Abstract
Background
Sarcoidosis is a chronic multisystem disease of unknown etiology characterized by noncaseating granulomas that most often involves the lungs, but frequently has extrapulmonary manifestations, which might be difficult to treat in individual patients.
Objective
To review different disease manifestations, focusing on extrapulmonary organ systems, and to provide treatment options for refractory cases.
Materials and methods
We performed a literature search using Medline and Google Scholar for individual or combined keywords of “sarcoidosis, extrapulmonary, treatment, kidney, neurosarcoidosis, cardiovascular, gastrointestinal, transplantation, musculoskeletal, rheumatology, arthritis, and skin”. Peer-reviewed articles, including review articles, clinical trials, observational trials, and case reports that were published in English were included. References from retrieved articles were also manually searched for relevant articles.
Results and conclusion
Isolated involvement of a single organ or organ system is rare in sarcoidosis, and thus all patients must be thoroughly evaluated for additional disease manifestations. Cardiac sarcoidosis and neurosarcoidosis may be life-threatening. Clinicians need to assess patients comprehensively using clinical, laboratory, imaging, and histopathological data to recommend competently the best and least toxic treatment option for the individual patient.
Introduction
“Sarcoidosis would be a benign and relatively unimportant disease but for three troublesome complications: pulmonary fibrosis, fibrotic uveitis, and nephrocalcinosis.” This notion by James et al,Citation1 published in the Lancet in 1967, illustrates that physicians treating sarcoidosis have been aware of its frequent extrapulmonary disease manifestations for decades. Sarcoidosis is a chronic inflammatory disorder of unknown etiology, characterized by noncaseating granulomas involving the lungs in more than 90% of patients.Citation2 Ocular, lymph-node, and cutaneous manifestations are next in frequency, but any organ system can be affected.Citation2 Extrapulmonary disease manifestations contribute to significant morbidity, but can easily be missed. The ACCESS study revealed that 50% of the 736 sarcoidosis patients studied had pulmonary sarcoidosis and coexisting extrapulmonary sarcoidosis compared to only 2% of patients who presented with isolated extrapulmonary disease.Citation3 Prevalence of extrapulmonary sarcoidosis was higher among African-Americans in comparison to Caucasians.Citation3 Remission may occur in nearly two-thirds of patients after 1–2 years; still, up to a third of patients will have chronic or progressive disease.Citation4
The diagnosis requires exclusion of other etiologies, and relies on compatible history, clinical findings, and noncaseating granulomas on histology. In patients with normal chest radiographs, evaluation and monitoring of extrapulmonary sarcoidosis with other diagnostic modalities, including ultrasound, chest computed tomography, gallium scintigraphy, magnetic resonance imaging (MRI), and positron-emission tomography (PET), can be helpful.Citation2
Despite the lack of evidence-based studies, glucocorticosteroids (GCs), namely prednisone, are the first-line therapy of sarcoidosis. Recommended starting doses vary between 20 and 40 mg/day, and are tapered slowly to a maintenance dose of less than 10 mg/day as the disease allows. However, lack of response or drug toxicity are limiting factors, especially with long-term treatment, and can result in significant disability. GC-sparing drugs, such as disease-modifying antisarcoid drugs or biological agents, are increasingly used in these instances.Citation5–Citation7 Clinicians are increasingly initiating methotrexate (MTX) along with GCs in clinical profiles that portend a poor prognosis.Citation8
Extrapulmonary disease manifestations
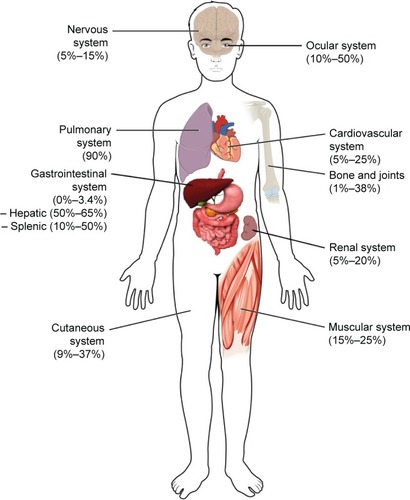
This review focuses on cutaneous, renal, central nervous system (CNS), cardiac, gastrointestinal, and musculoskeletal manifestations, though other organ systems not covered in this review can be involved. The following sections include the estimated frequencies, clinical presentation, relevant diagnostic tests, and management recommendations for the respective organ systems based on the current available evidence and the authors’ experience. gives an overview of the reported frequencies of manifestations of extrapulmonary sarcoidosis.
Figure 1 Estimated frequencies of extrapulmonary organ involvement.
Cutaneous sarcoidosis
Cutaneous lesions occur in 9%–37% of patients with extrapulmonary sarcoidosis.Citation9 Both specific skin lesions with the presence of granulomas and nonspecific skin lesions lacking granulomatous inflammation can be histologically detected in sarcoidosis.Citation10 One study found that 20.5% of 170 patients with sarcoidosis had erythema nodosum (EN), a nonspecific skin lesion lacking granulomas, as the most common skin manifestation.Citation11 Also detected were skin plaques (4.3%), subcutaneous nodules (4.3%), maculopapular eruptions (3.7%), scar lesions (2.9%), psoriasiform plaques (0.9%), and lupus pernio (2.7%).Citation11 The last, an indolent violaceous skin lesion affecting the face, had a higher association with pulmonary disease when compared to patients with EN or other lesions.Citation11 Cutaneous sarcoidosis has also been documented to develop in scars and tattoos.Citation12,Citation13 The presence of EN is frequently found in Löfgren’s syndrome, the acute form of sarcoidosis with bilateral hilar lymphadenopathy and ankle (peri)arthritis, and usually portends a good prognosis.Citation10 In addition, scarring and nonscarring alopecia,Citation14 as well as nail changes (such as onycholysis, dystrophy, hyperkeratosis, and longitudinal ridging) may occur. Interestingly, nail changes seem to indicate bone involvement, and appropriate workup should therefore be considered. The World Association of Sarcoidosis and Other Granulomatous Diseases has proposed various end points for cutaneous sarcoidosis to be assessed in clinical trials (Physician Global Assessment, Sarcoidosis Activity and Severity Index, Lupus Pernio Activity and Severity Index, photographs, lesion counts, and skin biopsies).
