Abstract
The Clinical Assessment Program and Teflaro® Utilization Registry (CAPTURE) is a multicenter study evaluating the clinical use of ceftaroline fosamil in patients with community-acquired bacterial pneumonia (CABP) or acute bacterial skin and skin structure infection. Data were collected between August 2011 and February 2013, from 398 evaluable patients receiving treatment at 33 sites in the USA. This manuscript presents data collected from patients with CABP who received care in an intensive care unit (ICU) or in general medical wards (35% and 64% of evaluable patients, respectively). The majority of ICU and general medical ward patients had underlying comorbidities (78% and 74%, respectively), with structural lung disease being the most common (42% in the ICU and 40% in general medical wards). Patients admitted to the ICU had a longer duration of stay, a longer duration of symptoms before treatment, and a longer duration of ceftaroline fosamil therapy than did general medical ward patients. Most patients treated in the ICU and in general medical wards were given ceftaroline fosamil as second-line therapy (87% and 80%, respectively). The overall rate of clinical success for patients treated with ceftaroline fosamil was 68% in the ICU and 85% in the general medical wards. Clinical success for patients receiving ceftaroline fosamil as a second-line agent was 84% in the ICU and 86% in general medical wards. These findings indicate that ceftaroline fosamil is a viable treatment option for CABP, both in the ICU and in general medical wards.
Introduction
Community-acquired bacterial pneumonia (CABP) remains a significant cause of morbidity and mortality in adults.Citation1 The initial clinical assessment defining disease severity is an important factor in determining optimal management, including antimicrobial treatment options and location of care (ie, outpatient clinic, general medical ward, or intensive care unit [ICU]). Between 10%–36% of patients with CABP require ICU admission,Citation2,Citation3 and these patients have mortality rates of 21%–58%.Citation4
Guidelines used to evaluate the severity of CABP include those published by a joint committee from the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS).Citation5 The IDSA/ATS consensus guidelines defined the major criteria for severe CABP and/or the need for ICU admission as patients in septic shock needing vasopressors or patients with acute respiratory failure requiring intubation.
Ceftaroline is a cephalosporin with broad-spectrum in vitro activity against multiple species of gram-positive and common gram-negative bacteria.Citation6–Citation8 Some of these bacteria, such as Streptococcus pneumoniae, Staphylococcus aureus, and Haemophilus influenzae, have been associated with severe CABP requiring admission to the ICU.Citation9 In addition, resistant phenotypes of the gram-positive pathogens, for example, methicillin-resistant S. aureus (MRSA) and penicillin-resistant S. pneumoniae, remain susceptible to ceftaroline in vitro.Citation7 Similar to other cephalosporins, ceftaroline does not possess in vitro activity against gram-negative organisms that produce extended-spectrum β-lactamases.
Ceftaroline is bactericidal in vitro by irreversibly binding penicillin-binding proteins (PBPs) to inhibit the biosynthesis of the bacterial cell wall. In addition, ceftaroline exhibits high binding affinity to PBPs in S. aureus (PBP1, -2 and -3), MRSA (PBP2A), and S. pneumoniae (PBP2X, -2A and -2B).Citation10
Ceftaroline is the active form of the prodrug, ceftaroline fosamil (Forest Laboratories, LLC, New York, NY, USA), which was approved by the Food and Drug Administration (FDA) in 2010 for the indications of CABP and acute bacterial skin and skin structure infections, in the USA,Citation11 and by the European Medicines Agency for similar indications. The recommended dosage of ceftaroline fosamil in patients ≥18 years of age is 600 mg every 12 hours, by intravenous (IV) injection; however, dose adjustment of ceftaroline fosamil is required in the setting of moderate-to-severe renal impairment. Ceftaroline fosamil demonstrated noninferiority compared with ceftriaxone in two Phase III clinical studies in patients with CABP.Citation12–Citation14 In both studies, ceftaroline fosamil was well-tolerated, with a safety profile similar to that of ceftriaxone.Citation12,Citation15
The Clinical Assessment Program and Teflaro® Utilization Registry (CAPTURE) is a multicenter registry study providing information on the routine clinical use of ceftaroline fosamil in the USA. This manuscript describes the use of ceftaroline fosamil among patients with CABP admitted to the ICU, and those treated in general medical wards.
