Abstract
Introduction
This retrospective cohort study evaluated whether education in combination with physiotherapy can reduce the risk of breast cancer-related lymphedema (BCRL).
Methods
We analyzed 1,217 women diagnosed with unilateral breast cancer between January 2007 and December 2011 who underwent tumor resection and axillary lymph node dissection. The patients were divided into three groups: Group A (n=415), who received neither education nor physiotherapy postsurgery; Group B (n=672), who received an educational program on BCRL between Days 0 and 7 postsurgery; and Group C (n=130), who received an educational program on BCRL between Days 0 and 7 postsurgery, followed by a physiotherapy program. All patients were monitored until October 2013 to determine whether BCRL developed. BCRL risk factors were evaluated using Cox proportional hazards models.
Results
During the follow-up, 188 patients (15.4%) developed lymphedema, including 77 (18.6%) in Group A, 101 (15.0%) in Group B, and 10 (7.7%) in Group C (P=0.010). The median period from surgery to lymphedema was 0.54 years (interquartile range =0.18–1.78). The independent risk factors for BCRL included positive axillary lymph node invasion, a higher (>20) number of dissected axillary lymph nodes, and having undergone radiation therapy, whereas receiving an educational program followed by physiotherapy was a protective factor against BCRL (hazard ratio =0.35, 95% confidence interval =0.18–0.67, P=0.002).
Conclusion
Patient education that begins within the first week postsurgery and is followed by physiotherapy is effective in reducing the risk of BCRL in women with breast cancer.
Introduction
Lymphedema refers to the accumulation of protein-rich fluid in the interstitial space caused by a compromised lymphatic system. Breast cancer-related lymphedema (BCRL) of the upper limbs is a common complication following breast cancer surgery. The incidence of BCRL was approximately 20% in breast cancer survivors who underwent tumor resection with axillary lymph node dissection (ALND).Citation1 BCRL may appear immediately or years posttreatment, with the majority diagnosed during the first 3 years.Citation2 Lymphedema causes limb and shoulder pain, heaviness, tightness, and decreased range of motion. Gross and fine motor skills are affected, the daily functions are restricted, and psychosocial relationship is impeded.Citation3
Previous studies have identified several risk factors associated with the occurrence of BCRL, which include the following: arm infection, inflammation, or injury;Citation2,Citation4–Citation6 over weight or weight gain;Citation2,Citation4–Citation7 aging;Citation4 surgery on the dominant arm;Citation4,Citation6 level of hand use;Citation5 a higher number of removed axillary lymph nodes;Citation7 being married;Citation7 having received a mastectomy,Citation8 ALND,Citation8 radiation therapy,Citation6,Citation8 or chemotherapy;Citation7 pathological status of the lymph nodes;Citation6,Citation8 and menopause.Citation6 These disease- and treatment-related factors cause tissue scarring and fibrosis. Fibrosis impairs the proliferation of lymphatic endothelial cells and compromises lymphatic regeneration.Citation9 Abnormal lymphatic microarchitecture and functions lead to a reversed lymphatic flow from the collecting vessels to the lymphatic capillaries and consequently the development of lymphedema.Citation10 At later stages, a complex interaction between lymphangiogenesis, inflammation, fibrosis, and lipid metabolism results in the proliferation and deposition of fibrotic and adipose tissues.Citation11,Citation12
Managing BCRL involves the practice of risk-reduction behavior, skin care, manual lymphatic drainage (MLD), exercise, and external compression devices.Citation13 The mainstay among these strategies continues to be symptom control. Early postoperative rehabilitation programs can improve the range of shoulder motion,Citation14 but whether they can reduce the risk of BCRL remains unclear. Only two prospective studies have shown that breast cancer patients who participated in early postoperative physiotherapy and an educational program had lower BCRL rates (5%–7%) compared with those who did not (20%–25%).Citation15,Citation16
The purpose of this study was to determine the effect of early postoperative education and physiotherapy in reducing the occurrence of secondary upper-limb lymphedema in women who underwent breast cancer surgery and ALND, and to compare this effect with those of education alone and complete nonintervention.