Treatment of cutaneous sarcoidosis
Cutaneous lesions are treated only if they cause significant cosmetic disfigurement, psychological effects, or if the lesions are uncontrollable. Local GC therapy, with intralesional injections for thick plaque lesions, is first-line treament.Citation15 Triamcinolone acetonide has reasonable availability, potency, and duration of action. Patients with progressive lesions will nevertheless require systemic therapy, most often GCs or hydroxychloroquine. Cases of lupus pernio achieved considerable improvement with halobetasol propionate 0.05% ointment twice daily for 10 weeks.Citation16 Mild improvements were seen in 72% of 54 patients, and near or complete resolution was observed in 20% with systemic GCs.Citation17 Antimalarials, antibacterials (tetracyclines, antimycobacterials), and immunosuppressive agents (MTX, thalidomide, apremilast, tumor necrosis factor alpha (TNFα) antagonists, such as infliximab and adalimumab, among others) have been investigated with variable success in refractory cases.Citation18–Citation26
Renal involvement
Although isolated renal involvement represents less than 5% of extrapulmonary sarcoidosis,Citation2 the rate of renal involvement throughout the disease course is probably much higher.Citation27 Sarcoidosis can involve the kidneys in three different ways: 1) hypercalciuria, which precedes the development of hypercalcemia, occurring in 50% and 10%, respectively, as a result of calcitriol-producing granulomatous burden, which may overwhelm the kidneys’ regulatory mechanisms and progress to renal insufficiency; 2) tubulointerstitial nephritis with or without granulomatous involvement; and 3) nephrocalcinosis and/or nephrolithiasis, which can occur as a result of hypercalcemia. These can occur independently or less frequently in combination.Citation28 Co-occurrence with other renal disease, primarily IgA nephropathy, is not uncommon.Citation28 Although sarcoidosis-associated end-stage renal disease is very uncommon and more likely due to other causes, a significant degree of renal insufficiency was common in one retrospective series, and hypercalcemia might be a contributing factor.Citation29,Citation30 Moreover, relapses may occur in up to a quarter of patients who undergo kidney transplantation because of sarcoidosis and may negatively impact graft survival.Citation31
One cohort study revealed that up to 3.6% of sarcoidosis cases had nephrolithiasis at first visit, and another 2.7% of these patients were asymptomatic.Citation32 Therefore, sarcoidosis should be considered if no other cause of nephrolithiasis is identified. Rare presentations include pseudotumors or mechanical complications, such as hydronephrosis, if retroperitoneal structures are affected by granulomatous inflammation.Citation33–Citation35
All patients diagnosed with sarcoidosis should be evaluated for the presence of renal involvement to prevent significant chronic kidney disease. Workup includes serum creatinine, blood urea nitrogen, estimated glomerular filtration rate, protein and calcium in both serum and urine, and screening of the urinary sediment for casts of red or white blood cells. 25-hydroxvitamin D3, 1,25-dihydroxyvitamin D3, and parathyroid hormone should be measured in patients with hypercalcemia or hypercalciuria to assess the degree and cause of calcium dysregulation. Renal biopsy remains the gold standard to confirm renal sarcoidosis. Nevertheless, histological findings are not specific for sarcoidosis, requiring infection and drug hypersensitivity to be excluded, as these are more frequent causes of interstitial nephritis.Citation36
Treatment of renal sarcoidosis
GCs are the mainstay of treatment in interstitial nephritis, and are often very effective.Citation36 They have also been used successfully in hypercalcemia and hypercalciuria, although many times discontinuation of vitamin D supplements is sufficient to normalize calcium levels.Citation30 When GCs fail, ketoconazole or hydroxychloroquine have been used successfully in such cases.Citation37 Additional immunosuppressive agents, such as azathioprine (AZA) or mycophenolate mofetil, have (albeit rarely) also been used in cases of granulomatous interstitial nephritis not responding to GCs.Citation37
The long-term prognosis of renal sarcoidosis seems to correlate with response to therapy after 1 month of treatment and inversely with the initial fibrosis score on histopathological examination.Citation29 In refractory renal sarcoidosis or failure of treatment leading to end-stage renal disease, renal transplantation is an option.Citation38 Recurrence of sarcoidosis in transplanted kidneys has been reported and requires close surveillance, as is necessary in other diseases. Relapse after transplantation has been managed with infliximab in a steroid-resistant case.Citation39
Neurosarcoidosis
Involvement of the CNS or peripheral nervous system is present in 5%–15% of cases, and represents one of the more serious complications of sarcoidosis.Citation40 Postmortem studies indicate that isolated neurosarcoidosis occurs in ~17% of cases and that subclinical neurosarcoidosis may be more prevalent but underdiagnosed, with a rate of antemortem diagnosis of ~50% of cases.Citation4,Citation41 Symptoms relating to involvement of the cranial nerves, hypothalamus, or pituitary glands have been reported to be the initial clinical manifestation in up to 50%–70% of neurosarcoidosis cases.Citation42 These can be present simultaneously in up to a third of patients or be isolated manifestations.Citation42 A biopsy of the CNS is required for a definitive diagnosis, especially in isolated cases.Citation43 However, in cases with biopsy-proven sarcoidosis of other sites, the diagnosis can be made without a CNS biopsy. The WASOG criteria for neurosarcoidosis are shown in .
