Abstract
Prostacyclin is an endogenous eicosanoid produced by endothelial cells; through actions on vascular smooth-muscle cells, it promotes vasodilation. Pulmonary arterial hypertension (PAH) is characterized by elevated mean pulmonary artery pressure due to a high pulmonary vascular resistance state. A relative decrease in prostacyclin presence has been associated with PAH; this pathway has thus become a therapeutic target. Epoprostenol, the synthetic equivalent of prostacyclin, was first utilized as short-term or bridging therapy in the 1980s. Further refinement of its long-term use via continuous intravenous infusion followed. A randomized controlled trial by Barst et al in 1996 demonstrated functional, hemodynamic, and mortality benefits of epoprostenol use. This work was a groundbreaking achievement in the management of PAH and initiated a wave of research that markedly altered the dismal prognosis previously associated with PAH. Analogs of prostacyclin, including iloprost and treprostinil, exhibit increased stability and allow for an extended array of parenteral and non-parenteral (inhaled and oral) therapeutic options. This review further examines the pharmacology and clinical use of epoprostenol and its analogs in PAH.
Introduction
Pulmonary arterial hypertension (PAH) describes a particular subset of pulmonary hypertension fitting within Group 1 of the World Health Organization (WHO) classification system. Hemodynamically, patients exhibit elevation in mean pulmonary artery pressure (mPAP) of ≥25 mmHg – with normal pulmonary artery wedge pressure (PAWP) (≤15 mmHg), and elevation in pulmonary vascular resistance (PVR) of >3 Wood units.Citation1 On a histologic level, PAH is characterized by abnormal remodeling of the pulmonary vasculature that may be idiopathic, heritable, or related to certain disease processes including connective tissue disease, congenital heart disease, portal hypertension, and drug exposure. Significant left heart, pulmonary parenchymal, and/or chronic thromboembolic disease is absent; patients with pulmonary hypertension of these types fall into separate WHO groups. Similarities in clinical manifestations and hemodynamics may exist between patients with PAH and other forms of pulmonary hypertension; however all prostaglandin-related therapies, as discussed in this review, are currently approved only for patients with WHO Group 1 PAH.
Prior to the introduction of advanced therapies, survival in PAH was poor. Early registry data following idiopathic PAH patients demonstrated overall survival of 2.8 years, with median 6-month survival in those with functional class IV disease (severe functional limitations and symptoms with any activity).Citation2 Over the last 20 years three major classes of PAH therapies have emerged, with clear impact on the morbidity and mortality of the disease. Current registry data now supports 1- and 3-year survival of 91% and 74%, respectively, for patients with idiopathic or heritable PAH.Citation3 While only one individual randomized controlled trial has shown a mortality benefit,Citation4 meta-analyses have supported a statistically significant improvement in survival with the use of PAH-specific therapy.Citation5,Citation6
Choice of initial therapy for PAH depends on several factors, most importantly the WHO functional class of the patient. Similar to the New York Heart Association classification for congestive heart failure, WHO functional classes are graded I to IV, from most mild to most severe limitations. Recommended treatment algorithms from the Fifth World Symposium on Pulmonary Hypertension support initiating an oral agent for those with mild (WHO functional class II) symptoms. Options include phosphodiesterase type-5 inhibitors (PDE-5Is: sildenafil, tadalafil), the related soluble guanylate-cyclase stimulator riociguat, and the endothelin-receptor antagonists (ERAs: bosentan, ambrisentan, and macitentan).Citation7
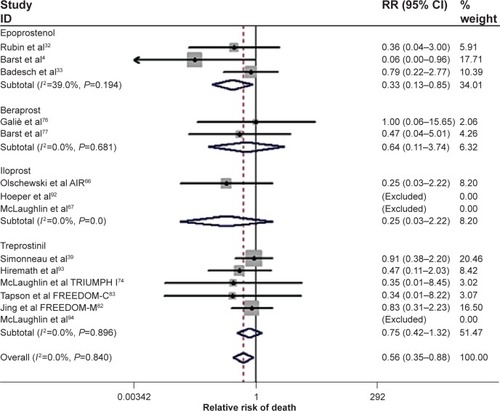
For patients with moderate to severe limitation (WHO functional class III or IV disease), prostacyclin analogs are often a key part of the treatment regimen. In the USA and Europe, options include intravenous epoprostenol, inhaled iloprost, and treprostinil, which can be administered via subcutaneous, intravenous, inhaled, and, most recently, oral routes. Combining data from all prostanoid trials, a recent meta-analysis supported improvements in mortality, clinical worsening, exercise capacity, and hemodynamics ().Citation8
Figure 1 Forest plot of randomized clinical trials utilizing prostanoid therapies: All cause mortality. Cumulative relative risk (RR) estimate of death in active treatment groups was compared with that in control groups, excluding non-event trials. No heterogeneity was found. Fixed effect model for combined effect size was adopted.
Abbreviations: RR, relative risk; CI, confidence interval; AIR, Aerosolized Iloprost Randomized study; TRIUMPH, TReprostinil sodium Inhalation Used in the Management of Pulmonary arterial Hypertension.
Prostanoid therapy in pulmonary arterial hypertension
Prostacyclin, or prostaglandin I2 (IP), is an endogenous eicosanoid produced by endothelial cells. Epoprostenol is the synthetic equivalent of prostacyclin, and treprostinil and iloprost are both stable synthetic analogs.
Deficiency of prostacyclin activity has long been identified as an important part of the pathobiology of PAH. Christman et al demonstrated decreased urinary excretion of prostacyclin metabolites in 34 patients with idiopathic or secondary pulmonary hypertension as compared with controls.Citation9 Loss of expression of prostacyclin synthase has also been observed in lung tissue of patients with PAH.Citation10
The primary target of prostacyclin and its analogs appears to be the IP receptor on vascular smooth-muscle cells. Activation of other vasodilatory prostaglandin receptors, such as EP2 and EP4 (prostaglandin E2 and E4 receptors, respectively), has also been observed, although this is of unclear importance.Citation11,Citation12 After prostacyclin binds target receptors on smooth-muscle cells, intracellular signaling leads to adenylate cyclase activation and an increase in cyclic adenosine monophosphate levels. Resulting smooth-muscle relaxation with vasodilation is one of the primary actions of prostacyclin. However, the important effects of the prostanoids expand well beyond pure vasodilation, targeting the pathologic vascular remodeling observed in PAH. Treprostinil and iloprost have been shown to suppress pulmonary artery smooth-muscle proliferation in vitro.Citation13,Citation14 Additional prostanoid effects include inhibition of platelet aggregation,Citation15 inhibition of inflammation,Citation16,Citation17 and augmentation of ventricular inotropy.Citation18
The pharmacokinetics, dosing, efficacy, and pertinent adverse effects for each agent are discussed following. Clinical efficacy data is further summarized in .