Methods
Study design
The objectives of CAPTURE were to determine patient, disease, and pathogen characteristics in patients diagnosed with CABP; to characterize the location of care and usage of ceftaroline fosamil; and to assess clinical response to treatment with ceftaroline fosamil. All study centers providing CAPTURE data were located in the USA. The data collected between August 2011 and February 2013 are presented for patients who received treatment for CABP in the ICU or in general medical wards.
CABP was defined as an acute illness with signs and symptoms consistent with a lower respiratory tract infection, and imaging consistent with bacterial pneumonia. ICU patients were defined as hospitalized patients who received any care in an ICU, and general medical ward patients were defined as hospitalized patients who received no ICU care.
Male and female patients who were enrolled had to be ≥18 years of age and have received treatment with ceftaroline fosamil for CABP. The study protocol initially required that patients had to receive treatment with two or more consecutive IV doses of ceftaroline fosamil. Subsequently, in August 2012, a protocol amendment increased the required number of consecutive doses of ceftaroline fosamil to four or more doses. Patient data were collected ≥30 days after the end of IV ceftaroline fosamil administration. Patients were excluded from the study if their charts were missing details of dosing with ceftaroline fosamil or discharge information. A further exclusion criterion was the previous extraction of data from a patient’s chart.
Ethics statement
This study was approved by the Institutional Review Board or ethics committee at each US study center providing data and was conducted in compliance with the International Conference on Harmonisation E6 Good Clinical Practice guidance.Citation16
Patient populations
All patients meeting the inclusion criteria and no exclusion criteria were defined as the enrolled population, and the evaluable population was defined by whether a clinical response (success or failure) was determined. Clinical success was defined as clinical cure with no further need for antibiotic, or clinical improvement with switch to oral antibiotic. Clinical failure was defined as discontinuation due to an adverse event or insufficient therapeutic effect, and switch to another IV antibiotic. In some cases, following a review of information where the patient was confirmed to be improving upon discontinuation of ceftaroline fosamil (with no evidence of failure), the clinical response was deemed as successful.
Data collection and analysis
As CAPTURE is a retrospective registry study collecting data on the contemporary clinical use of ceftaroline fosamil, no formal randomization or comparator group was included nor was the study powered for statistical inference. Categories of data collected included patient demographics, relevant medical history, disease characteristics, location of care, pathogens isolated, and antibiotic usage. For CABP patients, relevant medical history included congestive heart failure, stroke, lung cancer, structural lung disease, gastroesophageal reflux, chronic sinusitis, prior pneumonia, alcoholism, and smoking.
Data were collected by random selection and review of patient charts identified from pharmacy listings in the ICU or in the general medical wards. Data were summarized using descriptive statistics: mean, standard deviation (SD), median, and range for continuous variables; frequencies (n) and percentages for categorical variables. Data analyses were performed using SAS® Software Version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Sites and CABP patient populations
Between August 2011 and February 2013, 33 centers in the USA contributed CABP data to the CAPTURE study. The total number of enrolled patients with CABP was 418, of which 398 were evaluable (95%). The evaluable population was the focus of the data presented here.
Location of care
At the time of receiving ceftaroline fosamil therapy, 138 evaluable CABP patients were admitted to the ICU (35%) and 256 were treated in general medical wards (64%). Two patients received outpatient parenteral antimicrobial therapy or home IV therapy with ceftaroline fosamil, and information on location of care was missing for a further two patients, therefore, these four patients were excluded from this analysis. The mean (± SD) duration of patient stay was 21.1 (±21.5) days for the ICU patients and 11.1 (±10.3) days for patients treated in general medical wards. The median values for duration of stay were 14.0 days among ICU patients and 8.0 days among patients who received care in the general medical wards.