Table 1 The WASOG organ-assessment tool for neurosarcoidosis
The most common neurological manifestation is unilateral or bilateral cranial neuropathy of the facial or optic nerves.Citation40,Citation44 Cranial nerve palsy may be caused by nerve granulomas, increased intracranial pressure, or granulomatous basal meningitis. Moreover, acute or chronic aseptic meningitis may occur as well. Space-occupying cerebral lesions of sarcoidosis can involve the parenchyma and extradural or subdural spaces.Citation45 These lesions are most commonly found in the hypothalamus and pituitary glands, and may result in endocrine manifestations, including diabetes insipidus, adrenal and pituitary failure, and amenorrhea–galactorrhea syndrome.Citation46–Citation48 The significance of seizures on the overall prognosis is not clear, since reports have yielded conflicting results.Citation49,Citation50 Psychiatric manifestations, such as psychosis, may also be present, but are quite rare, whereas cognitive failure was more prevalent in one study.Citation51,Citation52 Spinal cord involvement is another rare manifestation, and usually presents with leg weakness and paresthesias; most often, the thoracic segments are involved.Citation53
Involvement of the peripheral nervous system may present with deficits of either sensory or motor function, and possibly both. Symptoms ranging from mononeuritis multiplex to Guillain–Barré-like syndromes, as well as polyradiculopathy or polyneuropathy, can occur. Electromyography often shows a multifocal axonal neuropathy pattern.Citation54
Small-fiber neuropathy (SFN) has been frequently reported in sarcoidosis over the past few years, and can easily be missed. Symptoms include pain and dysesthesia.Citation55 Standard nerve-conduction testing to measure large-nerve function and quantitative techniques for assessment of small-nerve fibers are not done routinely in all sarcoidosis patients. If collected carefully, skin-punch biopsy is helpful in diagnosing SFN by showing lower intraepidermal nerve-fiber density.Citation56
A definitive diagnosis of neurosarcoidosis is challenging, due to the difficulty of obtaining biopsies without causing any damage. Therefore, brain MRI is considered the most sensitive noninvasive test for neurosarcoidosis, but lacks specificity.Citation45 Lumbar punctures are reported to be normal in up to a third of patients, pathologic cerebrospinal fluid (CSF) findings include mild pleocytosis, increased protein levels, and mild hypoglycorrhachia, which are similar to those seen in multiple sclerosis or systemic lupus erythematosus.Citation45 Additionally, the usefulness of CSF angiotensin-converting enzyme (ACE) levels is still a matter of debate, because it can also be found in conditions other than sarcoidosis.Citation45,Citation57,Citation58
Treatment of neurosarcoidosis
Patients with confirmed systemic sarcoidosis and testing suggestive of CNS involvement should be treated immediately, due to the potentially high morbidity and mortality of neurosarcoidosis. GCs are first-line treatment, with a typical initial dose of 1 mg/kg/day. Severe cases may warrant a pulse of methylprednisolone at doses of 500–1,000 mg/day for 3 consecutive days and subsequent oral tapering. Alternative treatments, such as MTX, AZA, cyclosporine A (CsA), and cyclophosphamide (CYC), have been successfully used in refractory disease or when adverse effects to GCs are significant, but this is based on experience rather than evidence.Citation59 The response rate to MTX in neurosarcoidosis is nearly 60%, similar to that in pulmonary sarcoidosis.Citation60 CsA was found to reduce the GC dose to 30%–58% of the initial stabilization dose, but nonetheless deterioration occurred in four of the six reported patients.Citation61 The use of short-course pulse CYC given every 2–4 weeks demonstrated improvement of MRI or CSF abnormalities in seven of seven patients, and symptoms improved in four of seven and nine of ten patients in the respective studies.Citation60,Citation62 The dose used was initially 500 mg, then 750 mg for the second dose and a maintenance dose of 1 g given approximately every 3 weeks.Citation60,Citation62 Mycophenolate mofetil has been reported to be effective in CNS sarcoid, but not in sarcoid myopathy,Citation63 but this case series was small, and firm conclusions cannot be drawn.Citation63
In corticosteroid-refractory disease or when rapid response is needed to avoid permanent disability, several case reports have indicated positive responses to infliximab.Citation64 A review of 34 successful cases showed that 14 had improvement between the first and third infusions,Citation64 and infliximab might be more effective than CYC.Citation65 TNFα blockers also had positive effects on cognitive failure.Citation52 One double-blind randomized controlled trial found a positive effect of ARA 290, an immunomodulating erythropoietin-derived compound, on symptoms of SFN in 22 patients.Citation66 Lastly, radiation therapy with 20–25 Gy may be considered in patients who do not respond to drug therapy.Citation67
Cardiac sarcoidosis
Cardiac sarcoidosis commonly coexists with systemic sarcoidosis, rather than as an isolated finding. The presence of ocular sarcoidosis with choroidal involvement has, for example, been suggested to reflect vascular endothelial dysfunction and coexist with cardiac disease, thus requiring evaluation for cardiac sarcoidosis.Citation68
The ethnic background of patients is of importance, since cardiac sarcoidosis has been reported much more frequently in Japanese patients (~21%–58%) when compared to Caucasians or African-Americans (~13%–25%) based on autopsy studies.Citation69 There is a high rate of subclinical involvement, making overt cardiac sarcoidosis a rather rare manifestation.Citation3
Cardiac disease ranges from the incidental discovery in asymptomatic patients to life-threatening disease with sudden cardiac death. Patients commonly present with heart block, ventricular tachycardia/unsustained ventricular fibrillation, or heart failure due to cardiomyopathy.Citation70
History-taking is an essential element at both baseline and follow-up visits to detect symptoms that warrant further investigation.Citation71 Any structural component, as well as any layer (endocardium [heart valves], myocardium, and pericardium) of the heart can be affected. Clinical symptoms will depend on the structures involved, and may include palpitations, syncope, shortness of breath, chest pain, or peripheral edema.Citation69,Citation72 With the widespread use of implantable cardioverter defibrillators (ICDs), the main causes of death in patients with cardiac sarcoidosis is progressive heart failure due to granulomatous infiltration of the myocardium, the latter accounting for ~25% of deaths.Citation73,Citation74
The possibility of cardiac sarcoidosis should be considered in: 1) adults under 55 years of age with new-onset electrocardiogram abnormalities, decreased systolic ejection fraction, or cardiac symptoms after excluding coronary artery disease; 2) adults under 55 years of age with sustained atrioventricular block (second or third degree); 3) patients of any age with sustained ventricular tachycardia and/or cardiomyopathy; and 4) all patients with diagnosed sarcoidosis of other sites.Citation73,Citation75,Citation76 The different proposed diagnostic criteria for cardiac sarcoidosis are shown in .
Table 2 Criteria in use for the assessment of cardiac sarcoidosis
Workup commonly includes an electrocardiogram, 24-hour Holter monitoring and transthoracic echocardiography (TTE). The optimal diagnostic strategy still needs to be established; however, patients with cardiac sarcoidosis had significantly more abnormal Holter monitoring and TTE findings.Citation71 The sensitivity and specificity for the detection of cardiac sarcoidosis by Holter monitoring were reported with 67% and 80%, respectively, making its usefulness controversial, but it is relatively inexpensive.Citation77
TTE shows variable and nonspecific findings, including interventricular thinning, regional wall abnormalities, or aneurysmal distortion. These findings are similar to those found in ischemic heart disease; therefore, coronary angiography is recommended to exclude coronary artery disease.Citation69
Over the last few years, other noninvasive imaging modalities have emerged and been tested in cardiac sarcoidosis. Cardiac MRI (CMR) and 18F-fluorodeoxyglucose PET (FDG-PET) have high sensitivity and specificity and are of prognostic relevance.Citation78–Citation80 Especially, patients with late gadolinium enhancement detected on CMR are at risk for adverse events, even with preserved left ventricular ejection fraction.Citation81
Radionuclide imaging with gallium 67, thallium 201, technetium, or sestamibi are alternative tests to diagnose and monitor cardiac sarcoidosis that are useful when MRI is contraindicated or not available.Citation82 Nevertheless, due to radiation exposure and associated imaging artifacts that may produce nonspecific results, they have been replaced with CMR and FDG-PET in most centers.Citation82
Treatment of cardiac sarcoidosis
Cardiac sarcoidosis mandates rapid treatment, because of the risk of sudden death and progressive heart failure. GCs are the treatment of choice, and no difference in survival rates has been found between higher (>30 mg/day) and lower doses (<30 mg/day) of prednisone.Citation83 A recent systematic review found beneficial effects in the reported literature only for atrioventricular conduction disturbances.Citation84 The body of evidence is of very limited quality, since controlled trials for cardiac sarcoidosis have not been conducted.
