Abstract
The debate as to whether to administer ceftriaxone to neonates is likely to continue. Ceftriaxone has numerous advantages for critically ill pediatric patients. However, it is also known to contribute substantially to the development of biliary pseudolithiasis. Although pediatric patients rarely develop gallbladder disorders, this complication may lead to adverse events in high-risk patients with predisposing factors, particularly in neonates and infants treated with ceftriaxone. In this paper we present an interesting case report of a 28-day-old neonate with spontaneous severe epidural hematoma who developed biliary pseudolithiasis related to the use of ceftriaxone. We also discuss the efficacy of ceftriaxone in neonates and infants. Neonatologists and pediatric intensivists should be aware of the higher risk of co-existence of hyperbilirubinemia and gallbladder disorders while using ceftriaxone in pediatric settings.
Introduction
Recently, the pharmacokinetic and pharmacodynamic properties of a number of drugs have been studied in the pediatric population, including critically ill patients in neonatal and pediatric intensive care units. However, although the number of clinical studies has been increasing, there are still limited trial data on neonates and infants.Citation1–Citation5 For this reason, many questions regarding the individual properties of these drugs and their safety in the youngest, critically ill patients remain unanswered.
Antibiotics are a complex group of drugs and are commonly administered to children being managed in critical care. β-Lactam antibiotics, including cephalosporins, have an important role in the treatment of a variety of severe bacterial infections in infants and children. However, widespread use of these drugs may come with not only a higher risk of resistance but also a higher risk of adverse effects.Citation6–Citation11 Ceftriaxone, a third-generation cephalosporin, is commonly used for the treatment of severe infections in critically ill pediatric patients due to its advantages ie, a broad spectrum of activity, substantial resistance to β-lactamases, good penetration into tissues, predictable and adequate plasma concentrations for therapeutic effect, and a prolonged half-life. Ceftriaxone is highly bound to plasma proteins (97%), but in infants can displace bilirubin from its protein binding sites and exacerbate physiological jaundice. The kidneys (60%) and liver (40%) excrete this drug, but in patients with impaired renal function, biliary excretion may be increased. The rate of complications related to use of this drug may be higher in newborns, particularly those with low or very low birth weight, and may require intensive care.Citation12,Citation13 Age older than 24 months, Gram-negative sepsis, reduced visceral flow due to hypovolemic shock, total parenteral nutrition followed by reduced flow of bile prior surgery, hypercalcemia, and long-term treatment with high doses (>100 mg/kg/day) are the most common predisposing factors. The most common side effects associated with administration of ceftriaxone include allergic reactions (rash, eosinophilia, fever, anaphylactic shock), gastrointestinal disturbances, a transient increase in transaminases, hematological abnormalities (granulocytopenia, thrombocytopenia, hemolytic anemia) and gallbladder resolution deficiency.Citation14–Citation16 Gökçe et al suggested that the distribution of risk factors depends on both medical and non-medical changes, including geographic location, facilities available, and the reference status of the center.Citation15 Cholelithiasis, increased biliary sludge, and pseudolithiasis rarely occur in childhood, but there is a bimodal distribution characterized by two peaks, the first being in infancy and the second in early adolescence.Citation15,Citation17 In current practice, due to increased use of radiological imaging such as ultrasonography in neonatal and pediatric intensive care units this condition can be diagnosed more often.Citation18–Citation20 These diagnostic methods have contributed to an increased detection rate of this phenomenon, even in the youngest patients in pediatric intensive care unit, who may be asymptomatic.
This report describes specific considerations concerning the efficacy of ceftriaxone, a third-generation cephalosporin, in neonatal and pediatric practice, and follows the course of treatment of a 28-day-old neonate with life-threatening epidural bleeding followed by biliary pseudolithiasis due to administration of ceftriaxone as perioperative antibiotic prophylaxis.
Case report
A 28-day-old infant boy was referred from the district to the University Children’s Hospital to diagnose and treat severe spontaneous epidural bleeding, which was the original diagnosis. He was the fourth child of young parents, born from the fourth pregnancy, which ended at week 42. During the first 4 weeks of life, he did not show any symptoms of a congenital defect or any significant comorbidity.