Methods
Data source
We performed a retrospective cohort analysis based on cancer registry data from Chi-Mei Medical Center (CMMC). This registry has prospectively collected and followed up cancer patients diagnosed at CMMC since 2002 and at the center’s Liou-Ying branch since 2006. The demographics, diagnoses, and clinical characteristics of cancers, types of cancer treatment (operation, chemotherapy, or radiation), responsiveness to treatment (remission, recurrence, or metastasis), and outcome (survival or death) were recorded. Additional clinical information not included in the registry was obtained retrospectively from the medical charts. The CMMC Institutional Review Board reviewed the medical ethics of this study and approved the study before it was commenced.
Patients
This study included women who were diagnosed with Stages 0–3 breast cancer for the first time between January 1, 2007, and December 31, 2011, and who underwent tumor resection and ALND as their primary therapy. We excluded patients with bilateral breast cancer, patients who already developed lymphedema before surgery, and patients with neurological diseases that affected ipsilateral arm, shoulder, or axilla mobility.
During the study period, 1,233 women fulfilled our inclusion criteria. After the exclusion of the 11 women who had bilateral breast cancer, and five women who fulfilled other exclusion criteria, the remaining 1,217 patients qualified as our study population. These patients were further divided into three groups: Group A, who received neither education nor physiotherapy postsurgery; Group B, who received an educational program on lymphedema between Days 0 and 7 postsurgery; and Group C, who received an educational program between Days 0 and 7 postsurgery, followed by physiotherapy.
Educational program and physical therapy
Surgeons determined whether patients required rehabilitation services in the postoperative period, and the consulted rehabilitation physician selected the appropriate program for the patient. A patient-centered educational program, if requested, was conducted in a consistent manner. A specialized physiotherapist provided instructions with printed materials to the patients. The educational program was primarily based on a published guidelineCitation17 and materials from the National Lymphedema Network.Citation18 The educational program provided information on the lymphatic system, the symptoms and signs of lymphedema, and suggestions for preventing lymphedema, such as engaging in postoperative exercise, modifying activities, massaging the scar tissue, maintaining a healthy body weight, and avoiding trauma to or infection or venipuncture of the limb. The physiotherapy program, if requested, included the following treatments: breathing exercise, postsurgical positioning, massaging of scar tissue and stretching of soft tissue, mobilization of the shoulders, and shoulder and upper extremity exercises (Table S1). Physiotherapy was conducted under the instruction of physiotherapists, began during the first week postsurgery in the hospital, and was continued at outpatient clinics postdischarge. The duration of each session was 30 minutes, and the program was administered twice weekly. The total number of physiotherapy sessions varied according to the clinical condition of each patient.
Outcome
The outcome of this study was the occurrence of lymphedema in the upper extremity in the period after surgery to October 31, 2013. The diagnosis of lymphedema was based on clinical examination (ie, a limb-to-limb difference of ≥2 cm in circumference at any measurement site along the upper limb). The staging of lymphedema was performed according to the criteria defined by the International Society of Lymphology, which include the following: Stage 1: spontaneously reversible edema; Stage 2: spontaneously irreversible edema; and Stage 3: irreversible edema and fibrosis or lymphostatic elephantiasis.Citation19
Variables of interest
The variables of interest in this study can be categorized as patient-related, disease-related, and treatment-related variables. The patient-related variables included age at breast cancer diagnosis, body mass index (BMI), and menstrual status. The disease-related variables included the Classification of Malignant Tumors (TNM) stage (Stages 0–3), histologic grade of breast cancer (Grades 1–3), status of axillary lymph node invasion (negative or positive), and tumor size. TNM staging was based on the sixth edition of the American Joint Committee on Cancer’s AJCC Cancer Staging Manual,Citation20 and the histologic grading was based on the Nottingham Score of breast cancer.Citation21 The treatment-related variables included the type of surgery (breast-conserving surgery [BCS], simple mastectomy, and modified radical mastectomy [MRM]), the number of dissected axillary lymph nodes, and receiving adjuvant treatment (radiation therapy or chemotherapy).