Long-term improvement has been reported with the use of 6 mg MTX weekly in addition to prednisone versus prednisone alone. Ejection fraction and N-terminal prohormone brain natriuretic peptide levels were significantly better with combination therapy at 3 years, but not at 5 years.Citation85
Other alternative treatments, including MTX, CYC and CsA, have been studied retrospectively, and resulted in improvement or recovery in ten of eleven patients with cardiac sarcoidosis.Citation86 Infliximab has been reported as an effective therapy for cardiac sarcoidosis in case studies.Citation87,Citation88 TNFα blockers are, however, contraindicated in patients with advanced heart failure (New York Heart Association III or IV), as they may worsen symptoms.
Antiarrhythmic therapies have not been studied systematically, and firm recommendations cannot be made based on the available evidence. Amiodarone or sotalol are sometimes used.Citation69 Sudden cardiac death can be prevented by ICDs, and approximately a third of patients with ICDs receive appropriate therapies for ventricular arrhythmias.Citation89 The expert-consensus statement on management of arrhythmias associated with sarcoidosis by the Heart Rhythm SocietyCitation90 recommends ICD implantation in patients with: 1) spontaneous sustained ventricular arrhythmias, including prior cardiac arrest, and/or 2) left ventricular ejection fraction <35% despite optimal medical therapy and a period of immunosuppression (if there is active inflammation).Citation90
Ultimately, heart transplantation may be considered when all other therapies have failed, apparently without increased complications.Citation91,Citation92 Recurrence of sarcoidosis in the allograft has been reported, but seems to be rare.Citation93
Gastrointestinal and hepatic sarcoidosis
Gastrointestinal sarcoidosis is very rare, accounting for only 0%–3.4% of cases, based on autopsy studies. By contrast, autopsy frequently shows hepatic involvement in as many as 80%, depending on the series.Citation94 However, asymptomatic elevation in liver-function tests in the context of known sarcoidosis is the most common presentation in approximately a third of patients, and occurs more frequently in African-Americans than in Caucasians.Citation95 Of note, alkaline phosphatase seems to be more consistently elevated than aminotransferases in patients with hepatic sarcoidosis.Citation95 Clinical manifestations include hepatomegaly, fatigue, right upper-quadrant abdominal pain with pruritus (5%–15%), fever, jaundice, and weight loss (less than 5%).Citation10 Rare complications include Budd–Chiari syndrome or hilar lymphadenopathy, cholestatic liver disease mimicking primary biliary cirrhosis, primary sclerosing cholangitis, or obstructive jaundice.Citation96,Citation97
Splenic involvement is most often detected by imaging rather than symptoms or laboratory abnormalities, and has been reported with similar frequency as hepatic involvement. It may present with constitutional symptoms and marked splenomegaly in up to 6% of cases.Citation98,Citation99
Overt gastric or intestinal sarcoidosis presents with fullness, weight loss, protein-losing enteropathy, and signs of obstruction or gastrointestinal bleeding, findings also seen in Crohn’s disease.Citation100,Citation101 It is thus important to rule out other common granulomatous disorders that affect the intestines, as both have been reported to coexist in rare cases.Citation102,Citation103
A diagnosis of gastrointestinal or hepatosplenic involvement can be made by endoscopy, computed tomography, or ultrasound with or without contrast media and subsequent biopsy.Citation104–Citation106 Abdominal computed tomography shows hepatomegaly or nodules, and ultrasonography can reveal hypo- or hyperechoic nodules when compared to the liver parenchyma.Citation106 Noncaseating granulomas of the liver are present in up to 65% of patients in systemic sarcoidosis, and other causes, such as tuberculosis, fungal, or other infections, and drug toxicity, should be excluded.Citation107
Treatment of gastrointestinal sarcoidosis
Asymptomatic hepatic and splenic sarcoidosis does not require treatment. However, in symptomatic patients, GCs can reduce liver and spleen size and number of granulomas and improve organ function to some extent. Nevertheless, the effect on disease course and development of portal hypertension or hepatic fibrosis is limited.Citation108 Steroid-sparing agents have been used with some success, and agents such as MTX, AZA, antimalarials, and ursodeoxycholic acid all have been used.Citation109 The latter has been used in patients presenting with signs of cholestatic jaundice.Citation110,Citation111 AZA is preferred over MTX by some, although both are potentially hepatotoxic.Citation107
In end-stage liver disease, orthotopic liver transplantation has been successfully used as a treatment modality. Recurrences may occur in the allograft,Citation112 and allograft-survival rates have been reported to be comparable with other diseases (86% after 5 years).Citation113 Another study showed significantly lower rates compared to patients transplanted for primary sclerosing cholangitis or primary biliary cirrhosis (5-year graft-survival rates of 60% versus 75%).Citation114
Splenectomy is indicated only in rare situations after failure of medical treatment. These include massive splenomegaly, severe hypersplenism, suspicion of lymphoma or other malignancy, and as a preventive measure against splenic rupture.Citation115
Musculoskeletal sarcoidosis
Musculoskeletal sarcoidosis is reported in approximately 15%–25% of cases.Citation10 From a clinical perspective, differentiation of arthritis in acute/chronic and peripheral/axial types seems most useful. Acute arthritis in the context of sarcoidosis most frequently arises in Löfgren’s syndrome (bilateral hilar adenopathy, EN, and bilateral ankle swelling). Ultrasound-based studies have revealed that true arthritis of the ankles is the exception, and swelling is predominantly due to soft-tissue swelling and tenosynovitis.Citation116,Citation117 This has been confirmed in a small case series using MRI.Citation118 The presence of three of four criteria – 1) symmetrical ankle arthritis, 2) symptoms less than 2 months, 3) age below 40 years, and 4) EN reaching sensitivity and specificity of 93% and 99%, respectively – is used for diagnosing acute arthritis related to sarcoidosis.Citation119
Chronic sarcoidosis arthritis can mimic other forms of inflammatory arthritis; dactylitis, a feature typical of psoriatic arthritis and other seronegative spondylarthropathies, may also occur in sarcoidosis.Citation120 Conventional radiographs show a reticular, lacy pattern of the phalanges with singular or numerous cystic lesions, and in some cases, acroosteolysis may be observed and “punched-out lesions” due to granulomatous inflammations can occur.Citation121 A comprehensive assessment to differentiate other possible causes of arthritis is necessary, since sarcoidosis and other inflammatory diseases may simultaneously be present.Citation121
Axial sarcoidosis can involve the vertebral bodies or the sacroiliac joints. The frequency of sacroiliitis, the hallmark of seronegative spondylarthropathies, ranges from 6.6% to14.3% in the literature,Citation122,Citation123 and it may not always be possible to distinguish these two entities.Citation123 A negative human leukocyte antigen B27, however, would favor a diagnosis of sarcoidosis, especially when other sarcoidosis features are present.