On admission to the emergency department of a regional hospital, he was anxious and crying which was followed by a deep coma with a Glasgow Coma Scale score decreasing from 7 to 3 points. Anisocoria, bradycardia of 80–100 beats per minute, and bradypnea of 15 breaths per minute were also observed. Laboratory investigations revealed severe anemia and significant coagulopathy. No traumatic event or external action involving another person was reported in the anamnesis. Computerized tomography of the head revealed an intracranial hemorrhage and mass effect due to severe epidural hematoma of 0.11 cm with disseminated ischemic foci. The patient underwent urgent extensive frontoparietal craniotomy performed as a life-saving procedure by surgeons in the regional hospital. Broad-spectrum antibiotic prophylaxis was considered and ceftriaxone seemed to be the best choice. Ceftriaxone was administered intravenously at a dose of 100 mg/kg once a day. When the patient’s clinical condition was deemed to be compatible with stabilization of the respiratory and circulatory systems, he was transferred to the University Hospital for Children to continue advanced intensive neurosurgical treatment.
On admission to the pediatric intensive care unit, the severity of the neonate’s illness was assessed at 20 points according to PRISM (Pediatric Risk of Mortality), with a predicted risk of death of 34.4%. He was put into a barbiturate-induced coma as a neuroprotective strategy with supplementary sedation and analgesia due to the need for mechanical ventilation as a result of postoperative respiratory insufficiency. The patient was ventilated to normocapnia with pressure-controlled ventilation and parameters corresponding to his age and respiratory condition (Maquet ventilator, DrägerWerke AG, Lübeck, Germany). Empirical antibiotic therapy with ceftriaxone was continued, used previously as prophylaxis (100 mg/kg every 24 hours), because of its excellent penetration into the cerebrospinal fluid (CSF). The choice of ceftriaxone from the third-generation cephalosporins was consistent with the recommendations at our institution.
Laboratory investigations revealed deep, normocytic (mean corpuscular volume 82.9–84.6 fl) and normochromic (mean corpuscular hemoglobin concentration 34.4–36.9 g/dL) anemia (hemoglobin 7.8 g/dL, hematocrit 23%) as an effect of severe brain hemorrhage and blood loss. Additional results revealed a slightly elevated level of total bilirubin (3.7 mg/dL), a lower total protein (4.4 g/dL), and normal aspartate transaminase (36 IU/L) and alanine transaminase (11 IU/L) (). The coagulation screen showed normal antithrombin activity (70%) with an increased international normalized ratio (3.57) and a decreased prothrombin time (60%). Protein C and protein S levels were 44.7 U/dL and 98.8 U/dL, respectively. The patient required transfusions of red blood cells, fresh frozen plasma, and vitamin K supplementation. At the same time, a number of metabolic investigations were carried out in search of the primary cause of the unexplained intracranial bleeding. However, these excluded any congenital metabolic disorder.