Statistical analysis
The patient-related, disease-related, and treatment-related variables were summarized using descriptive statistics. Continuous variables were expressed as means with standard deviations or medians with interquartile ranges (IQRs) when appropriate. To compare the various patient groups, we employed analysis of variance on each patient’s age and BMI, and the Kruskal–Wallis test for follow-up duration and time to lymphedema occurrence. We analyzed the categorical variables by using Pearson’s chi-squared test or Fisher’s exact test and the log-rank test. These categorical variables included age group (<50 years, 50–65 years, or >65 years), BMI group (<27 kg/m2 or ≥27 kg/m2), menstrual status (reached menopause or not), TNM stage (Stages 0–2 or 3), histologic grade (Grade 1, 2, or 3), axillary lymph node invasion (negative or positive), tumor size (<2 cm, 2–5 cm, or >5 cm), surgery type (BCS, simple mastectomy, or MRM), number of dissected axillary lymph nodes (dichotomized as ≤20 or >20), and receiving adjuvant radiation therapy or chemotherapy (no or yes). Univariate and multivariate Cox proportional hazards regression models were used to evaluate the relative prognostic significance of the variables in predicting the occurrence of upper-limb lymphedema.Citation22 Based on 1-year steps, the entry time was the date of breast cancer surgery and the exit time was the occurrence of postsurgical upper-limb lymphedema during the follow-up. Only variables with statistical significance in the univariate analysis were included in the multivariate analysis. The results of the multivariate analysis were adjusted for all of the variables and presented as hazard ratios (HRs) and 95% confidence intervals (CIs). To estimate the probability of lymphedema occurrence over time, the Kaplan–Meier method was employed and compared using a log-rank test. Statistical significance was set at P<0.05 for all analyses, which were conducted using SPSS software, version 19.0 (SPSS Inc., Chicago, IL, USA).
Results
The 1,217 patients comprised 415 patients (34.1%) in Group A, who received neither the educational program nor physiotherapy, 672 patients (55.2%) in Group B, who received only the educational program, and 130 patients (10.7%) in Group C, who received both the educational program and physiotherapy. The median number of physiotherapy in Group C was 12 sessions (IQR =6–48). summarizes the patient demographics and clinical characteristics. The mean age for all patients when breast cancer was first diagnosed was 52.28±11.25 years (range =25–92), and their mean BMI was 24.12±3.70 kg⁄m2 (range =14.82–41.98). More than half (n=660, 54.2%) of the patients were postmenopausal. Most patients (n=873, 71.7%) received MRM as their primary surgery. The demographics and clinical characteristics did not differ substantially between the groups, except for surgery type ().
Table 1 Patient demographics and clinical characteristics
The patients were monitored postsurgery for a median duration of 2.88 years (IQR =1.78–4.33). During the follow-up, 188 patients (15.4%) developed lymphedema, comprising 116 patients (61.7%) with Stage 1 lymphedema, 60 patients (31.9%) with Stage 2 lymphedema, and 12 patients (6.4%) with Stage 3 lymphedema. The rates of lymphedema occurrence were 18.6% in Group A (n=77), 15.0% in Group B (n=101), and 7.7% in Group C (n=10) (P=0.010, ). The median period from surgery to initial lymphedema swelling was 0.54 years (IQR =0.18–1.78), and initial swelling most commonly occurred within the first year postsurgery (n=120, 63.8%), followed by the second year (n=30, 16.0%) and the third year (n=20, 10.6%).
Table 2 Patient outcomes
To evaluate the risk factors associated with the occurrence of postsurgical lymphedema, a univariate analysis was conducted (). The following factors were associated with lymphedema: a high BMI (≥27 kg/m2, HR =1.47), late TNM stage (Stage 3, HR =2.04), positive axillary lymph node invasion (HR =2.15), large tumor size (>5 cm, HR =2.00), a higher number (>20) of dissected axillary lymph nodes (HR =1.60), and receiving adjuvant radiation therapy (HR =1.99) and chemotherapy (HR =2.17). By contrast, receiving the postoperative educational program followed by physiotherapy was negatively associated with lymphedema occurrence (HR =0.39). Other factors, such as age, menstrual status, histologic grade of tumor, surgery type, and receiving the educational program alone, did not have predictive values for the occurrence of lymphedema ().