Table 1 Major randomized controlled trials of prostanoid therapy in pulmonary arterial hypertension (PAH)
Intravenous epoprostenol
Epoprostenol, the synthetically produced formulation of prostacyclin, was the first medication to be approved for the treatment of PAH by the US Food and Drug Administration (FDA) in 1995. Epoprostenol sodium for intravenous therapy is marketed as Flolan® (GlaxoSmithKline plc, London, UK) and is also available generic (Teva Pharmaceutical Industries Ltd, Petah Tikva, Israel). Since 2008 a room-temperature stable (RTS) formulation of epoprostenol (Veletri®, Actelion Pharmaceuticals Ltd, Allschwil, Switzerland) has also been available.
Pharmacokinetics
The in vivo half-life of epoprostenol is less than 6 minutes, thus necessitating drug delivery by continuous intravenous infusion. Epoprostenol is rapidly broken down, spontaneously or enzymatically, into two very weakly active primary metabolites. Metabolites are primarily excreted in the urine.Citation19,Citation20
Once infused, traditional and RTS preparations of epoprostenol are regarded as equivalent from biologic and efficacy standpoints.Citation21–Citation23
Dosing and administration
Epoprostenol is administered by continuous intravenous infusion using an ambulatory, battery-operated infusion pump. The drug is supplied as a sterile powder (0.5 or 1.5 mg per vial) that must be reconstituted and diluted to the desired concentration with a sterile diluent specific to epoprostenol. At room temperature, reconstituted epoprostenol loses stability and cannot be used for longer than 8 hours. Thus, epoprostenol for injection is administered with cold pouches to maintain solution temperature at 2°C to 8°C, extending use time to 24 hours. An extended-stability RTS epoprostenol has been subsequently introduced; this formulation combines epoprostenol with arginine (as opposed to glycine as in traditional epoprostenol), resulting in a more alkaline pH after reconstitution and consequently greater RTS.Citation19,Citation20 RTS epoprostenol must be reconstituted in sterile water or sodium chloride; following reconstitution and dilution it is stable at room temperature for 48 hours.Citation20
Intravenous epoprostenol is initiated in a monitored hospital setting, with a recommended starting dose of 2 ng/kg/min and titration rates variable according to disease severity and side effects. Long-term dosing goals are similarly individualized. In one observational study of 178 patients started on intravenous epoprostenol, as an example, mean doses at 3 months and 1 year were 14 and 21 ng/kg/min, respectively.Citation24
Clinical efficacy
The first published experience with epoprostenol in PAH occurred in 1980; an 8-year-old female with severe idiopathic PAH was administered prostacyclin with pulmonary artery catheter in place. Doses up to 44 ng/kg/min reduced mPAP and PVR and improved cardiac index.Citation25 Previous to this, successful use of prostacyclin in a neonate with severe hypoxemia and persistent fetal circulation had been described.Citation26 Further series of acute use in adults with PAH followed.Citation27–Citation29
Early adoption of epoprostenol as a therapy was limited by administration challenges. Higenbottam et al first described a 27-year-old female with idiopathic PAH and severe symptoms who noted significant improvement in symptoms and hemodynamics with acute epoprostenol while hospitalized.Citation30 A tunneled catheter was placed with delivery of epoprostenol by electric infusion pump, and she was able to be discharged home with great improvement in quality of life. The further courses of this patient and nine others were described separately.Citation31 These patients received long-term (1–25 months) epoprostenol infusions, with demonstrated improvements in exercise capacity and symptoms.
In the first randomized trial of continuous intravenous epoprostenol, Rubin et al randomized 23 patients to receive epoprostenol or conventional therapy.Citation32 After 8 weeks, total pulmonary resistance decreased in the epoprostenol group (21.7 Wood units at trial start, to 13.9 Wood units at trial end), with no significant change seen in the conventional therapy group. Functional class improvements were also more frequent in the epoprostenol group, and there were three deaths among those receiving conventional therapy versus one death in those receiving epoprostenol.Citation32
The landmark trial of epoprostenol in PAH randomized 81 patients with functional class III or IV idiopathic PAH to epoprostenol plus conventional therapy or conventional therapy alone.Citation4 At 12 weeks, 6-minute walk distance (6MWD) improved by 31 meters in the epoprostenol group, and decreased by 29 meters in the conventional therapy group. There was a between-treatment decrease in mPAP (−6.7 mmHg) and PVR (−4.9 Wood units) and improvement in cardiac index in favor of epoprostenol. Benefits on functional class, dyspnea scores, and quality of life scores were also noted. Eight patients died in the conventional therapy group, versus none in the epoprostenol group (P=0.003).Citation4 A similar randomized trial in 111 patients with scleroderma-related PAH showed that epoprostenol has positive impact on 6MWD, hemodynamics, and functional class in this population as well.Citation33
Long-term data have also supported the benefits of intravenous epoprostenol. McLaughlin et al followed 162 patients with idiopathic PAH on epoprostenol for a mean of 36 months. Survival at 1, 2, and 3 years was 88%, 76%, and 63%, respectively, which was above expected survival based on registry data (59%, 46%, and 35%, respectively, P<0.001 for each comparison).Citation2,Citation34 A separate long-term study of 178 treated idiopathic PAH patients demonstrated improved mortality compared with controls.Citation24
Safety and tolerability
Adverse effects associated with intravenous epoprostenol include flushing, headache, jaw pain (typically described with the first bite of food), nausea/vomiting, diarrhea, and flu-like symptoms.Citation19,Citation20 During initiation and dose escalation, the acute development of pulmonary edema suggests an underlying diagnosis of pulmonary veno-occlusive disease and can be fatal.Citation24
The presence of a chronic indwelling catheter predisposes patients to additional risk, including infection and thrombosis. From the 12-week randomized study treating 41 patients with epoprostenol, there were four episodes of catheter-related sepsis and one episode of thrombosis with paradoxical embolus.Citation4 Long-term observational work has found catheter-related infection/sepsis rates of 0.14 to 0.19 per patient-year.Citation24,Citation34 Drug interruption due to pump or catheter malfunction is another serious concern – due to rebound severe pulmonary hypertension and short drug half-life, this complication can be fatal if not immediately recognized and managed.Citation34,Citation35
Subcutaneous and intravenous treprostinil
The poor stability and short plasma half-life of epoprostenol stimulated interest in developing more stable analogs. Treprostinil, a benzindine prostanoid, was initially studied for potential use in congestive heart failure,Citation36 transplantation,Citation37 and peripheral vascular disease.Citation38 Support for treprostinil’s utility in PAH was sealed with a large subcutaneous infusion clinical trial.Citation39
Parenteral treprostinil (Remodulin®, United Therapeutics Corporation, Silver Spring, MD, USA) is supplied for administration via subcutaneous or intravenous route. Subcutaneous treprostinil was approved by the FDA in 2002; with scientific support for bioequivalence, intravenous use of treprostinil was subsequently approved in 2004.
