidney transplantation over dialysis in patients with hepatitis C: a systematic review and meta analysisTransplantation20139594394823425817
- EspinosaMMartin-MaloAAlvarez de LaraMARisk of death and liver cirrhosis in anti-HCV-positive long term hemodialysis patientsNephrol Dial Transplant2001161669167411477172
- SolezKColvinRBRacusenLCBanff´05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’)Am J Transplant2007751852617352710
- FabriziFDixitVMessaPMartinPInterferon monotherapy of chronic hepatitis C in patients: meta-analysis of clinical trialsJ Viral Hepat200815798818184190
- ChanTWuPLauJLokALaiCChengIInterferon treatment for hepatitis C virus infection in patients on haemodialysisNephrol Dial Transplant199712141414199249778
- LiuCLiangCLinJPegylated interferon alpha 2a versus standard interferon alpha-2a for treatment-naive dialysis patients with chronic hepatitis C: a randomised studyGut20085752553017881538
- GordonCUhligKLauJSchmidCLeveyAWongJInterferon for hepatitis C virus in hemodialysis – an individual patient meta-analysis of factors associated with sustained virological responseClin J Am Soc Nephrol200941449145819643927
- CovicAMafteiIMardareNAnalysis of safety and efficacy of PEGylated interferon alpha 2a in hepatitis C virus positive hemodialysis patients: results from a large, multicenter auditJ Nephrol20061979480117173254
- WangKLXingHQZhaoHEfficacy and tolerability of low dose interferon-α in hemodialysis patients with chronic hepatitis C virus infectionWorld J Gastroenterol2014204071407524744598
- FabriziFDixitVMessaPMartinPInterferon monotherapy of chronic hepatitis C in patients: meta-analysis of clinical trialsJ Viral Hepat200815798818184190
- LiuCLiangCLinJPegylated interferon alpha-2a versus standard interferon alpha-2a for treatment naive dialysis patients with chronic hepatitis C: a randomised studyGut20085752553017881538
- BruchfeldAStahleLAnderssonJSchvarezRRibavirin treatment in dialysis patients with chronic hepatitis C virus infection – a pilot studyJ Viral Hepat2001828729211454181
- TanABrouwerJGluePSafety of interferon and ribavirin therapy in haemodialysis patients with chronic hepatitis C: results of a pilot studyNephrol Dial Transplant20011619319511209032
- MousaDAbdallaAAl-ShoailGAl-SulaimanMAl-HawasFAl-KhaderAAlpha interferon with ribavirin in the treatment of hemodialysis patients with hepatitis CTransplant Proc2004361831183415350490
- FabriziFDixitVMartinPMessaPCombined antiviral therapy of hepatitis C virus in dialysis patients: meta analysis of clinical trialsJ Viral Hepat201118e253e269
- RendinaMSchenaACastellanetaNThe treatment of chronic hepatitis C with peginterferon alfa 2a (40 kDa) plus ribavirin in haemodialysed patients awaiting renal transplantJ Hepatol20074676877417383045
- DjordjevićVKostićSStefanovićVTreatment of chronic hepatitis C with interferon alpha in patients on maintenance hemodialysisNephron1998792292319647511
- LiuCLiangCLiuCPegylated interferon alfa 2 plus low dose ribavirin for the retreatment of dialysis chronic hepatitis C patients who relapsed from prior interferon monotherapyGut20095831431619136527
- HassanQRocheBBuffetCLiver-kidney recipient with chronic viral hepatitis C treated with interferon-alphaTranspl Int20122594194722882335
- Al-HarbiAMalikGSubaityYMansyHAbutalebNTreatment of acute hepatitis C virus infection with alpha interferon in patients with hemodialysisSaudi J Kidney Dis Transpl20051629329717642795
- FabriziFDixitVMessaPMartinPInterferon therapy of acute hepatitis C in dialysis patients: meta-analysisJ Viral Hepat20121978479123043385
- KamarNToupanceOBuchlerMSandres-SauneKIzopetJDurandDEvidence that clearance of hepatitis C virus RNA after interferon therapy in dialysis patients in sustained after renal transplantationJ Am Soc Nephrol2003122092209812874463
- RothDBloomRSelection and management of hepatitis C-virus infected patients for the kidney transplant waiting listContrib Nephrol2012176667622310782
- HassanQRocheBBuffetCSessadeTSamuelDCharpentierBDurrbachALiver-kidney recipient with chronic viral hepatitis C treated with interferon-alphaTranspl Int20122594194722882335
- WeiFLiuFHuHRenHHuPInterferon based antiviral therapy for hepatitis C virus infection after renal transplantation: an update meta-analysisPLoS One20149111
- GargVvan HeeswijkRLeeJEAlvesKNadkarniPLuoXThe effect of telaprevir on the pharmacokinetics of cyclosporine and tacrolimusHepatology201154202721618566
- WoodsideKJIdshiharaKTheisenJEUse of kidneys from hepatitis C seropositive donors shortens waitlist time but does not alter one-yr outcomeClin Transplant20031743343714703926
- KucirkaLMSingerALRosRAUnderutilization of hepatitis C-positive kidneys for hepatitis C positive recipientsAm J Transplant2010101238124620353475
- KucirkaLMPetersTGSegevDLImpact of donor hepatitis C virus infection status on death and need for liver transplant in hepatitis C virus-positive kidney transplant recipientsAm J Kidney Dis20126011212022560841
- MoralesJMCampistolJMDomínguez-GilBLong term experience with kidney transplantation from hepatitis C-positive donors into hepatitis C-positive recipientsAm J Transplant2010102453246220977636
- MoralesJMCampistolJMCastellanoGTransplantation of kidneys from donors with hepatitis C antibody into recipients with pre-transplantation anti-HCVKidney Int1995472362407537343
- VerouxPVerouxMSparacinoVKidney transplantation from donors with viral B and C hepatitisTransplant Proc20063899699816757242
- NatovSNLauJYRuthazerRSchmidCHLeveyASPereiraBJHepatitis C virus genotype does not affect patient survival among renal transplant candidates. The New England Organ Bank Hepatitis C Study GroupKidney Int19995670070610432411
- TreitelMMarburyTPrestonRASingle-dose pharmacokinetics of boceprevir in subjects with impaired hepatic or renal functionClin Pharmacokinet20125161962822799589
- DurmortierJGuillaudOGagnieuMCAnti-viral triple therapy with telaprevir in haemodialysed HCV patients: is it feasible?J Clin Virol20135614614923149155
- US Food and Drug AdministrationSovaldi prescribing information2013 Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/205123Orig1s000ClinPharmR.pdfAccessed July 16, 2014
- Sovaldi (sofosvubir) [package insert]Foster City, CA, USAGilead Sciences, Inc2013
- GhanyMNelsonDStraderDThomasDSeeffLAn update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guidelines by the American Association for the Study of Liver DiseasesHepatology2011541433144421898493
- GordonCEBalkEMBeckerBNKDOQI US Commentary on the KDIGO clinical practice guideline for the prevention diagnosis, evaluation, and treatment of hepatitis C in CKDAm J Kidney Dis200852815825