Table 1 Mean total bilirubin, direct bilirubin, albumin, ALT, and AST levels
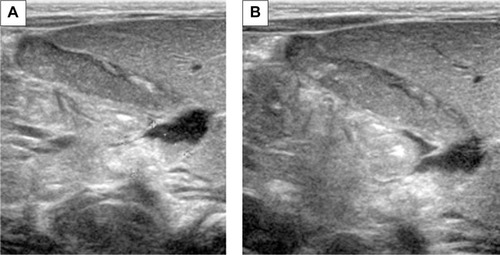
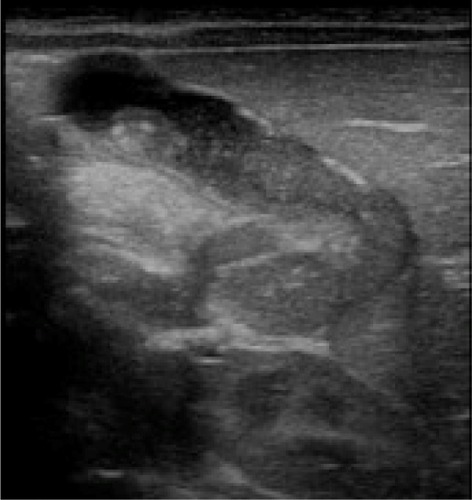
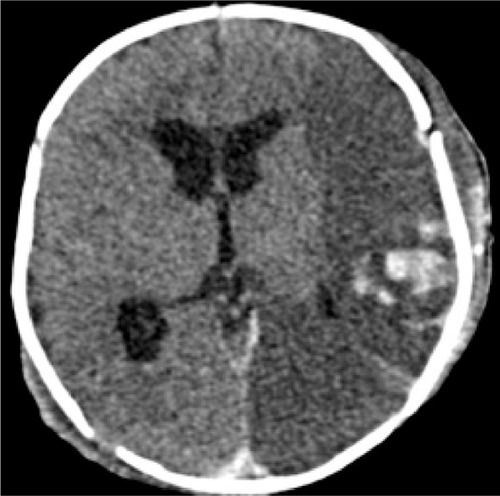
On the second day of hospital stay, the patient underwent computerized tomography of the head and ultrasound imaging of his abdomen as a routine pediatric intensive care procedure. Post-craniotomy axial computerized tomography scans revealed extensive hypodensity involving the gray as well as the white matter of the left hemisphere with bleeding foci. Midline shift and uncal herniation were also seen (). Also the following sonographic findings were observed in the abdomen: dilation of the biliary tree, thickening of the dilated cystic duct wall, common hepatic duct, and common bile duct () and mass-like sludge in the gallbladder ().
Figure 1 Post craniotomy axial CT shows extensive hypodensity involving the gray and white matter of the left hemisphere with bleeding foci. Midline shift and uncal herniation is also seen.
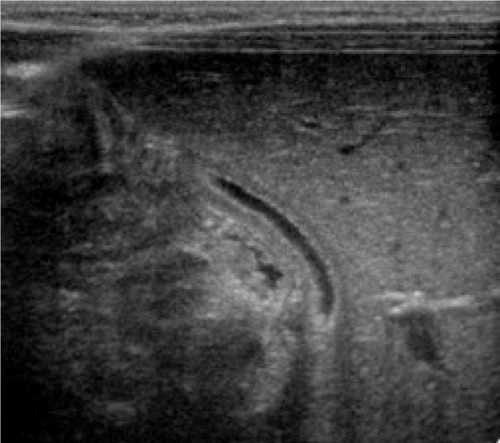
In view of the ceftriaxone therapy, the picture of biliary sludge suggested ceftriaxone-associated pseudolithiasis. Ceftriaxone was discontinued on the following day, after a total therapy that lasted 3 days. At this time, the ursodeoxycholic acid was administered to the child at a dose of 15 mg/kg twice a day and fat-soluble vitamins as supplementary therapy. After 2 weeks of treatment, ultrasonography revealed partial and gradual improvement and no surgical intervention was required (). Following another week, ultrasound examination showed complete normalization of image (). There was a gradual improvement of the radiological image over the course of 3 weeks and the bilirubin remained elevated for the first 14 days, reaching a maximum level of 8.38 mg/dL. Nevertheless, due to the prolonged hyperbilirubinemia, phototherapy was also introduced for the next 3 days, but no spectacular improvement was achieved. Despite this drug-related complication, the infant’s neurological status gradually improved, and he was able to be weaned and disconnected from the ventilator. He was successfully extubated on day 12 of his stay in pediatric intensive care. The first neurological examination was made when the child was conscious (Glasgow Coma Scale score of 12 points) and revealed signs of right-handed paresis. The patient was transferred to the surgical department to continue treatment and rehabilitation.