Table 3 Crude HRs for the occurrence of lymphedema following breast cancer surgery
In the multivariate analysis, the following variables were associated with an increased risk of lymphedema (): positive axillary lymph node invasion (HR =1.55, 95% CI =1.06–2.28, P=0.025), a higher (>20) number of dissected axillary lymph nodes (HR =1.40, 95% CI =1.05–1.88, P=0.024), and receiving radiation therapy (HR =1.53, 95% CI =1.11–2.11, P=0.010). Receiving the educational program with physiotherapy remained a protective factor against lymphedema (HR =0.35, 95% CI =0.18–0.67, P=0.002) (). The Kaplan–Meier plots indicated that the patients in Group C exhibited a significantly lower risk of lymphedema during the follow-up compared with Groups A and B (log-rank test: P=0.014, ).
Figure 1 Kaplan–Meier plot of lymphedema proportions estimated for patients on different treatment schedules.
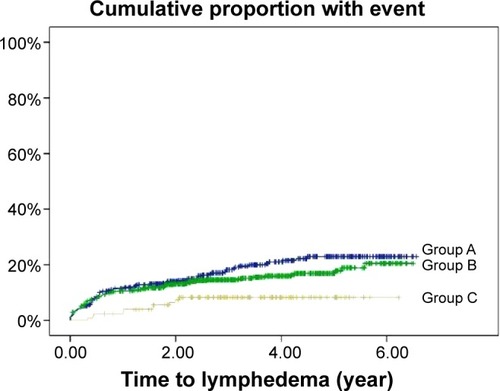
Table 4 Adjusted HRs for the occurrence of lymphedema following breast cancer surgery
Discussion
Among the women with breast cancer who were examined in this study, 15.4% developed upper-limb lymphedema postsurgery, including 18.6% in Group A, 15.0% in Group B, and 7.7% in Group C. Consistent with previous studies,Citation1 we found that having received a more extensive lymph node dissection, tumor invasion of the lymph nodes, and having received radiation therapy were significant risk factors for BCRL. Patients with any of these three risk factors may benefit from early intervention for BCRL. In addition, we demonstrated that early physiotherapy coupled with an educational program reduced the risk of BCRL. A higher percentage of the patients in Groups B and C received MRM, an aggressive procedure, whereas a higher percentage of those in Group A received the less aggressive BCS or simple mastectomy (). Therefore, patients in Groups B and C were at a higher risk of developing BCRL than those in Group A. However, our analysis showed that Group C exhibited the lowest lymphedema occurrence rate, indicating the beneficial effects of early physiotherapy and education on BCRL. By contrast, Group A (ie, no intervention) exhibited the highest lymphedema occurrence rate. The potential benefits of early education alone (Group B) versus no intervention (Group A) may have been obscured by the difference in the surgery type between the groups. Consequently, patient education was not an outcome factor for BCRL in the multivariate analysis.
Although some risk factors for BCRL are not modifiable, others are potentially preventable through patient education and physiotherapy. Their roles in lymphedema control are discussed as follows.
Effect of educational program
An educational program may increase the awareness of lymphedema and reduce the risk of BCRL through lifestyle modifications. A study of 136 breast cancer survivors demonstrated that patients who received lymphedema information reported significantly fewer symptoms and more practice of risk-reduction measures than those who did not.Citation23 In this study, however, we were unable to demonstrate the benefits of an educational program alone in preventing BCRL. One possible explanation is that patients did not implement the program’s advice into their daily lives. Poor adherence to lymphedema self-care programs is a major impediment to treatment success.Citation24 A prospective study showed that the average adherence to ten BCRL self-care modalities was suboptimal: only 31% of patients had ≥75% adherence over a 12-month period.Citation25 Some risk-reduction advice, such as avoiding venipuncture and blood pressure cuffs, are based on expert opinion and lack evidential support.Citation26,Citation27 Such advice (as well as other home-care programs) might become too complex and burdensome for breast cancer survivors to follow and maintain, leading to poor compliance. The method to deliver the educational materials is another issue. The timing, frequency, and delivery method of the risk-reduction advice, as well as the people who deliver it, may also be critical factors.Citation28 Further study is required to establish evidence-based recommendations regarding risk reduction and to investigate the optimal method for delivering educational materials to breast cancer patients.