Imaging is an important tool for the evaluation of musculoskeletal sarcoidosis. Conventional radiographs are typically the first method. To detect inflammatory changes, ultrasound may be used, but this has mostly been studied in the context of Löfgren’s syndrome.Citation116,Citation117 Other, potentially useful tests include MRI and FDG-PET.Citation124 When vertebral sarcoidosis is suspected and no other features of sarcoidosis are present, a histopathological confirmation is mandatory to exclude other possible causes, especially malignancy or tuberculosis, as these cannot be differentiated with imaging alone.
Treatment of musculoskeletal sarcoidosis
For the acute (peri)arthritis seen in Löfgren’s syndrome, nonsteroidal analgesics may be all that is necessary, but up to two-thirds of patients will nevertheless require additional treatment with GCs.Citation125 However, the chances of complete remission after a few weeks to months are very high in Löfgren’s syndrome.Citation10
Chronic arthritis may or may not be controlled with GCs alone. If GCs fail to control the symptoms, antimalarials and disease-modifying antisarcoid drugs have both been used with success, but controlled clinical trials for sarcoidosis arthritis have not been conducted. MTX is typically the preferred agent for these patients. In severe cases, TNFα antagonists, such as infliximab or adalimumab, may also be used. A management algorithm for patients unresponsive or intolerant to GCs has been proposed.Citation121
Conclusion
Extrapulmonary involvement is often underdiagnosed antemortem, but can be life-threatening if CNS or cardiac sarcoidosis occur. Subclinical involvement is common, making history-taking and a standardized review of systems essential. Diagnostic tests and appropriate imaging should be guided by history and clinical findings, and biopsies performed if necessary to exclude other causes of organ dysfunction.
The majority of patients will have either spontaneous remission or resolution within 1–2 years. However, up to a third of cases relapse, remain unresponsive, or progress despite treatment with GCs. These cases are considered to have refractory or chronic sarcoidosis. Guidelines are available for many of the manifestations, but firm treatment recommendations cannot be made, due to the lack of high-quality studies. Our suggested treatment approach for various extrapulmonary manifestations of sarcoidosis is depicted in .
Figure 2 Proposed management algorithm for extrapulmonary manifestations of sarcoidosis.
Abbreviations: ADA, adalimumab; AZA, azathioprine; CNS, central nervous system; CsA, cyclosporine A; CYC, cyclophosphamide; ESRD, end-stage renal disease; GCs, glucocorticoids; HCQ, hydroxychloroquine; HTx, heart transplantation; ICD, implantable cardioverter defibrillator; INF, infliximab; LTx, liver transplantation; MMF, mycophenolate mofetil; MTX, methotrexate; RTx, renal transplantation; THA, thalidomide; UDC, ursodeoxycholic acid; NSAID, non-steroidal anti-inflammatory drugs.
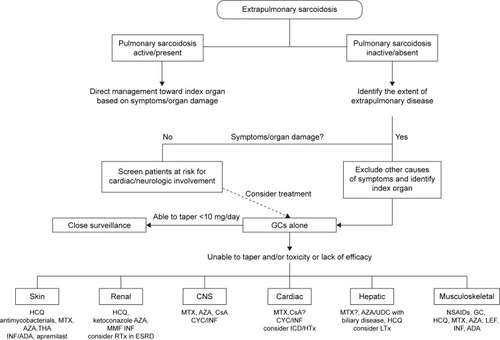
Adding or initiating disease-modifying antisarcoid drugs is necessary for many manifestations, and MTX is usually the first agent used by most sarcoidosis experts. Third-line or experimental therapies may be considered if other options fail. Ultimately, organ transplantation is a viable option for cardiac, renal, and liver sarcoidosis unresponsive to medical therapy, and outcomes seem to be comparable to other allograft recipients.
Acknowledgments
The work was supported by a grant from the Bernie Mac Foundation.
Disclosure
The authors report no conflicts of interest in this work.
References
- JamesDGCarstairsLSTrowellJSharmaOPTreatment of sarcoidosis: report of a controlled therapeutic trialLancet1967275155265284166889
- ValeyreDPrasseANunesHUzunhanYBrilletPYMüller-QuernheimJSarcoidosisLancet201438399231155116724090799
- BaughmanRPTeirsteinASJudsonMAClinical characteristics of patients in a case control study of sarcoidosisAm J Respir Crit Care Med200116410 Pt 11885188911734441
- HunninghakeGWCostabelUAndoMATS/ERS/WASOG statement on sarcoidosisSarcoidosis Vasc Diffuse Lung Dis199916214917310560120
- KorstenPMirsaeidiMSweissNJNonsteroidal therapy of sarcoidosisCurr Opin Pulm Med201319551652323884295
- KorstenPStrohmayerKBaughmanRPSweissNJRefractory pulmonary sarcoidosis: proposal of a definition and recommendations for the diagnostic and therapeutic approachClin Pulm Med2016232677526973429
- BaughmanRPGruttersJCNew treatment strategies for pulmonary sarcoidosis: antimetabolites, biological drugs, and other treatment approachesLancet Respir Med201531081382226204816
- WijsenbeekMSCulverDATreatment of sarcoidosisClin Chest Med201536475176726593147
- MangasCFernández-FiguerasMTFitéEFernández-ChicoNSàbatMFerrándizCClinical spectrum and histological analysis of 32 cases of specific cutaneous sarcoidosisJ Cutan Pathol2006331277277717177936
- JudsonMAExtrapulmonary sarcoidosisSemin Respir Crit Care Med20072818310117330194
- YanardağHPamukONKarayelTCutaneous involvement in sarcoidosis: analysis of the features in 170 patientsRespir Med200397897898212924527
- AntonovichDDCallenJPDevelopment of sarcoidosis in cosmetic tattoosArch Dermatol2005141786987216027303
- DittlerELBoeck’s sarcoid in postoperative abdominal scarN Y State J Med19545419273813194153
- HouseNSWelshJPEnglishJCSarcoidosis-induced alopeciaDermatol Online J20121884
- CallenJPIntralesional corticosteroidsJ Am Acad Dermatol1981421491517217384
- KhatriKAChotzenVABurrallBALupus pernio: successful treatment with a potent topical corticosteroidArch Dermatol19951315617618