Discussion
Similarly to other third-generation cephalosporins, ceftriaxone has many attractive properties as an antibiotic ie, a broad spectrum of action, a long half-life, once-a-day dosing, and good tissue penetration in the central nervous system. This drug has proved to be clinically effective, relatively safe and easy to use so is the most common antibiotic used as an initial empiric therapy for critically ill patients in intensive care.Citation21 It may be difficult to diagnose pathogens especially in neonatal intensive care unit and due to the absence of such data, local epidemiology and susceptibility patterns may contribute to the selection of empiric therapy. The latest evidence-based recommendation is that ceftriaxone should be used as an initial therapy because of its broad spectrum of action and favorable benefit-risk ratio.Citation22 For these reasons, our patient was a candidate for empiric ceftriaxone therapy after craniotomy for a very severe brain injury.
The World Health Organization (WHO) and US Food Drug Administration (FDA) suggest a combination of ceftriaxone and gentamicin for the treatment of neonates aged 0–28 days who suffer from a severe illness.Citation23,Citation24 The advantages of ceftriaxone in neonates with severe infection include its high effectiveness, good tolerance and relative safety. The doses recommended for neonates younger than 72 hours are lower (50 mg/kg) than those administered to infants older than 28 days (100 mg/kg).Citation25 These higher doses were used in our patient due to the excellent blood–brain barrier penetration.
Both the WHO and FDA do not recommend simultaneous administration of ceftriaxone and solutions containing calcium to neonates, especially via intravenous infusion and using the same catheter line because of the risk of precipitation of ceftriaxone–calcium salts in the lungs and kidneys. Recent in vitro studies of neonatal and adult plasma did not reveal a direct correlation between ceftriaxone and infusions of calcium-containing solutions. This allowed the FDA in 2009 to modify its earlier restriction for ceftriaxone administration in both adult and pediatric patients (>28 days). The most recent recommendation is based on the Safety Information and Adverse Event Reporting Program.Citation26 The main change did ease the restriction of the sequential (48 hours) and separate administration of ceftriaxone and calcium-containing fluids to avoid calcium precipitation and to minimize serious organ toxicity caused by ceftriaxone–calcium precipitates.Citation6,Citation27 Concomitant use of ceftriaxone and intravenous calcium-containing products is still contraindicated in neonates (≤28 days of age). In this study, the patient’s serum calcium was normal and calcium-containing fluids were administered separately.
Hyperbilirubinemia is an important contraindication to administration of ceftriaxone in neonates, especially preterm newborns, because of the displacement of bilirubin from albumin-binding sites and an increase in serum concentrations of free bilirubin. However, risk factors for biliary pseudolithiasis include age older than 24 months and higher doses of ceftriaxone (≥2 g/day) used as a long-term treatment. Neonates and infants in particular are at higher risk of a poor outcome because of bilirubin encephalopathy.Citation28–Citation30 Therefore, intensivists should be aware of hyperbilirubinemia, pseudolithiasis and biliary sludge as an adverse reaction to ceftriaxone therapy in neonatal and pediatric intensive care unit patients. Indeed, our patient demonstrated slightly elevated bilirubin levels on the second day of treatment with ceftriaxone, but this therapy was started earlier in the surgical department as antibiotic prophylaxis and was only discontinued following an abnormal ultrasound examination. Gulian et al investigated physiological jaundice in newborns and examined three forms of bilirubin, ie, bound to albumin, unbound, and linked to cellular membranes (to erythrocytes in particular). The authors found a significantly increased number of bilirubin–erythrocyte complexes after exposure to ceftriaxone. Furthermore, the study has shown that binding of bilirubin by erythrocytes could be used as a determinant of bilirubin displacement by drugs.Citation28