Effect of physiotherapy
In this study, physiotherapy, which included scar massage and upper-limb and shoulder exercises, combined with patient education reduced the risk of BCRL by 65% (HR =0.35) (). This finding is comparable with those of two previous studies.Citation15,Citation16 A study in Italy compared two groups of breast cancer patients: the first group (n=25) was provided information on lymphedema presurgery, and the second group (n=58) received information on lymphedema presurgery and an early rehabilitation program postsurgery; the program included deep breathing, relaxation, neck muscle stretching, and shoulder exercise. At Day 180, 5.3% of the patients in the second group exhibited a lower incidence of BCRL, whereas that in the first group was 20.0% (P=0.036).Citation16 Another study in Spain randomized breast cancer patients to receive either early physiotherapy and patient education (n=60) or only education (n=60).Citation15 Their physiotherapy programs were similar to ours, including scar massage and shoulder exercises, as well as MLD, which our study did not include. The study found that patients receiving both physiotherapy and education had a lower lymphedema occurrence rate (6.8%) at the 1-year follow-up compared with those receiving education alone (24.5%).Citation15 The authors partially attributed the benefits of physiotherapy to MLD. However, the findings of the study conducted in Italy and those from our study indicate that scar massage and shoulder exercise programs might play active roles in reducing the risk for BCRL because both studies did not include MLD. Scar massage induces matrix remodeling of scar tissue,Citation29,Citation30 and exercise can compress the lymphatic vessels,Citation31 thereby improving lymphatic drainage and reducing the occurrence of lymphedema. Furthermore, a study has indicated that MLD had no major effect in preventing lymphedema.Citation32
Limitations
The advantages of this study are that it involved a larger sample and a longer follow-up period compared with the two discussed previous prospective studies.Citation15,Citation16 However, our study had limitations. First, this was a hospital-based retrospective cohort study. The hospital sample comprised only patients in a designated region, rendering the generalizability of results a major challenge. Second, the cancer registry may not have included all the variables of outcome significance; therefore, estimates from the multivariate analysis are subject to confounding bias from these unavailable covariates, or to residual confounding of the measured variables. However, we endeavored to include most clinically critical variables into our analyses through medical chart reviews. Third, we were unable to access information from other hospitals; hence, we could not exclude the possibility that patients in Group A (no intervention) or Group B (educational program only) received physiotherapy programs outside our hospital. Fourth, the median duration until lymphedema onset was marginally longer in Group C (1.29 years) than in Groups A (0.55 years) and B (0.44 years). Physiotherapy coupled with patient education might only delay the onset rather than prevent BCRL. A longer follow-up period could facilitate answering this question. Finally, the decision to request rehabilitation services was determined by the surgeon rather than through a randomized process. Surgeons might think that patients who have received a conservative procedure do not require rehabilitation. Such bias might explain why the surgery type was distributed unevenly among Groups A–C.
Conclusion
Patient education that begins within the first week postsurgery and is followed by physiotherapy is effective in reducing the risk of lymphedema in women who undergo breast cancer surgery with ALND.
Supplementary material
Table S1 Physiotherapy program
Disclosure
The authors have no financial relationships to disclose with regard to this study. The authors report no conflicts of interest in this work.
References
- DiSipioTRyeSNewmanBHayesSIncidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysisLancet Oncol201314650051523540561
- PetrekJASenieRTPetersMRosenPPLymphedema in a cohort of breast carcinoma survivors 20 years after diagnosisCancer20019261368137711745212
- HayesSCJohanssonKStoutNLUpper-body morbidity after breast cancer: incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of careCancer20121188 suppl2237224922488698
- MakSSYeoWLeeYMPredictors of lymphedema in patients with breast cancer undergoing axillary lymph node dissection in Hong KongNurs Res200857641642519018216
- SoranAD’AngeloGBegovicMBreast cancer-related lymphedema – what are the significant predictors and how they affect the severity of lymphedema?Breast J200612653654317238983