Patient demographics, medical history, and disease characteristics
The majority of patients with CABP who received care in an ICU were aged <65 years (57%) and were male (59%) (). Most of the patients treated in general medical wards were aged ≥65 years (53%) and were female (55%). In the ICU, 78% of patients had relevant medical histories compared with 74% of patients in general medical wards. Structural lung disease was the most common underlying comorbidity (42% in the ICU and 40% in general medical wards) (). Other common comorbidities included smoking, congestive heart failure, gastroesophageal reflux, and prior pneumonia (18%–31%, in both the ICU and general medical wards). Of the ICU patients with a history of smoking, 58% were current smokers. Of the patients in the general medical wards with a history of smoking, 44% were current smokers.
Table 1 Baseline demographic data for evaluable CABP patients treated in the ICU (n=138) and in general medical wards (n=256)
Table 2 Relevant medical history for evaluable CABP patients treated in the ICU (n=138) and in general medical wards (n=256)
The mean (± SD) duration of symptoms prior to treatment was 5.9 (±7.7) days for ICU patients and 3.4 (±5.0) days for general medical ward patients. On the day of diagnosis, the majority of patients in the ICU and in the general medical wards had two or more signs and symptoms of CABP (88% and 85%, respectively).
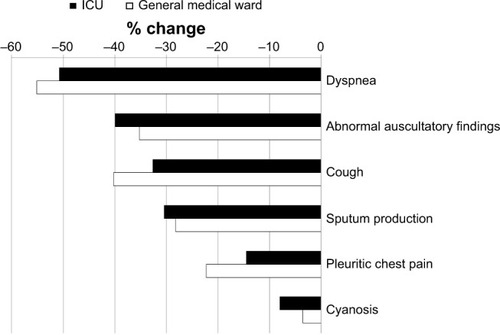
The most prevalent sign and symptom was dyspnea, regardless of location of care (79% in ICU and 71% in general medical wards). There was usually a higher frequency of each sign and symptom in the ICU than in general medical wards, for example, dyspnea (shown above), abnormal auscultatory findings (59% and 51%, respectively), sputum production (41% and 39%, respectively), and cyanosis (9% and 6%, respectively). The frequency of cough was 52% in the ICU and 66% in general medical wards, and for pleuritic chest pain, was 17% in the ICU and 27% in the general medical wards.
At the end of treatment with ceftaroline fosamil there was a decrease in the percentage of patients with each sign and symptom, when compared with those on the day of diagnosis ().
Figure 1 Change in clinical signs and symptoms at the end of treatment versus the day of diagnosis for evaluable CABP patients treated in the ICU (n=138) and in general medical wards (n=256).
Abbreviations: CABP, community-acquired bacterial pneumonia; ICU, intensive care unit.
Pathogens isolated
Pathogens were isolated from 59% of patients treated in the ICU and 29% of patients treated in general medical wards. Among the ICU patients with pathogens isolated, S. aureus was the organism most commonly recovered (57%, comprising 41% MRSA and 16% methicillin-susceptible S. aureus [MSSA]), followed by S. pneumoniae (12%). When pathogens were recovered from patients in the general medical wards, S. aureus was also most frequently isolated organism (52%, comprising 39% MRSA and 13% MSSA), followed by S. pneumoniae (8%).
Antibiotic usage
Patients were treated with ceftaroline fosamil for a mean (± SD) duration of 6.6 (±4.7) days in the ICU and 5.9 (±4.1) days in general medical wards. The number of mean (± SD) doses of ceftaroline fosamil given to patients treated in the ICU was 11.8 (±9.3) compared with 10.3 (±8.2) mean doses for patients treated in the general medical wards.
The majority of ICU and general medical ward patients had received other antibiotics before treatment with ceftaroline fosamil (87% and 80%, respectively) (). The most common types of prior antibiotics given to ICU patients were glycopeptides (40%), followed by other cephalosporins and quinolones (37% each). Other cephalosporins were the prior antibiotics most frequently administered to general medical ward patients (38%), followed by quinolones (27%) and macrolides (25%).