- StagakiEMountfordWKLacklandDTJudsonMAThe treatment of lupus pernio: results of 116 treatment courses in 54 patientsChest2009135246847618812454
- VeienNKBrodthagenHCutaneous sarcoidosis treated with methotrexateBr J Dermatol1977972213216911683
- DrakeWPOswald-RichterKRichmondBWOral antimycobacterial therapy in chronic cutaneous sarcoidosis: a randomized, single-masked, placebo-controlled studyJAMA Dermatol201314991040104923863960
- BaughmanRPJudsonMAIngledueRCraftNLLowerEEEfficacy and safety of apremilast in chronic cutaneous sarcoidosisArch Dermatol2012148226226422004880
- BaughmanRLoKGuzzoCBarnathanEEfficacy of infliximab in improving lupus pernio, in patients receiving treatment for chronic pulmonary sarcoidosisJ Am Acad Dermatol2006543AB13
- PariserRJPaulJHiranoSToroskyCSmithMA double-blind, randomized, placebo-controlled trial of adalimumab in the treatment of cutaneous sarcoidosisJ Am Acad Dermatol201368576577323276549
- DroitcourtCRybojadMPorcherRA randomized, investigator-masked, double-blind, placebo-controlled trial on thalidomide in severe cutaneous sarcoidosisChest201414641046105424945194
- SteenTEnglishJCOral minocycline in treatment of cutaneous sarcoidosisJAMA Dermatol2013149675876023783160
- JonesECallenJPHydroxychloroquine is effective therapy for control of cutaneous sarcoidal granulomasJ Am Acad Dermatol1990233 Pt 14874892212149
- BaughmanRPJudsonMALowerEEInfliximab for chronic cutaneous sarcoidosis: a subset analysis from a double-blind randomized clinical trialSarcoidosis Vasc Diffuse Lung Dis Off J WASOG World Assoc Sarcoidosis Granulomatous Disord324289295
- BergnerRHoffmannMWaldherrRUppenkampMFrequency of kidney disease in chronic sarcoidosisSarcoidosis Vasc Diffuse Lung Dis200320212613212870722
- LöfflerCLöfflerUTuleweitAWaldherrRUppenkampMBergnerRRenal sarcoidosis: epidemiological and follow–up data in a cohort of 27 patientsSarcoidosis Vasc Diffuse Lung Dis201531430631525591142
- MahévasMLescureFXBoffaJJRenal sarcoidosis: clinical, laboratory, and histologic presentation and outcome in 47 patientsMedicine (Baltimore)20098829810619282700
- BaughmanRPJanovcikJRayMSweissNLowerEECalcium and vitamin D metabolism in sarcoidosisSarcoidosis Vasc Diffuse Lung Dis201330211312024071882
- AouizerateJMatignonMKamarNRenal transplantation in patients with sarcoidosis: a French multicenter studyClin J Am Soc Nephrol20105112101210820671220
- RizzatoGColomboPNephrolithiasis as a presenting feature of chronic sarcoidosis: a prospective studySarcoidosis Vasc Diffuse Lung Dis19961321671728893387
- DuveauASayeghJBeloncleFMoreauASubraJFAugustoJFPseudotumours: an atypical presentation of renal sarcoidosisQJM20131061094794923667183
- MiyazakiETsudaTMochizukiASarcoidosis presenting as bilateral hydronephrosisIntern Med19963575795828842767
- GodinMFillastreJPDucastelleTHemetJMorerePNouvetGSarcoidosis. Retroperitoneal fibrosis, renal arterial involvement, and unilateral focal glomerulosclerosisArch Intern Med19801409124012427406624
- KorstenPMüllerGAInterstitial nephritis in rheumatic diseasesZ Rheumatol2015744290299 German25962450
- HildersonIVan LaeckeSWautersADonckJTreatment of renal sarcoidosis: is there a guideline? Overview of the different treatment optionsNephrol Dial Transplant201429101841184710.1093/ndt/gft44224235078
- GöbelUKettritzRSchneiderWLuftFThe protean face of renal sarcoidosisJ Am Soc Nephrol200112361662311181812
- SrivastavaSRajakariarRAshmanNRafteryMBrownHMartinJEInfliximab as long-term maintenance in steroid-resistant and recurrent sarcoidosis in a renal transplant with central nervous system involvementClin Kidney J201251535526069750
- SternBJKrumholzAJohnsCScottPNissimJSarcoidosis and its neurological manifestationsArch Neurol19854299099173896208
- IwaiKTachibanaTTakemuraTMatsuiYKitaichiMKawabataYPathological studies on sarcoidosis autopsy. I. Epidemiological features of 320 cases in JapanActa Pathol Jpn1993437–83723768372682
- NozakiKJudsonMANeurosarcoidosis: clinical manifestations, diagnosis and treatmentPresse Méd2012416 Pt 2e331e34822595777
- ZajicekJPScoldingNJFosterOCentral nervous system sarcoidosis – diagnosis and managementQJM199992210311710209662
- PawateSMosesHSriramSPresentations and outcomes of neurosarcoidosis: a study of 54 casesQJM2009102744946019383611
- HoitsmaEFaberCGDrentMSharmaOPNeurosarcoidosis: a clinical dilemmaLancet Neurol20043739740715207796
- MurialdoGTamagnoGEndocrine aspects of neurosarcoidosisJ Endocrinol Invest200225765066212150344
- TamagnoGMurialdoGAmenorrhea-galactorrhea syndrome as an uncommon manifestation of isolated neurosarcoidosisAnn Ital Med Int200116426026611799635
- BullmannCFaustMHoffmannAFive cases with central diabetes insipidus and hypogonadism as first presentation of neurosarcoidosisEur J Endocrinol2000142436537210754478
- KrumholzASternBJSternEGClinical implications of seizures in neurosarcoidosisArch Neurol19914888428441898259
- JosephFGScoldingNJNeurosarcoidosis: a study of 30 new casesJ Neurol Neurosurg Psychiatry200980329730418977817
- O’BrienGMBaughmanRPBroderickJPArnoldLLowerEEParanoid psychosis due to neurosarcoidosisSarcoidosis199411134368036341
- ElfferichMDNelemansPJPondsRWDe VriesJWijnenPADrentMEveryday cognitive failure in sarcoidosis: the prevalence and the effect of anti-TNF-α treatmentRespir Int Rev Thorac Dis2010803212219
- SohnMCulverDAJudsonMAScottTFTaveeJNozakiKSpinal cord neurosarcoidosisAm J Med Sci2014347319519823364469
- ScottTSBrillmanJGrossJASarcoidosis of the peripheral nervous systemNeurol Res19931563893907907406