- van der VeenPDe VoogdtNLievensPDuquetWLamoteJSacreRLymphedema development following breast cancer surgery with full axillary resectionLymphology200437420620815693539
- PaskettEDNaughtonMJMcCoyTPCaseLDAbbottJMThe epidemiology of arm and hand swelling in premenopausal breast cancer survivorsCancer Epidemiol Biomarkers Prev200716477578217416770
- TsaiRJDennisLKLynchCFSnetselaarLGZambaGKScott-ConnerCThe risk of developing arm lymphedema among breast cancer survivors: a meta-analysis of treatment factorsAnn Surg Oncol20091671959197219365624
- AvrahamTClavinNWDaluvoySVFibrosis is a key inhibitor of lymphatic regenerationPlast Reconstr Surg2009124243845019644258
- BlumKSProulxSTLucianiPLerouxJCDetmarMDynamics of lymphatic regeneration and flow patterns after lymph node dissectionBreast Cancer Res Treat20131391818623613202
- StamatakosMStefanakiCKontzoglouKLymphedema and breast cancer: a review of the literatureBreast Cancer201118317418021331463
- LinSKimJLeeMJProspective transcriptomic pathway analysis of human lymphatic vascular insufficiency: identification and validation of a circulating biomarker panelPLoS One2012712e5202123272198
- Fialka-MoserVKorpanMVarelaEThe role of physical and rehabilitation medicine specialist in lymphoedemaAnn Phys Rehabil Med201356539641023727074
- ChanDNLuiLYSoWKEffectiveness of exercise programmes on shoulder mobility and lymphoedema after axillary lymph node dissection for breast cancer: systematic reviewJ Adv Nurs20106691902191420626480
- Torres LacombaMYuste SánchezMJZapico GoñiAEffectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: randomised, single blinded, clinical trialBMJ2010340b539620068255
- ScaffidiMVulpianiMCVetranoMEarly rehabilitation reduces the onset of complications in the upper limb following breast cancer surgeryEur J Phys Rehabil Med201248460161122510674
- HarrisSRHugiMROlivottoIALevineMSteering Committee for Clinical Practice Guidelines for the Care and Treatment of Breast CancerClinical practice guidelines for the care and treatment of breast cancer: 11. LymphedemaCMAJ2001164219119911332311
- Position statement of the National Lymphedema NetworkTopic: Lymphedema Risk Reduction Practices2011 Available from: http://www.lymphnet.org/pdfDocs/nlnriskreduction.pdfAccessed December 1, 2014
- Internaltional Society of LymphologyThe diagnosis and treatment of peripheral lymphedema: 2013 Consensus Document of the International Society of LymphologyLymphology201346111123930436
- American Joint Commmittee on CancerAJCC Cancer Staging Manual6th edHeidelbergSpringer2002
- GaleaMHBlameyRWElstonCEEllisIOThe Nottingham prognostic index in primary breast cancerBreast Cancer Res Treat19922232072191391987
- KimMKimSWLeeSUA model to estimate the risk of breast cancer-related lymphedema: combinations of treatment-related factors of the number of dissected axillary nodes, adjuvant chemotherapy, and radiation therapyInt J Radiat Oncol Biol Phys201386349850323541809
- FuMRChenCMHaberJGuthAAAxelrodDThe effect of providing information about lymphedema on the cognitive and symptom outcomes of breast cancer survivorsAnn Surg Oncol20101771847185320140528
- VignesSPorcherRArraultMDupuyAFactors influencing breast cancer-related lymphedema volume after intensive decongestive physiotherapySupport Care Cancer201119793594020495983
- BrownJCChevilleALTchouJCHarrisSRSchmitzKHPrescription and adherence to lymphedema self-care modalities among women with breast cancer-related lymphedemaSupport Care Cancer201422113514324013569
- CemalYPusicAMehraraBJPreventative measures for lymphedema: separating fact from fictionJ Am Coll Surg2011213454355121802319
- ShowalterSLBrownJCChevilleALFisherCSSataloffDSchmitzKHLifestyle risk factors associated with arm swelling among women with breast cancerAnn Surg Oncol201320384284923054109
- NielsenIGordonSSelbyABreast cancer-related lymphoedema risk reduction advice: a challenge for health professionalsCancer Treat Rev200834762162818691823
- ChanMWHinzBMcCullochCAMechanical induction of gene expression in connective tissue cellsMethods Cell Biol20109817820520816235
- RenòFSabbatiniMLombardiFIn vitro mechanical compression induces apoptosis and regulates cytokines release in hypertrophic scarsWound Repair Regen200311533133612950636
- TartaglioneGPaganMMoreseRIntradermal lymphoscintigraphy at rest and after exercise: a new technique for the functional assessment of the lymphatic system in patients with lymphoedemaNucl Med Commun201031654755120215978
- Reul-HircheHManual lymph drainage when added to advice and exercise may not be effective in preventing lymphoedema after surgery for breast cancerJ Physiother201157425822093127