Pharmacokinetics
Subcutaneous treprostinil is rapidly absorbed with complete bioavailability.Citation40,Citation41 As a result of this documented bioequivalence, from an efficacy standpoint both routes of administration are considered similar, though important differences in risks and adverse effects remain. The relationship between infused dose and measured plasma concentration is linear in healthy volunteersCitation42 and in PAH patients treated intravenously or subcutaneously over a dosing range of 10 to 125 ng/kg/min.Citation43
With chronic intravenous or subcutaneous therapy, the plasma half-life of treprostinil has ranged from 2.9 to 4.6 hours, significantly longer than that of epoprostenol.Citation41,Citation42 Metabolism of treprostinil occurs primarily in the liver by CYP2C8, with the majority of metabolites inactive and excreted in the urine. Medication interactions with cytochrome P450 inhibitors or inducers should be noted. Rifampin, a CYPC28 inducer, decreases exposure to treprostinil, while the inhibitor gemfibrozil increases exposure.Citation44
Dosing and administration
Treprostinil sodium is supplied in vials containing four possible drug concentrations, from 1 to 10 mg/mL. For patients using subcutaneous therapy, treprostinil is continuously administered undiluted via a self-inserted subcutaneous catheter with an appropriate infusion pump. Undiluted treprostinil from a single syringe is used for up to 72 hours. For patients using intravenous therapy, treprostinil is diluted in sterile water, saline, or epoprostenol diluent, with administration similar to that of epoprostenol solution. After dilution, intravenous treprostinil solution at room temperature can be administered for up to 48 hours.Citation44
Parenteral treprostinil is initiated at a dose of 1.25 ng/kg/min, with dose escalation dependent on efficacy and side effects. For subcutaneous treprostinil, a rapid escalation plan (starting dose of 2.5 ng/kg/min, with increases of 2.5 ng/kg/min twice in the first week and then weekly or biweekly thereafter) led to greater improvements in exercise capacity at 12 weeks and actually decreased infusion-site pain.Citation45 Of patients involved in randomized clinical trials of subcutaneous treprostinil, those remaining on therapy at 1 and 3 years were treated with a mean dose of 26 and 42 ng/kg/min, respectively.Citation46 Escalation of intravenous treprostinil dosing can typically be achieved more rapidly. In 16 treatment-naïve patients initiating intravenous treprostinil, the mean dose was 41 ng/kg/min at 12 weeks, and 98 ng/kg/min at 48 weeks.Citation47
A novel, completely implantable delivery system for intravenous treprostinil has been under investigation, with prospects of improving patient convenience and safety.Citation48,Citation49
Clinical efficacy
The pivotal study of subcutaneous treprostinil in PAH was reported by Simonneau et al in 2002.Citation39 A total of 470 patients from 40 international centers were randomized to receive subcutaneous treprostinil, starting at a dose of 1.25 ng/kg/min, or infused placebo. The primary outcome was 6MWD at 12 weeks, with secondary outcomes including signs and symptoms of pulmonary hypertension, hemodynamics, dyspnea scores, and quality of life measures. After 12 weeks, the median between-treatment difference in 6MWD was 16 meters, in favor of treprostinil (95% confidence interval 4.4–27.6 meters, P=0.006). The mean treprostinil dose was 9.3 ng/kg/min, and there was a significant relationship between achieved treprostinil dose and improvement in 6MWD (those in the highest quartile of treprostinil dosing had a median 6MWD improvement of 36 meters). Secondary outcomes were also in favor of treprostinil.Citation39 Further analysis of the subset with connective tissue disease-associated PAH also confirmed benefits in this population.Citation50
An open-label study described outcomes of 860 PAH patients (including subjects from the randomized clinical trial) treated long-term with subcutaneous treprostinil. Survival at 1, 2, 3, and 4 years was 87%, 78%, 71%, and 68%, respectively, which was well above anticipated survival rates from historical data. Fifteen percent of patients had other PAH-specific agents added during the study period.Citation46 Another long-term study of 99 patients with PAH and 23 with chronic thromboembolic pulmonary hypertension (CTEPH) using subcutaneous treprostinil reported similar survival – 89% at 1 year and 71% at 3 years. Significant improvements in 6MWD (305–445 meters, P=0.0001), dyspnea scores, and functional class were noted.Citation51
Efficacy of intravenous treprostinil has also been independently supported by clinical studies. Tapson et al treated 16 PAH patients with open-label intravenous treprostinil (mean dose 41 ng/kg/min at end of study) over 12 weeks, with noted improvements in 6MWD, dyspnea scores, and hemodynamics.Citation52 Subjects were then followed on therapy for a 48-week period; further improvements in exercise capacity and hemodynamics were demonstrated. In the same study an additional 31 patients were transitioned from intravenous epoprostenol (mean baseline epoprostenol dose 41 ng/kg/min), of whom 23 remained on treprostinil at 1 year (mean treprostinil dose 111 ng/kg/min). Exercise parameters and hemodynamics were similar at 48 weeks compared with baseline on epoprostenol.Citation47
Safety and tolerability
Diarrhea, jaw pain, flushing, and edema were all more common with treprostinil in comparison to placebo.Citation39 Delivery system problems (pump malfunction or infusion-set complications) occurred in 30% during the long-term subcutaneous study; no serious complications developed as a result of these.Citation46
Infusion-site pain was common (85%, versus 27% with placebo) in patients administered subcutaneous treprostinil in the large randomized trial;Citation39 this led to drug discontinuation in 8%. In the long-term continuation study, site pain was responsible for almost all of the drug withdrawals due to adverse events (23% of the total study population).Citation46 Infusion-site pain and reactions can be decreased with appropriate site selection; as pain peaks in the first few days after a site change, use of a single site for 4 weeks or more can be helpful and safe. Additional topical and/or systemic therapies, including topical anesthetics and steroids, analgesics, and histamine antagonists, are often employed to improve tolerance.Citation53
While intravenous therapy eliminates the problem of infusion-site pain, as with intravenous epoprostenol, the risks of infection and thrombosis must be considered. Bloodstream infections, with Gram-negative infections in particular, occur more commonly in patients receiving intravenous treprostinil compared with intravenous epoprostenol.Citation54–Citation56 Data from 1,146 patients who received intravenous prostanoid therapy enrolled in the REVEAL Registry™ were reviewed by Kitterman et al.Citation56 Treatment with treprostinil was associated with a significant increase in bloodstream infection rates in comparison with epoprostenol (0.36 vs 0.12 infections per 1,000 treatment days, P<0.001); for Gram-negative bloodstream infections a 6.86-fold increased incidence was found.Citation56 Diluting treprostinil with the basic pH epoprostenol diluent instead of sterile water or saline was associated with a decreased risk of Gram-negative infections in one study.Citation57
Inhaled iloprost
Due to the complexities and risks associated with parenteral prostanoids, development of alternative modes of administration, including oral and inhaled routes, has additionally been pursued. Inhalational therapy also has the benefit of reducing systemic side effects. Success with two nebulized prostanoids, iloprost and treprostinil, was achieved. Iloprost for inhalation (Ventavis®, Actelion Pharmaceuticals Ltd) was approved by the FDA for the treatment of PAH in 2004.