Table 3 Antibiotic usage for evaluable CABP patients treated in the ICU (n=138) and in general medical wards (n=256)
Ceftaroline fosamil was given as monotherapy to 32% of ICU patients and to 35% of patients in general medical wards. The remaining patients were treated with ceftaroline fosamil as concurrent therapy. When ceftaroline fosamil was given concurrently, quinolones were the most commonly coadministered antibiotics among ICU patients (26%), followed by macrolides (21%) (). Among general medical ward patients, ceftaroline fosamil was most commonly coadministered with macrolides and quinolones (25% and 20%, respectively). Other antibiotics given concurrently with ceftaroline fosamil in both locations of care included other cephalosporins, glycopeptides, and penicillins (6%–15%).
After ceftaroline fosamil treatment, ICU and general medical ward patients were most commonly switched to quinolones (15% in each location of care) ().
Clinical response
The rates of clinical success with ceftaroline fosamil were 68% in the ICU patients and 85% among patients in general medical wards. In the ICU, clinical success was 56%, 69%, and 90%, respectively, among patients with MRSA, MSSA, or S. pneumoniae. The rates of clinical success in the general medical wards were 76%, 80%, and 83%, respectively, for patients with MRSA, MSSA, or S. pneumoniae.
Clinical success was similar among patients receiving ceftaroline fosamil as first-line therapy or second-line therapy in the ICU (61% and 69%, respectively) and in the general medical wards (84% and 86%, respectively). For patients receiving ceftaroline fosamil as monotherapy, clinical success was observed in 72% of ICU patients and 81% of patients in general medical wards. For patients treated with ceftaroline fosamil as concurrent therapy, the rates of clinical success were 66% in the ICU and 88% in general medical wards.
A total of seven patients discontinued treatment with ceftaroline fosamil because of adverse events (2%); four of these patients were treated in the ICU, and three were in general medical wards.
Hospital discharge
After receiving ceftaroline fosamil, 50% of ICU patients were transferred to another care facility (including general medical ward, nursing home, rehabilitation facility, or skilled nursing facility) compared with 27% of general medical ward patients. The proportion of ICU patients discharged to home was 39%, and was 71% among general medical ward patients.
A total of eight patients died; six of these patients were being treated in the ICU, and two patients were in general medical wards (4% and 1%, respectively). Nine ICU patients and three general medical ward patients had missing information on their destination upon discharge (7% and 1%, respectively).
Discussion
Patient demographic data from the CAPTURE study showed that 59% of CABP patients treated in the ICU were male, with structural lung disease cited as the most frequent comorbidity (42%), followed by smoking (31%) and congestive heart failure (22%). These findings are supported by those from a US study comparing patients with CABP admitted to the ICU versus those who received care in general medical wards.Citation17 The authors reported that ICU patients with CABP were mostly men (88%) and had comorbidities such as chronic obstructive pulmonary disease (35%), smoking (32%), or congestive heart failure (21%). ICU patients in CAPTURE had a longer duration of stay than did general medical ward patients. Restrepo and AnzeutoCitation18 also found that patients with CABP requiring ICU admission had a longer duration of hospitalization than did those treated in general medical wards.
Clinical success among patients treated with ceftaroline fosamil was lower in the ICU (68%) than in the general medical wards (85%). Even though patients in both locations of care had comparable frequencies of underlying comorbidities, the severity of those comorbidities, and potentially, the severity of disease, may have been greater among ICU patients than those in general medical wards. In addition, patients in the ICU commonly had a higher frequency of each sign and symptom upon diagnosis than did patients in the general medical wards. Restrepo et alCitation17 concluded that ICU patients presented with more severe disease than did patients in general medical wards, based on pneumonia severity index scoring.Citation19 The CAPTURE study did not collect the results of such scoring systems as a means of comparing disease severity in patients since these scores are intended for use upon admission, whereas the majority of patients in CAPTURE received ceftaroline fosamil subsequent to the time of admission.