- HoitsmaEMarziniakMFaberCGSmall fibre neuropathy in sarcoidosisLancet200235993232085208612086764
- MyersMIPeltierACUses of skin biopsy for sensory and autonomic nerve assessmentCurr Neurol Neurosci Rep201313132323250768
- TahmoushAJAmirMSConnorWWCSF-ACE activity in probable CNS neurosarcoidosisSarcoidosis Vasc Diffuse Lung Dis200219319119712405488
- DaleJCO’BrienJFDetermination of angiotensin-converting enzyme levels in cerebrospinal fluid is not a useful test for the diagnosis of neurosarcoidosisMayo Clin Proc199974553510319092
- HoitsmaEDrentMSharmaOPA pragmatic approach to diagnosing and treating neurosarcoidosis in the 21st centuryCurr Opin Pulm Med201016547247920671516
- LowerEEBroderickJPBrottTGBaughmanRPDiagnosis and management of neurological sarcoidosisArch Intern Med199715716186418689290546
- SternBJSchonfeldSASewellCKrumholzAScottPBelendiukGThe treatment of neurosarcoidosis with cyclosporineArch Neurol19924910106510721329698
- DotyJDMazurJEJudsonMATreatment of corticosteroid-resistant neurosarcoidosis with a short-course cyclophosphamide regimenChest200312452023202614605084
- AndrodiasGMailletDMarignierRMycophenolate mofetil may be effective in CNS sarcoidosis but not in sarcoid myopathyNeurology201176131168117221444902
- LorentzenAOSvebergLMidtvedtØKertyEHeuserKOvernight response to infliximab in neurosarcoidosis: a case report and review of infliximab treatment practiceClin Neuropharmacol201437514214825229171
- SodhiMPearsonKWhiteESCulverDAInfliximab therapy rescues cyclophosphamide failure in severe central nervous system sarcoidosisRespir Med2009103226827318835147
- HeijLNiestersMSwartjesMSafety and efficacy of ARA 290 in sarcoidosis patients with symptoms of small fiber neuropathy: a randomized, double-blind pilot studyMol Med2012181430143623168581
- MenningerMDAmdurRJMarcusRBRole of radiotherapy in the treatment of neurosarcoidosisAm J Clin Oncol2003264e115e11812902908
- LiuDBirnbaumADUpdate on sarcoidosisCurr Opin Ophthalmol201526651251626448043
- DubreySWSharmaRUnderwoodRMittalTCardiac sarcoidosis: diagnosis and managementPostgrad Med J201591107738439426130811
- DubreySShahSHardmanTSharmaRSarcoidosis: the links between epidemiology and aetiologyPostgrad Med J201490106858258925230946
- MehtaDLubitzSAFrankelZCardiac involvement in patients with sarcoidosis: diagnostic and prognostic value of outpatient testingChest200813361426143518339784
- MorimotoTAzumaAAbeSEpidemiology of sarcoidosis in JapanEur Respir J200831237237917959635
- LynchJPHwangJBradfieldJFishbeinMShivkumarKTungRCardiac involvement in sarcoidosis: evolving concepts in diagnosis and treatmentSemin Respir Crit Care Med201435337239025007089
- SekiguchiMYazakiYIsobeMHiroeMCardiac sarcoidosis: diagnostic, prognostic, and therapeutic considerationsCardiovasc Drugs Ther19961054955108950063
- HamzehNSteckmanDASauerWHJudsonMAPathophysiology and clinical management of cardiac sarcoidosisNat Rev Cardiol201512527828825707386
- SoejimaKYadaHThe work-up and management of patients with apparent or subclinical cardiac sarcoidosis: with emphasis on the associated heart rhythm abnormalitiesJ Cardiovasc Electrophysiol200920557858319175448
- SuzukiTKandaTKubotaSImaiSMurataKHolter monitoring as a noninvasive indicator of cardiac involvement in sarcoidosisChest19941064102110247523035
- BlanksteinROsborneMTDorbalaSDi CarliMFCardiac positron emission tomography as a prognostic indicator of cardiac sarcoidosisJ Am Coll Cardiol20146323259024727253
- AhmadianABroganABermanJQuantitative interpretation of FDG PET/CT with myocardial perfusion imaging increases diagnostic information in the evaluation of cardiac sarcoidosisJ Nucl Cardiol201421592593924879453
- IseTHasegawaTMoritaYExtensive late gadolinium enhancement on cardiovascular magnetic resonance predicts adverse outcomes and lack of improvement in LV function after steroid therapy in cardiac sarcoidosisHeart2014100151165117224829369
- MurtaghGLaffinLJBeshaiJFPrognosis of myocardial damage in sarcoidosis patients with preserved left ventricular ejection fraction: risk stratification using cardiovascular magnetic resonanceCirc Cardiovasc Imaging201691e00373826763280
- KouranosVWellsAUSharmaRUnderwoodSRWechalekarKAdvances in radionuclide imaging of cardiac sarcoidosisBr Med Bull2015115115116326311504
- YazakiYIsobeMHiroeMPrognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisoneAm J Cardiol20018891006101011703997
- SadekMMYungDBirnieDHBeanlandsRSNeryPBCorticosteroid therapy for cardiac sarcoidosis: a systematic reviewCan J Cardiol20132991034104123623644
- NagaiSYokomatsuTTanizawaKTreatment with methotrexate and low-dose corticosteroids in sarcoidosis patients with cardiac lesionsIntern Med201453542743324583430
- Chapelon-AbricCde ZuttereDDuhautPCardiac sarcoidosis: a retrospective study of 41 casesMedicine (Baltimore)200483631533415525844
- Chapelon-AbricCSaadounDBiardLLong-term outcome of infliximab in severe chronic and refractory systemic sarcoidosis: a report of 16 casesClin Exp Rheumatol201533450951526120779
- BarnabeCMcMeekinJHowarthAMartinLSuccessful treatment of cardiac sarcoidosis with infliximabJ Rheumatol20083581686168718671332
- SchullerJLZipseMCrawfordTImplantable cardioverter defibrillator therapy in patients with cardiac sarcoidosisJ Cardiovasc Electrophysiol201223992592922812589
- BirnieDHSauerWHJudsonMAConsensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosisHeart2016102641141426743924
- ValantineHATazelaarHDMacoviakJCardiac sarcoidosis: response to steroids and transplantationJ Heart Transplant1987642442503312535