Pharmacokinetics
After inhalation, an early peak in iloprost levels in the serum is seen with undetectable levels 30 to 60 minutes after 5 μg dosing.Citation58,Citation59 Half-life is reported to be 20 to 30 minutes.Citation58 In one clinical study, however, serum half-life was shorter (6.5 to 9.4 minutes); the half-life of physiologic effect (PVR decrease) was considerably longer, from 21 to 25 minutes.Citation59 The discrepancy between molecular half-life and physiologic effect is also noted with inhaled treprostinil and may suggest storage of the drug in the perivascular lung tissue.Citation60 Duration of molecular and cellular effects after receptor binding also likely outlasts the prostanoid’s presence within the microvasculature.
Iloprost is metabolized by β-oxidation and the major metabolite is excreted primarily through the urine.Citation58
Dosing and administration
Inhaled iloprost is introduced at a dose of 2.5 μg and up-titrated to 5 μg if well tolerated. Inhalations are recommended six to nine times daily (at least 2 hours apart), inhaled via a specific nebulizer system, the I-neb® Adaptive Aerosol Delivery (AAD®) system (Philips Healthcare, Amsterdam, the Netherlands).
Clinical efficacy
Several uncontrolled case reports and series described substantial benefits of inhaled iloprost in PAH.Citation61–Citation65 In the largest, a 1-year study of inhaled iloprost in 24 idiopathic PAH patients, significant improvements in 6MWD (278 to 363 meters) and hemodynamics (mPAP 59 to 52 mmHg, with cardiac output improvement) were noted.Citation65
Positive uncontrolled data led the way for the first placebo-controlled study of inhaled iloprost, Aerosolized Iloprost Randomized (AIR). AIR studied the efficacy of iloprost (2.5 or 5.0 μg per dose, six to nine times daily, median daily dose 30 μg) versus inhaled placebo in 203 patients with PAH or inoperable CTEPH. The primary endpoint, requiring a 10% increase in 6MWD, improvement in functional class, and absence of clinical deterioration, occurred in 16.8% versus 4.9% of the iloprost and placebo groups, respectively (P=0.007).Citation66 A subsequent randomized controlled trial using iloprost as add-on therapy to bosentan demonstrated near-significant improvements in 6MWD (placebo-adjusted difference 26 meters, P=0.051) and improvement in functional class and hemodynamics.Citation67 However, long-term stability on iloprost monotherapy has been questioned. In one study, only 42% of 76 patients on monotherapy were still stable on iloprost alone after 1 year.Citation68
Safety and tolerability
Safety data from AIR suggested several relevant adverse effects, including flushing (27%), cough (39%), headache (30%), and jaw pain (12%). Serious syncopal episodes were also more common in the iloprost group (five patients [5%]).Citation66 A 2-year, prospective, open-label study of iloprost in 63 patients with PAH showed similar findings; during the study only two patients discontinued therapy – one due to back pain, headache, and vertigo, and the other due to throat irritation.Citation69
Inhaled treprostinil
Relative to iloprost, the half-life of treprostinil is prolonged, and inhalations of treprostinil can therefore be dosed on a less frequent basis (four times daily versus six to nine times daily). Treprostinil for oral inhalation (Tyvaso®, United Therapeutics Corporation) was approved by the FDA for the treatment of PAH in 2009.
Pharmacokinetics
Systemic concentrations of treprostinil peak within 15 minutes after inhalation, with the maximal concentration proportional to the inhaled dose.Citation70,Citation71 In a pilot study of PAH patients administered a single 60 μg dose of inhaled treprostinil, peak concentration (Cmax) was 1.59 ng/mL, similar to the plasma concentration in healthy subjects administered intravenous treprostinil at a dose of 15 ng/kg/min.Citation40,Citation71 As serum concentrations decrease steadily following inhalation (less than 0.5 ng/mL by 120 minutes),Citation71 total drug exposure (area under the curve [AUC]) will be considerably lower with inhalational therapy than with most clinically relevant doses of subcutaneous or intravenous treprostinil. Plasma half-life, at 44 to 52 minutes, is longer than that of iloprost after inhalation.Citation72
Similar to inhaled iloprost, plasma concentration and physiologic effect profiles are offset with inhaled treprostinil. Two studies described mPAP and PVR profiles following a single inhaled dose of treprostinil (15, 30, or 45 mcg) in PAH patients.Citation60,Citation72 The hemodynamic effects of treprostinil continued out to 180 minutes after inhalation, a time when serum concentrations are negligible based on the data by Voswinckel et al.Citation71
Similar to parenteral therapy, inhaled treprostinil is metabolized predominantly in the liver by CYP2C8.Citation70
Dosing and administration
Treprostinil for inhalation is administered using a specific ultrasonic pulsed delivery system, the Tyvaso® Inhalation System, with either the newer TD-100 or older Optineb models (United Therapeutics Corporation). Doses are administered four times daily, spaced throughout waking hours, at least 4 hours apart. At initiation, patients are delivered three breaths (18 μg) per session; this is serially increased to the full dose of nine breaths (54 μg) four times daily.