Changes in the management of CABP over the last 20 years indicate that the initial focus of disease treatment has shifted from diagnostic microbiological testing to early and empiric antimicrobial therapy.Citation20 Principles on the appropriateness of antimicrobial use have been discussed since the discovery of these lifesaving agents, and the need is ever more urgent in the era of increasing antimicrobial resistance. A known modifiable risk factor in dealing with the issue of resistance is the judicious prescribing of antimicrobial therapy. Many institutions have implemented antimicrobial stewardship programs to optimize favorable clinical outcomes, to improve patient safety, and to decrease the costs associated with indiscriminate antimicrobial use.Citation21
In the treatment of CABP, severity of illness and location of care are the major determinants of the antibiotics that should be prescribed to patients. In the IDSA/ATS consensus guidelines, the empiric therapy recommended for CABP patients admitted to the ICU is combination therapy comprising a β-lactam plus either a macrolide or a fluoroquinolone.Citation5 In the CAPTURE study, the majority of patients received other antibiotics prior to treatment with ceftaroline fosamil, and this likely represents second-line or use as salvage therapy.
One multicenter study compared the efficacy of levofloxacin monotherapy with the combination therapy of cefotaxime and ofloxacin among ICU patients with CABP, in France, Tunisia, and South Africa.Citation22 In this study and that of Restrepo et alCitation17 S. pneumoniae was the pathogen most frequently identified among CABP patients (27% and 8%, respectively), whereas in the CAPTURE study, S. aureus was the organism most often recovered. The difference between the most prevalent pathogen encountered in CAPTURE likely reflects the prescribing practices of clinicians in switching to ceftaroline fosamil upon the detection of S. aureus after diagnostic testing. S. aureus is also commonly identified in patients with structural lung disease,Citation5 which was the most frequent comorbidity among ICU and general medical ward patients in CAPTURE (42% and 40%, respectively). The contrast between the most prevalent pathogens recovered in each study may also indicate changing epidemiology since the studies by Restrepo et alCitation17 and Leroy et alCitation22 which most commonly identified S. pneumoniae, were conducted several years ago. Furthermore, the microbiological confirmation of S. pneumoniae can be difficult due to its fastidious nature, which may result in a low yield of this organism in culture.Citation23
The CAPTURE data showed that MRSA was isolated more frequently than MSSA. If community-acquired MRSA is suspected or identified as the causative pathogen of CABP for a patient in the ICU, then vancomycin or linezolid may be administered as part of the currently recommended empiric therapy.Citation5 In CAPTURE, ICU patients had received glycopeptides (40%) and oxazolidinones (3%) prior to ceftaroline fosamil, which suggests that they were used as therapy for MRSA.
Registry studies such as CAPTURE, which are based on the collection of data from patient charts, have limitations, including the retrospective study design, and the lack of randomization or a comparator group to control possible bias. These studies do, however, describe multiple informative factors relating to patient and disease demographics, antibiotic treatment, and clinical response. These types of studies also describe current clinical use.
Data collected from the CAPTURE study indicated that ceftaroline fosamil was used in treating CABP in the ICU and in general medical wards, including patients with underlying comorbidities and those who had received prior antibiotics. These findings further describe the use of ceftaroline fosamil in contemporary clinical practice, for the treatment of patients hospitalized with CABP.
Acknowledgments
We would like to thank the participating sites, investigators, and staff for their contributions to this study.
CAPTURE is funded by Cerexa, Inc., a wholly owned subsidiary of Forest Laboratories, LLC.
Micron Research Limited assisted in the preparation of this manuscript. This assistance was funded by Forest Laboratories, LLC.
Disclosure
C Maggiore, JA Vazquez, DJ Guervil and A Ramani are study investigators for CAPTURE. JA Vazquez and A Ramani have both received consultancy fees (speaker and advisory board) from Forest Laboratories, LLC. A Jandourek, P Cole and HD Friedland are employees of Cerexa, Inc., a wholly owned subsidiary of Forest Laboratories, LLC.