Ceftriaxone-induced pseudolithiasis and biliary sludge were first described by Schaad et al.Citation30,Citation31 Since that time, numerous case reports and prospective studies have described the relationship between ceftriaxone, pseudolithiasis, and biliary sludge in both adults and children.Citation16,Citation32–Citation34 To our knowledge, there are still limited data concerning this phenomenon in neonates and infants, requiring specific factors that are typical for this extreme age group of patients.Citation12 Firstly, biliary cholelithiasis and nephrolithiasis are asymptomatic and diagnosed by means of sonographic examination rather than by laboratory tests or clinical signs.Citation35,Citation36 Secondly, it may be impossible to diagnose an adverse event in the neonatal and pediatric setting using the Naranjo adverse drug reaction probability scale despite its usefulness in adults. Some questions included in this paper, ie, those assessing retrospective events related to drugs used previously as well as assessing previous reports of adverse patient–drug reactions remained unanswered. Finally, re-administration of the same potentially toxic drug in this group of patients requires further research as it is not fully explained.Citation37
Unlike biliary pseudolithiasis, nephrolithiasis induced by kidney calcifications occurs much more rarely in children treated with ceftriaxone. The mechanism of formation of ceftriaxone precipitates in the kidneys can be explained with an elevated level of urinary uric acid. Moreover, being an organic anion, ceftriaxone behaves like a calcium-sensitive anion, which is implicated in the development of nephrolithiasis.Citation38
The pathogenesis of biliary and urinary precipitates has not been well investigated yet. The fact that only a limited number of patients treated with ceftriaxone develop pseudolithiasis indicates that individual predisposition does exist, particularly in the genetic profile. Fratzayas et al studied three pediatric patients aged 5.5, 18, and 48 months, who were receiving ceftriaxone at doses of 100 mg/kg/day and developed pseudolithiasis in the gallbladder.Citation39 All of these patients were carriers of the A(TA)7TAA polymorphism of the UGT1A1 gene encoding uridine diphosphate (UDP)-glucuronosyltransferase, the enzyme involved in glucuronidation of bilirubin.
Although ceftriaxone can interact with calcium and displace bilirubin from albumin-binding sites, increasing the risk of adverse events, one may still consider use of the drug in the neonatal period due to the considerable CSF concentrations reached.Citation21 Thus, it is possible to extend the indications to the youngest patients with primary brain injury, particularly after neurosurgical procedures. The pharma-cokinetic properties of ceftriaxone are of interest due to the high drug concentrations reaching the CSF after intravenous administration. Steele et al found ceftriaxone concentrations in the CSF of infants to be 5.4 μg/mL and 6.4 μg/mL after doses of 50 mg/kg and 70 mg/kg, respectively, with CSF to peak serum concentrations of 2.2%–2.3%.Citation40 McCracken et al assessed ceftriaxone in newborns weighing less than 1,500 g and observed significant differences ie, the mean plasma half-life was longer and the volume of distribution was larger. No other differences were found in older neonates and infants.Citation13 However, the clearance of ceftriaxone is highly dependent on developmental age and the clearance of cephalosporin increases as postnatal maturation proceeds.Citation21
The major limitation of this study is the lack of monitoring of plasma ceftriaxone concentration. However, biliary pseudolithiasis appeared as an unexpected adverse event very early on and ceftriaxone was discontinued after the third dose (48 hours after the start of therapy) of the drug. Cephalosporins like other β-lactams, macrolides and quinolones have a wide therapeutic indexCitation41,Citation42 and it is neither recommended nor possible to monitor plasma concentrations of cephalosporins in standard daily practice.
Conclusion
Ceftriaxone can cause transient reversible biliary pseudolithiasis infrequently but rarely causes nephrolithiasis or ceftriaxone–calcium precipitates in the lungs and kidneys. The incidence of this phenomenon in the pediatric population has been reported to be in the wide range from 3% to 50%, particularly in older children receiving higher doses of ceftriaxone (>2 g/kg/day) as long-term therapy (5–6 days) with spontaneous disappearance of symptoms within 1–2 weeks after cessation of the drug administration.Citation30,Citation43,Citation44 Neonates and infants are at higher risk of hyperbilirubinemia and are more vulnerable to the side effects of ceftriaxone which include pseudolithiasis and biliary sludge that can occur even in the first 2–3 days of therapy. Further research is necessary to ensure that the dosing regimens of ceftriaxone used in neonates with brain injury are entirely evidence-based.Citation21 Neonatologists and pediatric intensivists need to be aware of the higher risk of hyperbilirubinemia and gallbladder disorders associated with ceftriaxone therapy. These conditions require adequate monitoring and early detection. The protocol of repeat ultrasound monitoring seems to be the gold standard for neonates and infants undergoing ceftriaxone therapy and having a higher risk of hyperbilirubinemia and biliary pseudolithiasis.Citation44,Citation45
Disclosure
The authors report no conflicts of interest in this work.