Clinical efficacy
Acute hemodynamic benefits of treprostinil inhalation were first described in three patients with PAH; two went on to be treated long-term, with benefits on functional class and 6MWD seen at 3 months.Citation60 Positive effects of inhaled treprostinil on mPAP were also noted acutely in PAH patients on background bosentanCitation72 or sildenafilCitation73 therapy. Channick et al further continued the former study out to 12 weeks, with improvements in mPAP, 6MWD, and functional class also found at that time point.Citation72
The TReprostinil sodium Inhalation Used in the Management of Pulmonary arterial Hypertension (TRIUMPH I) study randomized 235 PAH patients at 31 centers to inhaled treprostinil (escalated up to 54 μg/dose, four times daily) or placebo. The majority of subjects were functional class III and all were on background therapy with bosentan or sildenafil. After 12 weeks, the median between-treatment increase in post-dose 6MWD was 20 meters (P=0.0004). Quality of life measures and brain natriuretic peptide levels also improved compared with placebo.Citation74 Extension data from TRIUMPH I, out to 24 months, were also presented. Improvements in 6MWD at 12 and 24 months were 31 and 18 meters, respectively. Survival in those on therapy was 97% at 12 months and 91% at 24 months.Citation75
Safety and tolerability
Cough (54% vs 29% taking placebo in TRIUMPH I), headache (41%), nausea (19%), dizziness (19%), flushing (15%), and throat irritation (14%) were the more common side effects noted in the clinical trial. Adverse effects lead to study withdrawal in 6%.Citation74 In the 24-month extension study, 19% discontinued treprostinil due to adverse events.Citation75
Due to inhibition of platelet function, bleeding is a concern with all formulations of prostanoids. Most relevant to inhaled treprostinil, during an open-label study, hemoptysis occurred in three patients and was fatal in one case.Citation70
Oral treprostinil
Potential for an oral prostacyclin derivative has long been of great interest. Investigations using oral beraprost, another prostacyclin analog, were initially encouraging, with a 25-meter improvement in 6MWD, the primary outcome, noted.Citation76 However, a subsequent 12-month study failed to show such efficacy beyond the 6-month mark, and beraprost was never approved in the USA or Europe as a result.Citation77
Oral treprostinil (Orenitram®, United Therapeutics Corporation) is the most recent of the prostanoid formulations to gain approval in the USA.
Pharmacokinetics
Treprostinil diolamine is provided in sustained-release tablets designed to supply the systemic circulation with a more constant concentration of the drug. In patients with PAH receiving chronic twice-daily dosing, Cmax increased with increasing doses of oral treprostinil, with time to maximum concentration within 8 hours; median 4 hours. Total exposure to treprostinil (AUC) increases linearly with increasing oral treprostinil dose.Citation78 The oral bioavailability is approximately 17%.Citation79 In comparison to the fasted state, Cmax and AUC are increased and time to maximum concentration delayed when the medication is ingested with a high-fat, high-calorie meal, though no significant difference in absorption kinetics was seen in healthy volunteers taking in four different meal types.Citation80
Treprostinil is metabolized primarily by the liver, with similar metabolic pathways as in parenteral therapy. Administration to patients with mild, moderate, and severe hepatic impairment resulted in significantly increased drug exposure.Citation81 Oral treprostinil is contraindicated in those with severe hepatic impairment.Citation79
Dosing and administration
The recommended starting dose for oral treprostinil is 0.25 mg twice daily, 12 hours apart, with food, with dose escalations of 0.25 to 0.50 mg twice daily every 3 to 4 days. However, patients not tolerant of initial dosing or escalation can be provided with 0.125 mg tablets and can be dosed three times daily (every 8 hours).Citation79 Similar to parenteral formulations, the maximum dose is limited by tolerability. The mean dose at 12 weeks in the monotherapy clinical trial was 3.4 mg twice daily.Citation82
Clinical efficacy
The FREEDOM-C study investigated effect of oral treprostinil in 350 patients with PAH on background therapy with a PDE-5I, an ERA, or both, over the 16-week study period. At study initiation the starting dose of treprostinil was 1 mg twice daily with escalation of 1 mg per dose; protocol amendments subsequently allowed for lower starting doses and incremental increases due to difficulties with tolerance of side effects. Lower discontinuation rates were observed with availability of these lower doses. A nonsignificant between-treatment improvement in 6MWD of 11 meters (P=0.07) occurred in those on treprostinil; suggestion of improved efficacy in those achieving higher treprostinil doses and with access to smaller dosing increments was observed.Citation83 Due to the described dosing and tolerance problems, FREEDOM-C2 enrolled 310 further subjects on background therapy; subjects were initiated at 0.25 mg twice daily, with incremental increases of 0.25 or 0.50 mg. Using the new protocol, the mean dose in the treatment group at 16 weeks was 3.1 mg twice daily, and difference in 6MWD between groups was again not statistically significant (10.0 meters, 95% confidence interval −2.2 to 22.0 meters, P=0.089).Citation84
Efficacy in a population not on background therapy was studied in FREEDOM-M, a randomized, double-blind, placebo-controlled trial enrolling 349 subjects with PAH not on other advanced therapies. Subjects were WHO functional class II (33%) and III (66%) and the majority (75%) had idiopathic or heritable PAH. Similar protocol amendments were enacted to allow access to lower-dose tablets for 228 patients. In the intention-to-treat analysis, at 12 weeks a mean improvement in 6MWD of 26 meters (P=0.0001) was noted.Citation82
Safety and tolerability
The most common noted adverse reactions in FREEDOM-M included headache (69% in the intention-to-treat population), diarrhea (37%), nausea (39%), jaw pain (25%), and flushing (21%). In subjects with access to the lower-dose tablets, 4% discontinued due to adverse effects.Citation82
As with other prostanoid formulations, due to platelet aggregation inhibition, an increased risk of bleeding is anticipated. Specific to the oral formulation, caution should be taken in patients with diverticulosis, as the tablet shell does not dissolve and can lodge in a diverticulum.Citation79
Transitioning between prostanoids
As the number of available PAH therapies has expanded in recent years, transitions from one line of therapy to another have become more familiar scenarios. Within prostanoid-based therapy, switching from one modality to another may be needed due to progression of symptoms (eg, change from inhaled therapy to subcutaneous or intravenous) or intolerance of mode of delivery (cough with inhaled therapy, site pain with subcutaneous, or bloodstream infections with intravenous). As newer agents have been introduced, transitions for patient safety or convenience are also recognized.