- TheofilogiannakosEKPettitSJGhaziARasslDLewisCParameshwarJHeart transplantation for advanced heart failure due to cardiac sarcoidosisSarcoidosis Vasc Diffuse Lung Dis201532320821426422565
- OniAAHershbergerRENormanDJRecurrence of sarcoidosis in a cardiac allograft: control with augmented corticosteroidsJ Heart Lung Transplant1992112 Pt 13673691576143
- HerculesHDBethlemNMValue of liver biopsy in sarcoidosisArch Pathol Lab Med1984108108318346548124
- VattiRSharmaOPCourse of asymptomatic liver involvement in sarcoidosis: role of therapy in selected casesSarcoidosis Vasc Diffuse Lung Dis199714173769186992
- AlamILevensonSDFerrellLDBassNMDiffuse intrahepatic biliary strictures in sarcoidosis resembling sclerosing cholangitis: case report and review of the literatureDig Dis Sci1997426129513019201098
- DelfosseVde LevalLDe RooverABudd-Chiari syndrome complicating hepatic sarcoidosis: definitive treatment by liver transplantation – a case reportTransplant Proc20094183432343419857764
- WarshauerDMDumbletonSAMolinaPLYankaskasBCParkerLAWoosleyJTAbdominal CT findings in sarcoidosis: radiologic and clinical correlationRadiology1994192193988208972
- BrittARFrancisIRGlazerGMEllisJHSarcoidosis: abdominal manifestations at CTRadiology1991178191941984330
- InomataMIkushimaSAwanoNUpper gastrointestinal sarcoidosis: report of three casesIntern Med201251131689169422790127
- MunkerMSharmaOPFatal gastrointestinal haemorrhage in sarcoidosis: a previously unreported occurrenceSarcoidosis19874155573495838
- ShethSPatelRBaddouraWNathanSMetastatic sarcoidosis of terminal ileum and colon: very rare, but important to differentiate from Crohn’s diseasePoster presented at: American College of Gastroenterology Annual Scientific MeetingOctober 17–22, 2014Philadelphia, PA
- SmiéjanJMCosnesJChollet-MartinSSarcoid-like lymphocytosis of the lower respiratory tract in patients with active Crohn’s diseaseAnn Intern Med1986104117213940500
- CaguiatKAbramowitzMDubrovskayaVRekaSGarciaRChenNNGastric sarcoidosis: a case reportPoster presented at: American College of Gastroenterology Annual Scientific MeetingOctober 17–22, 2014Philadelphia, PA
- TanaCDietrichCFSchiavoneCHepatosplenic sarcoidosis: contrast-enhanced ultrasound findings and implications for clinical practiceBiomed Res Int2014201492620325215299
- WarshauerDMLeeJKImaging manifestations of abdominal sarcoidosisAJR Am J Roentgenol20041821152814684507
- Modaresi EsfehJCulverDPlesecTJohnBClinical presentation and protocol for management of hepatic sarcoidosisExpert Rev Gastroenterol Hepatol20159334935825473783
- EbertECKiersonMHagspielKDGastrointestinal and hepatic manifestations of sarcoidosisAm J Gastroenterol20081031231843192 quiz 319318853979
- AyyalaUSPadillaMLDiagnosis and treatment of hepatic sarcoidosisCurr Treat Options Gastroenterol20069647548317081481
- AleneziBLamoureuxEAlpertLSzilagyiAEffect of ursodeoxycholic acid on granulomatous liver disease due to sarcoidosisDig Dis Sci200550119620015712660
- BécheurHDall’ostoHChatellierGEffect of ursodeoxycholic acid on chronic intrahepatic cholestasis due to sarcoidosisDig Dis Sci19974247897919125650
- FidlerHMHadziyannisSJDhillonAPSherlockSBurroughsAKRecurrent hepatic sarcoidosis following liver transplantationTransplant Proc1997295250925109270828
- LipsonEJFielMIFlormanSSKorenblatKMPatient and graft outcomes following liver transplantation for sarcoidosisClin Transplant200519448749116008593
- VanattaJMModanlouKADeanAGOutcomes of orthotopic liver transplantation for hepatic sarcoidosis: an analysis of the United Network for Organ Sharing/Organ Procurement and Transplantation Network data files for a comparative study with cholestatic liver diseasesLiver Transpl20111791027103421594966
- SharmaOPVucinicVJamesDGSplenectomy in sarcoidosis: indications, complications, and long-term follow-upSarcoidosis Vasc Diffuse Lung Dis2002191667012002389
- Le BrasEEhrensteinBFleckMHartungWEvaluation of ankle swelling due to Lofgren’s syndrome: a pilot study using B-mode and power Doppler ultrasonographyArthritis Care Res2014662318322
- KellnerHSpäthlingSHerzerPUltrasound findings in Löfgren’s syndrome: is ankle swelling caused by arthritis, tenosynovitis or periarthritis?J Rheumatol199219138411556697
- AnandacoomarasamyAPedutoAHoweGManoliosNSpencerDMagnetic resonance imaging in Löfgren’s syndrome: demonstration of periarthritisClin Rheumatol200726457257516897117
- VisserHVosKZanelliESarcoid arthritis: clinical characteristics, diagnostic aspects, and risk factorsAnn Rheum Dis200261649950412006321
- MatuszakJDurckelJSibiliaJLipskerDBlondetCImperialeAIs sarcoid dactylitis worse than we expect?Arthritis Rheumatol201668241726473297
- SweissNJPattersonKSawaqedRRheumatologic manifestations of sarcoidosisSemin Respir Crit Care Med201031446347320665396
- ErbNCushleyMJKassimosDGShaveRMKitasGDAn assessment of back pain and the prevalence of sacroiliitis in sarcoidosisChest2005127119219615653983
- KobakSSeverFInceOOrmanMThe prevalence of sacroiliitis and spondyloarthritis in patients with sarcoidosisInt J Rheumatol2014201428945424899899
- MostardRLPrompersLWeijersREF-18 FDG PET/CT for detecting bone and bone marrow involvement in sarcoidosis patientsClin Nucl Med2012371212522157023
- TejeraBHolgadoSMateoLPego-ReigosaJMCarniceroMOlivéALöfgren syndrome: a study of 80 casesMed Clin (Barc)20141434166169 Spanish24855899
- JudsonMACostabelUDrentMThe WASOG Sarcoidosis Organ Assessment Instrument: An update of a previous clinical toolSarcoidosis Vasc Diffuse Lung Dis2014311192724751450
- OpenStax College, Anatomy and PhysiologyOpenStax CNX Available from: http://cnx.org/contents/[email protected]