References
- BlumerJLClinical pharmacology of midazolam in infants and childrenClin Pharmacokinet199835237479673833
- PeetersMYPrinsSAKnibbeCAPharmacokinetics and pharmacodynamics of midazolam and metabolites in nonventilated infants after craniofacial surgeryAnesthesiology200610561135114617122576
- de WildtSNde HoogMVinksAAvan der GiesenEvan den AnkerJNPopulation pharmacokinetics and metabolism of midazolam in pediatric intensive care patientsCrit Care Med20033171952195812847388
- DavisPJCookDRStillerRLDavin-RobinsonKAPharmacodynamics and pharmacokinetics of high-dose sufentanil in infants and children undergoing cardiac surgeryAnesth Analg19876632032082950809
- Woloszczuk-GebickaBGrabowskiTBoruckaBKaras-TrzeciakMPharmacokinetics of sufentanil administered with 0.2% ropivacaine as a continuous epidural infusion for postoperative pain relief in infantsPaediatr Anaesth201424996296724824135
- BradleyJSWasselRTLeeLNambiarSIntravenous ceftraxione and calcium in the neonate: assessing the risk for cardiopulmonary adverse eventsPediatrics20091234609613
- MonteSVPrescottWAJohnsonKKKuhmanLPaladinoJASafety of ceftriaxone sodium at extremes of ageExpert Opin Drug Saf20087551552318759704
- YaoYZhouRWangYFatal adverse effects of injected ceftriaxone sodium in ChinaPharmacoepidemiol Drug Saf201221111197120122761158
- Atanasković-MarkovićMVelickovićTCGavrović-JankulovićMVuckovićONestorovićBImmediate allergic reactions to cephalosporins and penicillins and their cross-reactivity in childrenPediatr Allergy Immunol200516434134715943598
- BelliardCRSibilleGAnaphylactoid shock or precipitation of calcium-ceftriaxone in a premature newborn. A case reportArch Pediatr200714219920017166703
- RomanoACaubetJCAntibiotic allergies in children and adults: from clinical symptoms to skin testing diagnosisJ Allergy Clin Immunol Pract20142131224565763
- MartinEFanconiSKälinPCeftriaxone-bilirubin-albumin interactions in the neonate an in vivo studyEur J Pediatr199315265305348335024
- McCrackenGHJrSiegelJDThrelkeldNThomasMCeftriaxone pharmacokinetics in newborn infantsAntimicrob Agents Chemother19832323413436301369
- European Medicines AgencyAnnex III. Summary of product characteristics, labelling and package leaflet Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Rocephin_30/WC500160113.pdfAccessed May 15, 2015
- GökçeSYildirimMErdoğanDA retrospective review of children with gallstones: single-center experience from Central AnatoliaTurk J Gastroenterol2014251465324918130
- RivkinAMHepatocellular enzyme elevations in a patient receiving ceftriaxoneAm J Health Syst Pharm200562192006201016174837
- AlehosseinMSotoudehKNasoohiSSalamatiPAkhtare-KhavariHCeftriaxone induced biliary pseudocholelithiasis in children. Report of 14 casesIran J Pediatr20081813137
- SoysalAErasovKAkpinarIBakirMBiliary precipitation during ceftriaxone therapy: frequency and risk factorsTurk J Pediatr200749440440718246742
- BinerBOnerNCeltikCCeftriaxone-associated biliary pseudolithiasis in childrenJ Clin Ultrasound200634521722216673364
- KutuyaNOzakiYOkazakiTSymptomatic child with ceftriaxone-associated biliary pseudocholithiasisJ Med Ultrason (2001)200835125128
- PacificiGMPharmacokinetics of cephalosporins in the neonate: a reviewClinics (Sao Paulo)20116671267127421876985
- HamiltonJLJohnSPEvaluation of fever in infants and young childrenAm Fam Physician201387425426023418797