Transition from intravenous epoprostenol to parenteral treprostinil has been well described and is successful in most patients. Hospitalization is required during this change, which is typically done with stepwise increases in treprostinil combined with decreases in epoprostenol, over 1 to 2 days for change to intravenous treprostinil.Citation44,Citation85 Several days may be needed for conversion to subcutaneous therapy.Citation86,Citation87 At the end of the transition period, the treprostinil dose is typically equal to or greater than the initial epoprostenol dose and often requires further up-titration to a dosing range approximately double the starting epoprostenol dose. In one series of 31 patients making an epoprostenol to intravenous treprostinil transition, mean epoprostenol dose started at 40 ng/kg/min; at the time of hospital discharge treprostinil was administered at an average of 47 ng/kg/min, and by week 12 the mean dose was up to 83 ng/kg/min.Citation85
Select patients on low to moderate doses of parenteral prostanoids who also display reasonable functional status and right ventricular function may be appropriate to consider for transition to inhaled prostanoid therapy. In a few small series this has been successful in many patients, though some do not tolerate such a change.Citation88–Citation90 In the largest, a multicenter observational study of 37 PAH patients on parenteral epoprostenol or treprostinil (mean dose 16.8 ng/kg/min), 29 (78.4%) successfully transitioned to and remained on inhaled iloprost at 90 days. Six of these patients also had changes made to their oral therapies; background use of an ERA or a PDE-5I was additionally associated with greater likelihood of persisting on inhaled therapy.Citation89 Transitioning between inhaled therapies (iloprost and treprostinil) appears to be well tolerated and safe.Citation91
Conclusion
Associated with deficiencies in production or action, prostacyclin has been identified as having an important role in the pathobiology of PAH. The treatment of PAH patients with epoprostenol and related molecules takes advantage of their vasodilatory properties; antiproliferative and other secondary actions of these prostanoids are also noteworthy and gaining increasing attention. In clinical trials, the prostanoid agents – namely epoprostenol, treprostinil, and iloprost delivered via intravenous, subcutaneous, inhaled, and now oral routes – confer benefits in function, morbidity, and mortality to PAH patients. Much about the use of these agents, from analog selection to route of delivery to dose titration, is highly individualized and requires experience and understanding on the part of both the patient and the provider. Particularly for patients with the most severe disease, prostanoid-based therapy remains a critical component of the optimal management of PAH.
Disclosure
The author declares no conflicts of interest in this work.
References
- HoeperMMBogaardHJCondliffeRDefinitions and diagnosis of pulmonary hypertensionJ Am Coll Cardiol20136225 SupplD42D5024355641
- D’AlonzoGEBarstRJAyresSMSurvival in patients with primary pulmonary hypertension. Results from a national prospective registryAnn Intern Med199111553433491863023
- BenzaRLMillerDPBarstRJBadeschDBFrostAEMcGoonMDAn evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL RegistryChest2012142244845622281797
- BarstRJRubinLJLongWAA comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertensionN Engl J Med199633452963018532025
- GalièNManesANegroLPalazziniMBacchi-ReggianiMLBranziAA meta-analysis of randomized controlled trials in pulmonary arterial hypertensionEur Heart J200930439440319155250
- RyersonCJNayarSSwistonJRSinDDPharmacotherapy in pulmonary arterial hypertension: a systematic review and meta-analysisRespir Res2010111220113497
- GalièNCorrisPAFrostAUpdated treatment algorithm of pulmonary arterial hypertensionJ Am Coll Cardiol20136225 SupplD60D7224355643
- ZhengYYangTChenGHuEGuQXiongCProstanoid therapy for pulmonary arterial hypertension: a meta-analysis of survival outcomesEur J Clin Pharmacol2014701132124026627
- ChristmanBWMcPhersonCDNewmanJHAn imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertensionN Engl J Med1992327270751603138
- TuderRMCoolCDGeraciMWProstacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertensionAm J Respir Crit Care Med199915961925193210351941
- WhittleBJSilversteinAMMottolaDMClappLHBinding and activity of the prostacyclin receptor (IP) agonists, treprostinil and iloprost, at human prostanoid receptors: treprostinil is a potent DP1 and EP2 agonistBiochem Pharmacol2012841687522480736
- LaiYJPullamsettiSSDonyERole of the prostanoid EP4 receptor in iloprost-mediated vasodilatation in pulmonary hypertensionAm J Respir Crit Care Med2008178218819618467507
- ClappLHFinneyPTurcatoSTranSRubinLJTinkerADifferential effects of stable prostacyclin analogs on smooth muscle proliferation and cyclic AMP generation in human pulmonary arteryAm J Respir Cell Mol Biol200226219420111804870
- FalcettiEHallSMPhillipsPGSmooth muscle proliferation and role of the prostacyclin (IP) receptor in idiopathic pulmonary arterial hypertensionAm J Respir Crit Care Med201018291161117020622039
- MoncadaSHiggsEAVaneJRHuman arterial and venous tissues generate prostacyclin (prostaglandin x), a potent inhibitor of platelet aggregationLancet197718001182063657
- ZhouWHashimotoKGoleniewskaKProstaglandin I2 analogs inhibit proinflammatory cytokine production and T-cell stimulatory function of dendritic cellsJ Immunol2007178270271017202330
- RaychaudhuriBMalurABonfieldTLThe prostacyclin analogue treprostinil blocks NFkappaB nuclear translocation in human alveolar macrophagesJ Biol Chem200227736333443334812082102
- FontanaMOlschewskiHOlschewskiASchluterKDTreprostinil potentiates the positive inotropic effect of catecholamines in adult rat ventricular cardiomyocytesBr J Pharmacol2007151677978617533419
- Flolan® [package insert]LondonGlaxoSmithKline2011 Available from: https://http://www.gsksource.com/gskprm/htdocs/documents/FLOLAN.PDFAccessed September 1, 2014
- Veletri® [package insert]AllschwilActelion Pharmaceuticals Ltd2012 Available from: http://www.veletri.com/pdf/veletri_full_prescribing_information_2nd_gen.pdfAccessed September 1, 2014
- NicolasLBGutierrezMMDingemanseJComparative pharmacokinetic, pharmacodynamic, safety, and tolerability profiles of 3 different formulations of epoprostenol sodium for injection in healthy menClin Ther201335444044923498778