References
- FileTMMarrieTJBurden of community-acquired pneumonia in North American adultsPostgrad Med2010122213014120203464
- MarrieTJShariatzadehMRCommunity-acquired pneumonia requiring admission to an intensive care unit: a descriptive studyMedicine (Baltimore)200786210311117435590
- RestrepoMIMortensenEMRelloJBrodyJAnzuetoALate admission to the ICU in patients with community-acquired pneumonia is associated with higher mortalityChest2010137355255719880910
- DíazLAMortensenEMAnzuetoARestrepoMINovel targets in the management of pneumoniaTher Adv Respir Dis20082638740019124384
- MandellLAWunderinkRGAnzuetoAInfectious Diseases Society of AmericaAmerican Thoracic SocietyInfectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adultsClin Infect Dis200744Suppl 2S27S7217278083
- IshikawaTMatsunagaNTawadaHTAK-599, a novel N-phosphono type prodrug of anti-MRSA cephalosporin T-91825: synthesis, physicochemical and pharmacological propertiesBioorg Med Chem200311112427243712735989
- SaderHSFritscheTRKanigaKGeYJonesRNAntimicrobial activity and spectrum of PPI-0903M (T-91825), a novel cephalosporin, tested against a worldwide collection of clinical strainsAntimicrob Agents Chemother20054983501351216048970
- MushtaqSWarnerMGeYKanigaKLivermoreDMIn vitro activity of ceftaroline (PPI-0903M, T-91825) against bacteria with defined resistance mechanisms and phenotypesJ Antimicrob Chemother200760230031117548456
- WatkinsRRLemonovichTLDiagnosis and management of community-acquired pneumonia in adultsAm Fam Physician201183111299130621661712
- MoisanHPruneauMMalouinFBinding of ceftaroline to penicillin-binding proteins of Staphylococcus aureus and Streptococcus pneumoniaeJ Antimicrob Chemother201065471371620097788
- Teflaro® (ceftaroline fosamil) injection for intravenous (IV) use [package insert]St Louis, MOForest Laboratories, Inc2013 Available from: http://www.frx.com/pi/teflaro_pi.pdfAccessed March 11, 2014
- FileTMLowDEEckburgPBIntegrated analysis of FOCUS 1 and FOCUS 2: randomized, doubled-blinded, multicenter Phase 3 trials of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in patients with community-acquired pneumoniaClin Infect Dis201051121395140521067350
- FileTMJrLowDEEckburgPBFOCUS 1 Investigators. FOCUS 1: a randomized, double-blinded, multicentre, Phase III trial of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in community-acquired pneumoniaJ Antimicrob Chemother201166Suppl 3iii19iii3221482566
- LowDEFileTMJrEckburgPBFOCUS 2 Investigators. FOCUS 2: a randomized, double-blinded, multicentre, Phase III trial of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in community-acquired pneumoniaJ Antimicrob Chemother201166Suppl 3iii33iii4421482568
- RankDRFriedlandHDLaudanoJBIntegrated safety summary of FOCUS 1 and FOCUS 2 trials: Phase III randomized, double-blind studies evaluating ceftaroline fosamil for the treatment of patients with community-acquired pneumoniaJ Antimicrob Chemother201166Suppl 3iii53iii5921482570
- International Conference on HarmonisationGuidance for Industry. E6 Good Clinical Practice: Consolidated GuidanceRockville, MDDrug Information Branch (HFD-210), Center for Drug Evaluation and Research (CDER)1996 Available from: http://www.fda.gov/downloads/Drugs/Guidances/ucm073122.pdfAccessed March 11, 2014
- RestrepoMIMortensenEMVelezJAFreiCAnzuetoAA comparative study of community-acquired pneumonia patients admitted to the ward and the ICUChest2008133361061717989157
- RestrepoMIAnzuetoASevere community-acquired pneumoniaInfect Dis Clin North Am200923350352019665080
- FineMJAubleTEYealyDMA prediction rule to identify low-risk patients with community-acquired pneumoniaN Engl J Med199733642432508995086
- BartlettJGDiagnostic tests for agents of community-acquired pneumoniaClin Infect Dis201152Suppl 4S296S30421460288
- FileTMSrinivasanABartlettJGAntimicrobial stewardship: importance for patient and public healthClin Infect Dis201459Suppl 3S93S9625261547
- LeroyOSauxPBédosJPCaulinEComparison of levofloxacin and cefotaxime combined with ofloxacin for ICU patients with community-acquired pneumonia who do not require vasopressorsChest2005128117218316002932
- BlaschkeAJInterpreting assays for the detection of Streptococcus pneumoniaeClin Infect Dis201152Suppl 4S331S33721460292