- http://www.fda.gov Available from: http://www.fda.gov/downloads/Safety/MedWatch/safetyInformation/Safetyalertsforhumanmedical-products/ucm165639.pdfAccessed May 15, 2015
- World Health OrganizationCeftriaxone – safety in neonates Available from: http://www.who.int/selection_medicines/committees/subcommittee/2/Ceftriaxone.pdfAccessed May 15, 2015
- Van ReemptsPJVan OvermeireBMahieuLMVanckerKJClinical experience with ceftriaxone treatment in the neonateChemotherapy19954143163227555213
- BradleyJSBocchiniJAFDA eases restrictions on use of ceftriaxone in infantsAAP News2009302810.1542/aapnews.2009306-28
- SteadmanERaischDWBennettCLEvaluation of a potential interaction between ceftriaxone and calciumAntimicrob Agents Chemother20105441534154020086152
- GulianJMDalmassoCPontierFGonardVDisplacement effect of ceftriaxone on bilirubin bound to human serum albuminChemotherapy19863253994033757585
- Abu TeirMMGhitanJAbu-TahaMIDarwishSMAbu-HadidMMSpectroscopic approach of the interaction study of ceftriaxone and human serum albuminJ Biophys Struct Biol201461112
- SchaadUBTschäppelerHLentzeNJTransient formation of precipitations in the gallbladder associated with ceftriaxone therapyPediatr Infect Dis1986567087103540889
- SchaadUBWedgwood-KruckoJTschäppelerHReversible ceftriaxone-associated biliary pseudolithiasis in childrenLancet198828625141114132904533
- AlamMKBaslul KarimASMKabirMHHuqueSSSamsuzzamanMCeftriaxone associated biliary sludge in children – a study in Bangabandhu Sheikh Mujib Medical UniversityBangladesh J Child Health2013373142145
- BickfordCLSpencerAPBiliary sludge and hyperbilirubinemia associated with ceftriaxone in an adult: case report and review of the literaturePharmacotherapy200525101389139516185184
- SchaadUBStoeckelKSingle-dose pharmacokinetics of ceftriaxone in infants and young childrenAntimicrob Agents Chemother19822122482536280597
- PrinceJSSenacMOJrCeftriaxone-associated nephrolithiasis and biliary pseudolithiasis in a childPediatr Radiol200333964865112830336
- MilohTKotlus RosenbergHKochinIKerkarNInspissated bile syndrome in a neonate treated with ceftriaxoneJ Ultrasound Med20092854154419321684
- NaranjoCABustoUSellersEMA method for estimating the probability of adverse drug reactionsClin Pharmacol Ther19813022392457249508
- AklKFMasriATHjazeenMMAcute urinary retention induced by ceftriaxoneSaudi J Kidney Dis Transpl20112261226122822089789
- FratzayasALiapiOPapadopoulouANicolaidouPStamoulakatouAIs ceftriaxone-induced biliary pseudocholithiasis influenced by UDP-glucuronosyltransferase 1A1 gene polymorphisms?Case Rep Med2011201173025022110515
- SteeleRWEyreLBBradsherRWWeinfeldREPatelIHSpicehandlerJPharmacokinetics of ceftriaxone in pediatric patients with meningitisAntimicrob Agents Chemother19832321911946301362
- Hammett-StablerCAJohnsTLaboratory guidelines for monitoring of antimicrobial drugsClin Chem1998445112911409590397
- BeggEJBarclayMLKirkpatrickCJMThe therapeutic monitoring of antimicrobial agentsBr J Clin Pharmacol1999471233010073735
- PapadopoulouFEfremidisSKarydaSIncidence of ceftriaxone-associated gallbladder pseudolithiasisActa Paediatr1999881352135510626521
- AlemayehuHDesaiAAThomasPSharpSWSt PeterSDCeftriaxone-induced pseudolithiasis in children treated for perforated appendicitisPediatr Surg Int20143033233224464035
- AndruszkiewiczPSobczykDUltrasound in critical careAnaesthesiol Intensive Ther20134517718124092516