- SitbonODelcroixMBergotEEPITOME-2: An open-label study assessing the transition to a new formulation of intravenous epoprostenol in patients with pulmonary arterial hypertensionAm Heart J2014167221021724439982
- ChinKMBadeschDBRobbinsIMTwo formulations of epoprostenol sodium in the treatment of pulmonary arterial hypertension: EPITOME-1 (epoprostenol for injection in pulmonary arterial hypertension), a phase IV, open-label, randomized studyAm Heart J20141672218225.e124439983
- SitbonOHumbertMNunesHLong-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survivalJ Am Coll Cardiol200240478078812204511
- WatkinsWDPetersonMBCroneRKShannonDCLevineLProstacyclin and prostaglandin E1 for severe idiopathic pulmonary artery hypertensionLancet19801817710836103414
- LockJEOlleyPMCoceaniFSwyerPRRoweRDUse of prostacyclin in persistent fetal circulationLancet197918130134387797
- SzczeklikJSzczeklikANizankowskiRProstacyclin for pulmonary hypertensionLancet19802820310766107694
- GuadagniDNIkramHMaslowskiAHHaemodynamic effects of prostacyclin (PGI2) in pulmonary hypertensionBr Heart J19814543853887013775
- RubinLJGrovesBMReevesJTFrosolonoMHandelFCatoAEProstacyclin-induced acute pulmonary vasodilation in primary pulmonary hypertensionCirculation19826623343387046988
- HigenbottamTWheeldonDWellsFWallworkJLong-term treatment of primary pulmonary hypertension with continuous intravenous epoprostenol (prostacyclin)Lancet198418385104610476143976
- JonesDKHigenbottamTWWallworkJTreatment of primary pulmonary hypertension intravenous epoprostenol (prostacyclin)Br Heart J19875732702783552006
- RubinLJMendozaJHoodMTreatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol). Results of a randomized trialAnn Intern Med199011274854912107780
- BadeschDBTapsonVFMcGoonMDContinuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease. A randomized, controlled trialAnn Intern Med2000132642543410733441
- McLaughlinVVShillingtonARichSSurvival in primary pulmonary hypertension: the impact of epoprostenol therapyCirculation2002106121477148212234951
- BarstRJRubinLJMcGoonMDCaldwellEJLongWALevyPSSurvival in primary pulmonary hypertension with long-term continuous intravenous prostacyclinAnn Intern Med199412164094158053614
- PattersonJHAdamsKFJrGheorghiadeMAcute hemodynamic effects of the prostacyclin analog 15AU81 in severe congestive heart failureAm J Cardiol199575326A33A7801859
- DumbleLJGibbonsSTejpalN15 AU81, a prostacyclin analog, potentiates immunosuppression and mitigates renal injury due to cyclosporineTransplantation1993555112411288497892
- MohlerER3rdKlugherzBGoldmanRKimmelSEWadeMSehgalCMTrial of a novel prostacyclin analog, UT-15, in patients with severe intermittent claudicationVasc Med20005423123711213235
- SimonneauGBarstRJGalièNContinuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trialAm J Respir Crit Care Med2002165680080411897647
- WadeMBakerFJRoscignoRDellaMaestraWHuntTLLaiAAAbsolute bioavailability and pharmacokinetics of treprostinil sodium administered by acute subcutaneous infusionJ Clin Pharmacol2004441838814681345
- LaliberteKArnesonCJeffsRHuntTWadeMPharmacokinetics and steady-state bioequivalence of treprostinil sodium (Remodulin) administered by the intravenous and subcutaneous route to normal volunteersJ Cardiovasc Pharmacol200444220921415243302
- WadeMBakerFJRoscignoRPharmacokinetics of treprostinil sodium administered by 28-day chronic continuous subcutaneous infusionJ Clin Pharmacol200444550350915102871
- McSwainCSBenzaRShapiroSDose proportionality of treprostinil sodium administered by continuous subcutaneous and intravenous infusionJ Clin Pharmacol2008481192518094217
- Remodulin® [package insert]Silver Spring, MDUnited Therapeutics Corporation2013 Available from: https://http://www.remodulin.com/downloads/remodulin-prescribinginformation.pdfAccessed September 1, 2014
- Skoro-SajerNLangIMHarjaEKneusslMPSingWGGibbsSJA clinical comparison of slow- and rapid-escalation treprostinil dosing regimens in patients with pulmonary hypertensionClin Pharmacokinet200847961161818698881
- BarstRJGalieNNaeijeRLong-term outcome in pulmonary arterial hypertension patients treated with subcutaneous treprostinilEur Respir J20062861195120316899485
- BenzaRLTapsonVFGomberg-MaitlandMPomsABarstRJMcLaughlinVVOne-year experience with intravenous treprostinil for pulmonary arterial hypertensionJ Heart Lung Transplant201332988989623953817
- DesoleSVelik-SalchnerCFraedrichGEwertRKahlerCMSubcutaneous implantation of a new intravenous pump system for prostacyclin treatment in patients with pulmonary arterial hypertensionHeart Lung201241659960522920608
- EwertRHalankMBruchLGhofraniHAA case series of patients with severe pulmonary hypertension receiving an implantable pump for intravenous prostanoid therapyAm J Respir Crit Care Med2012186111196119823204382
- OudizRJSchilzRJBarstRJTreprostinil, a prostacyclin analogue, in pulmonary arterial hypertension associated with connective tissue diseaseChest2004126242042715302727
- LangIGomez-SanchezMKneusslMEfficacy of long-term subcutaneous treprostinil sodium therapy in pulmonary hypertensionChest200612961636164316778286
- TapsonVFGomberg-MaitlandMMcLaughlinVVSafety and efficacy of IV treprostinil for pulmonary arterial hypertension: a prospective, multicenter, open-label, 12-week trialChest2006129368368816537868
- MathierMAMcDevittSSaggarRSubcutaneous treprostinil in pulmonary arterial hypertension: Practical considerationsJ Heart Lung Transplant201029111210121720855220
- Bloodstream infections among patients treated with intravenous epoprostenol or intravenous treprostinil for pulmonary arterial hypertension – seven sites, United States, 2003–2006MMWR Morb Mortal Wkly Rep200756817017217332729
- KallenAJLedermanEBalajiABloodstream infections in patients given treatment with intravenous prostanoidsInfect Control Hosp Epidemiol200829434234918462147
- KittermanNPomsAMillerDPLombardiSFarberHWBarstRJBloodstream infections in patients with pulmonary arterial hypertension treated with intravenous prostanoids: insights from the REVEAL REGISTRY®Mayo Clinic Proc2012879825834
- RichJDGlassnerCWadeMThe effect of diluent pH on bloodstream infection rates in patients receiving IV treprostinil for pulmonary arterial hypertensionChest20121411364221659437
- Ventavis® [package insert]AllschwilActelion Pharmaceuticals Ltd112013 Available from: http://www.4ventavis.com/pdf/Ventavis_PI.pdfAccessed September 1, 2014
- OlschewskiHRohdeBBehrJPharmacodynamics and pharmacokinetics of inhaled iloprost, aerosolized by three different devices, in severe pulmonary hypertensionChest200312441294130414555558
- VoswinckelRGhofraniHAGrimmingerFSeegerWOlschewskiHInhaled treprostinil [corrected] for treatment of chronic pulmonary arterial hypertensionAnn Intern Med2006144214915016418424
- OlschewskiHWalmrathDSchermulyRGhofraniAGrimmingerFSeegerWAerosolized prostacyclin and iloprost in severe pulmonary hypertensionAnn Intern Med199612498208248610951
- OlschewskiHGhofraniHAWalmrathDTemmesfeld-WollbruckBGrimmingerFSeegerWRecovery from circulatory shock in severe primary pulmonary hypertension (PPH) with aerosolization of iloprostIntensive Care Med19982466316349681789
- StrickerHDomenighettiGFioriGMombelliGSustained improvement of performance and haemodynamics with long-term aerosolised prostacyclin therapy in severe pulmonary hypertensionSchweiz Med Wochenschr19991292492392710413827
- OlschewskiHGhofraniHASchmehlTInhaled iloprost to treat severe pulmonary hypertension. An uncontrolled trial. German PPH Study GroupAnn Intern Med2000132643544310733442
- HoeperMMSchwarzeMEhlerdingSLong-term treatment of primary pulmonary hypertension with aerosolized iloprost, a prostacyclin analogueN Engl J Med2000342251866187010861321
- OlschewskiHSimonneauGGalièNAerosolized Iloprost Randomized Study GroupInhaled iloprost for severe pulmonary hypertensionN Engl J Med2002347532232912151469
- McLaughlinVVOudizRJFrostARandomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertensionAm J Respir Crit Care Med2006174111257126316946127
- OpitzCFWenselRWinklerJClinical efficacy and survival with first-line inhaled iloprost therapy in patients with idiopathic pulmonary arterial hypertensionEur Heart J200526181895190215888496
- OlschewskiHHoeperMMBehrJLong-term therapy with inhaled iloprost in patients with pulmonary hypertensionRespir Med2010104573174020153158
- Tyvaso® [package insert]Silver Spring, MDUnited Therapeutics Corporation2014 Available from: https://http://www.tyvaso.com/pdf/Tyvaso-PI.pdfAccessed September 1, 2014
- VoswinckelREnkeBReichenbergerFFavorable effects of inhaled treprostinil in severe pulmonary hypertension: results from randomized controlled pilot studiesJ Am Coll Cardiol20064881672168117045906
- ChannickRNOlschewskiHSeegerWStaubTVoswinckelRRubinLJSafety and efficacy of inhaled treprostinil as add-on therapy to bosentan in pulmonary arterial hypertensionJ Am Coll Cardiol20064871433143717010807
- VoswinckelRReichenbergerFEnkeBAcute effects of the combination of sildenafil and inhaled treprostinil on haemodynamics and gas exchange in pulmonary hypertensionPulm Pharmacol Ther200821582483218657627
- McLaughlinVVBenzaRLRubinLJAddition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trialJ Am Coll Cardiol201055181915192220430262
- BenzaRLSeegerWMcLaughlinVVLong-term effects of inhaled treprostinil in patients with pulmonary arterial hypertension: the Treprostinil Sodium Inhalation Used in the Management of Pulmonary Arterial Hypertension (TRIUMPH) study open-label extensionJ Heart Lung Transplant201130121327133322055098
- GalièNHumbertMVachiéryJLEffects of beraprost sodium, an oral prostacyclin analogue, in patients with pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled trialJ Am Coll Cardiol20023991496150211985913
- BarstRJMcGoonMMcLaughlinVBeraprost therapy for pulmonary arterial hypertensionJ Am Coll Cardiol200341122119212512821234
- WhiteRJTorresFAllenRPharmacokinetics of oral treprostinil sustained release tablets during chronic administration to patients with pulmonary arterial hypertensionJ Cardiovasc Pharmacol201361647448123328389
- Orenitram® [package insert]Silver Spring, MDUnited Therapeutics Corporation2013 Available from: http://www.orenitram.com/dtc/pdf/Orenitram_Full_Prescribing_Information.pdfAccessed September 1, 2014
- LimAWang-SmithLKatesJLaurentAKumarPLaliberteKThe effect of different meal compositions on the oral bioavailability of treprostinil diolamine in healthy volunteersJ Clin Pharm Ther201338645045523927483
- PetersonLMarburyTMarierJLaliberteKAn evaluation of the pharmacokinetics of treprostinil diolamine in subjects with hepatic impairmentJ Clin Pharm Ther201338651852324033615
- JingZCParikhKPulidoTEfficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trialCirculation2013127562463323307827
- TapsonVFTorresFKermeenFOral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C study): a randomized controlled trialChest201214261383139022628490
- TapsonVFJingZCXuKFFREEDOM-C2 Study TeamOral treprostinil for the treatment of pulmonary arterial hypertension in patients receiving background endothelin receptor antagonist and phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C2 study): a randomized controlled trialChest2013144395295823669822
- Gomberg-MaitlandMTapsonVFBenzaRLTransition from intravenous epoprostenol to intravenous treprostinil in pulmonary hypertensionAm J Respir Crit Care Med2005172121586158916151039
- VachieryJLHillNZwickeDBarstRBlackburnSNaeijeRTransitioning from i.v. epoprostenol to subcutaneous treprostinil in pulmonary arterial hypertensionChest200212151561156512006444
- RubenfireMMcLaughlinVVAllenRPTransition from IV epoprostenol to subcutaneous treprostinil in pulmonary arterial hypertension: a controlled trialChest2007132375776317400684
- de Jesus PerezVARosenzweigERubinLJSafety and efficacy of transition from systemic prostanoids to inhaled treprostinil in pulmonary arterial hypertensionAm J Cardiol2012110101546155022853986
- ChannickRNFrantzRPKawutSMA multicenter, retrospective study of patients with pulmonary arterial hypertension transitioned from parenteral prostacyclin therapy to inhaled iloprostPulm Circ20133238138824015339
- EnderbyCYSoukupMAl OmariMZeigerTBurgerCTransition from intravenous or subcutaneous prostacyclin therapy to inhaled treprostinil in patients with pulmonary arterial hypertension: a retrospective case seriesJ Clin Pharm Ther201439549650024806480
- BourgeRCTapsonVFSafdarZRapid transition from inhaled iloprost to inhaled treprostinil in patients with pulmonary arterial hypertensionCardiovasc Ther2013311384422970909
- HoeperMMLeuchteHHalankMCombining inhaled iloprost with bosentan in patients with idiopathic pulmonary arterial hypertensionEur Respir J200628469169417012628
- HiremathJThanikachalamSParikhKExercise improvement and plasma biomarker changes with intravenous treprostinil therapy for pulmonary arterial hypertension: a placebo-controlled trialJ Heart Lung Transplant201029213714920022264
- McLaughlinVVGaineSPBarstRJEfficacy and safety of treprostinil: an epoprostenol analog for primary pulmonary hypertensionJ Cardiovasc Pharmacol200341229329912548091
